Abstract
Objective
To report on a pilot study of an inpatient intervention for suicidal adolescents, As Safe as Possible [ASAP], supported by a smartphone app [BRITE] to reduce post-discharge suicide attempts.
Method
Across two sites, 66 adolescents hospitalized for suicidal ideation (n=26) or a recent suicide attempt (n=40) were randomized to ASAP + Treatment as Usual (TAU) or TAU alone. ASAP, which focused on emotion regulation, and safety planning, was a 3 hour intervention delivered on the inpatient unit. BRITE prompted participants to rate their level of emotional distress on a daily basis, and provided personalized strategies for emotion regulation and safety planning. A blind, independent evaluator assessed post-discharge suicide attempts and ideation at 4, 12, and 24 weeks post-discharge.
Results
ASAP did not have a statistically significant effect on suicide attempt, although findings were in the hypothesized direction for occurrence of (16% vs. 31%; χ21=1.86, p=0.17; g=−0.36) and time to event (hazard ratio=0.49, 95% CI:0.16, 1.47, z=−1.27, p=0.20). Past history of an attempt moderated treatment outcome (p=0.03), with a stronger, albeit non-significant effect of ASAP in those with a history of an attempt (hazard ratio=0.23, 95% CI: 0.05, 1.09, z=−1.85, p=0.06). There were no treatment effects on suicidal ideation. The majority of participants (70%) used BRITE, with an average use of the app of a median of 19 times. Participants reported high satisfaction with the intervention and app.
Conclusions
ASAP shows promise in reducing the incidence of post-discharge attempts in hospitalized suicidal adolescents and merits further study.
Keywords: suicide prevention, inpatient intervention, adolescents, phone application
Introduction
Adolescent suicide and suicidal behavior have shown dramatic increases within the past decade (1,2). From 2007 to 2015, the adolescent suicide rate increased 30% in males and doubled in females, making suicide the second-leading cause of death in this age group (1,2). Parallel changes have been reported in emergency room visits for adolescent self-harm behavior, which has been found to have shown an annual rate of increase of 5.7% from 2009-2015, with the greatest increases in younger adolescent females (3,4).
The standard of care is to hospitalize adolescents deemed to be at highest imminent risk for a suicide attempt (5,6). However, the risk for suicidal behavior after discharge from the hospital is extraordinarily high (7,8), and currently, there are no extant interventions designed to decrease the risk of suicide attempt during this high-risk time period that encompasses the transition from inpatient to outpatient care (9).
Researchers have developed interventions for suicidal adolescents that include distress tolerance, emotion regulation, and safety planning with some promising results (10–15). Nevertheless, even with specialized interventions designed to target suicidal behavior, a large proportion of suicidal events (i.e., increase in suicidal ideation or suicide attempt) occur within the first 3 weeks of outpatient treatment following discharge (16,17), meaning that even rapid referral to outpatient care may only partially obviate the high rate of suicidal behavior post-discharge. As suicidal events commonly occur early in outpatient care following hospitalization, one possible strategy for reducing risk for these early events is to provide an intervention during the hospitalization designed to protect suicidal patients as they transition to outpatient care (15).
To address this critical gap in clinical care, we developed and tested a brief inpatient intervention designed to decrease the risk of suicide attempts post-discharge, and herein, we report on the results from a two-site, NIMH-funded treatment development randomized controlled trial of this brief intervention for suicidal, psychiatrically hospitalized adolescents. This intervention, “As Safe as Possible (ASAP)”, is designed to augment protective factors against recurrent suicidal behavior. The intervention includes a phone app (BRITE) that promotes emotion regulation and provides access to a personalized safety plan during transition from inpatient to outpatient care.
Method
Participants
Participants were adolescents (12-18 years) who presented to psychiatric inpatient units at two academic medical centers with recent suicidal ideation with plan or intent and/or a recent suicide attempt. The study was approved by both sites’ IRBs and written informed assent and consent were obtained from adolescents and their parents/guardians, respectively, upon admission. Exclusion criteria included need for residential treatment, active involvement of child protective services, mania, psychosis, autism, and intellectual disability. As shown in the CONSORT diagram (Figure 1), 104 inpatients were evaluated for eligibility, 93 were eligible, and 68 (73.1%) of those who were eligible consented. Two of these 68 were excluded after baseline (one discharged prior to completing baseline measures; one declined assessment), yielding 66 randomized. Participants were mid-adolescents (mean [M]=15.1 years, standard deviation [SD]=1.5), and largely female (89.4%) and Caucasian (77.3%). Median income bracket was 3.5 (IQR=4) corresponding to a median income range of $50,000 - $74,999. Participants had moderate to severe depression (M=18.4, SD=5.3) on the Patient Health Questionnaire (PHQ-9) (18), and significant suicidal ideation (M=66.6, SD=22.0) on the Suicidal Ideation Questionnaire-Junior (SIQ-Jr) (19). While 60% were hospitalized for a recent suicide attempt, 80% had a lifetime history of attempt as per the Columbia Suicide Severity Rating Scale (C-SSRS) (20), and 40% were hospitalized because of suicidal ideation. Most participants had a clinical diagnosis of major depression (86.4%), often comorbid with an anxiety disorder (57.6%). Diagnoses were also obtained using the Youth Self-Report (YSR) (21) with similar results.
Figure 1. Consort Diagram.
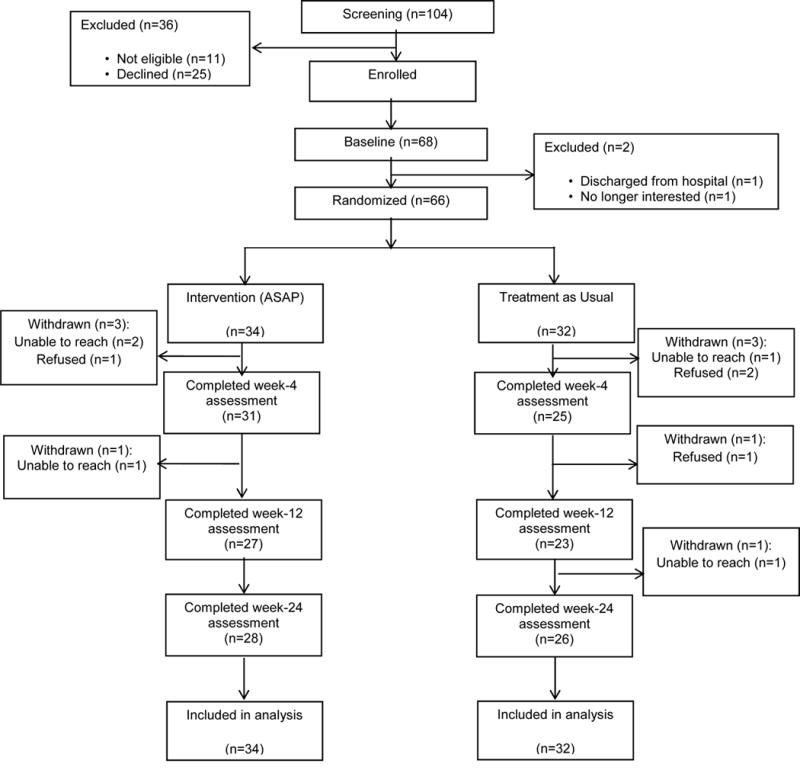
Consolidated Standards of Reporting Trials Diagram of Flow of Participants: Screening to Analysis
Study Design
Participants were randomized to ASAP or Treatment as Usual using a web-based computer program based on Efron’s biased coin toss (22). Participants were balanced both within and across sites on sex, history of past attempt, and drug/alcohol use. Positive drug/alcohol use was defined as an admitting diagnosis or positive screen on the CRAFFT (Car, Relax, Alone, Forget, Friends, and Trouble) questionnaire (23).
Treatment Intervention (ASAP)
ASAP consisted of four modules including chain analysis and safety planning; distress tolerance and emotional regulation; increasing positive affect through savoring/switching; and review of the skills, safety plan and app, and was delivered using a motivational interviewing framework on the inpatient unit (Table 1; 24, 25). The intervention used in this study (including the app described below) was first piloted in two open trials of 17 participants, and modified based on clinician and participant feedback (26).
Table 1.
Motivational Interviewing -Guided ASAP: Treatment Manual Outline
| Module 1: Adherence and Safety Plan |
| Motivational Interviewing |
| Used as framework for all modules |
| Adherence |
| Promote engagement with patient and family by developing truce and increasing supportive behaviors and positive communication; enhance motivation for behavior change and treatment adherence |
| Psychoeducation |
|
| Safety Plan |
|
|
|
| Module 2: Affect Protection: Reasons for Living, Mood monitoring, Pleasant events |
| Review Safety Plan and orient to BRITE (in this and each subsequent session) |
| Behavioral Activation and pleasant event scheduling |
|
|
|
| Module 3: Affect Protection: Savoring |
| Affect Regulation Strategies: Savoring, Switching, Distress Tolerance |
|
|
|
| Consolidation and Review |
|
|
|
| Bridging Calls (2 calls within 1-2 weeks) |
|
| Case Management/Liaison (continuing through transition to community provider) |
|
The ASAP therapist contacted the participant by phone at 1 and 2 weeks after discharge to review use of safety plan, ASAP components, app use, and adherence to recommended care.
Phone App (See Fig. 2): BRITE
Figure 2.
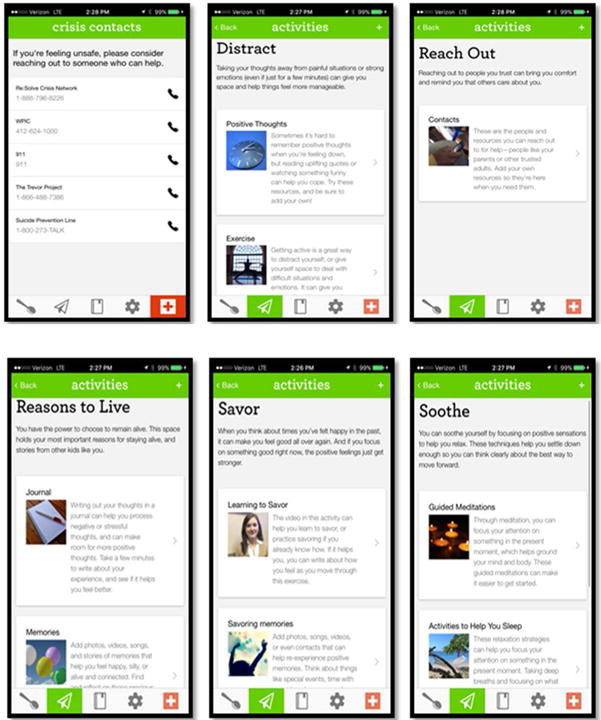
Phone App Examples
A HIPAA-compliant phone application compatible with IOS and Android platforms provided participants with convenient access to distress tolerance strategies, emotion regulation skills, and safety plan via the participant’s phone, personalized to the preferences of the participant and populated by the therapist in collaboration with the participants (26). Participants received daily texts to rate their level of emotional distress (1-5, 5=most upsetting). Based on their level of distress, participants were offered a range of distress tolerance and emotion regulation skills, with the ability to upload support materials (videos, websites, photos). For participants at the highest level of distress, the app presented the safety plan, including interpersonal support and clinical contact options.
Treatment as Usual (TAU)
Inpatient care across sites focused on diagnosis, safety assessment, stabilization, pharmacotherapy, psychoeducation, and disposition. Referrals for outpatient treatment were provided prior to discharge. Unit therapists developed a safety plan with the patient and family, although no standard protocol was followed.
Treatment Fidelity and Quality Assurance
Therapists (n=5) had at least master’s level training in psychology/counseling or were enrolled in a clinical psychology doctoral program. Therapists received training on the intervention including training in motivational interviewing with expert co-investigators (AD, TG). All treatment sessions were audio-recorded. Weekly supervision phone calls were held to review cases and monitor treatment quality. The major components of the treatment (motivational interviewing, chain analysis, distress tolerance, savoring, and safety planning) were quality rated for 20% of the ASAP sessions by study coauthors with expertise in each component. Quality rating of Motivational Interviewing was derived from Motivational Interviewing Treatment Integrity Code (MITI 3.1.1) (27); chain analysis, distress tolerance, and savoring quality ratings were derived the Cognitive Therapy Rating Scale (28); and quality of the safety planning was reviewed using the Safety Plan Rating Scale (SPRS) (29) (scales available in supplementary materials). Eighty percent or above of all sessions (N=29) were rated as adequate.
Assessments
Demographic information, intake diagnoses, and length of hospital stay were obtained from the medical record. Assessments included dimensional measures of psychopathology (YSR) (21), anxiety (the Screen for Anxiety Related Disorders [SCARED, 5-item scale]) (30), depression (PHQ-9) (18), and alcohol and drug use (CRAFFT) (22). Clinical treatment targets were reasons for living, assessed with the Reasons for Living Inventory for Adolescents (31), emotion regulation, assessed with the Regulation of Emotions Questionnaire (32), distress tolerance, assessed with the Distress Tolerance Scale (33), and social support, assessed with the Multidimensional Scale of Perceived Social Support (34).
Assessments were conducted at baseline and weeks 4, 12, and 24 by an independent evaluator (IE) blind to study condition. IEs were supervised by trained and experienced evaluators. Independent ratings of audio-taped IE evaluations on the C-SSRS showed excellent inter-rater reliability (kappa’s from 0.63, standard error [SE] = 0.27 to 0.83, SE = 0.28).
Outcome measures
Suicidal ideation and behavior
The primary and secondary outcomes were time to suicide attempt and severity of suicidal ideation, respectively. Past and current suicidal behavior and nonsuicidal self-injury were assessed with the Columbia–Suicide Severity Rating Scale, and current suicidal ideation was assessed with the self-reported Suicidal Ideation Questionnaire–Junior Highschool Version (19, 20). Time to attempt was calculated from the initiation of the intervention.
Treatment Utilization
Treatment history was obtained using week-by-week ratings on items derived from the Child and Adolescent Services Assessment (CASA) (35).
Self-reported Client Satisfaction
Satisfaction ratings with the phone app and with the ASAP intervention were obtained from the participant and parent using an adaptation of the Post-Study Satisfaction and Usability Questionnaire (PSSUQ) (36) and Client Satisfaction Questionnaire-8 (CSQ-8)(37), respectively.
Data Analysis
We aimed to recruit 80 participants, 40 in each cell, with an alpha of 0.05 (two-sided), anticipating a power of 0.80 (1–beta) to detect an effect size (Cohen’s d) of 0.63. With a recruited sample of 66 participants and 60 participants retained for follow-up (ASAP plus treatment as usual group, N=31; treatment as usual group, N=29),we were able to detect an effect size (Cohen’s d) of 0.74 and a hazard ratio of 0.48 or less for survival models.
We conducted our primary analyses with all 66 inpatients enrolled. We followed the analytic plan of our protocol by first comparing the participants’ baseline characteristics by group, by site, and by patients retained compared with patients not retained by using standard univariate statistics. We compared the rates of suicidal behavior by intervention group over the 24-week follow-up with chi-square and Fisher’s exact tests and the time to suicide attempt with Kaplan-Meier curves. We identified variables associated with time to attempt and controlled for these variables, along with age, sex, income, and site, by using Cox proportional hazards models. As per our protocol, we tested for moderation on all stratification variables (age, sex, drug or alcohol abuse, past history of suicide attempt), along with site. Mixed-effects regression with group, time (weeks since baseline), and group-by-time interaction was used to examine the effects of treatment on the course of suicidal ideation over time, and moderation was tested on all stratification variables. The impact of the intervention on putative targets (reasons for living, emotion regulation, distress tolerance, social support) was assessed using mixed effects linear regression. Effect sizes were calculated as Hedges’ g (38). All analyses were intent-to-treat, significance level was set at α=0.05, and analyses were conducted with STATA 12.1.
Results
Of the 66 randomized participants, 34 were randomized to ASAP+ Treatment as Usual (TAU) and 32 were randomized to TAU. Since the timing of our assessments was from baseline rather than discharge, 3 participants completed week 4 assessments while still in the hospital, and one of the week 12 assessments was conducted on a participant during a re-admission hospitalization. Six participants (9.1%, 3 in each treatment group) did not complete any follow-up assessments. There were no site differences in loss to follow-up (3.5% vs 13.5%, Fisher’s exact test (FET), p = 0.22). Those who were lost to follow-up, compared to those retained for at least one assessment had higher baseline suicidal ideation (M=78.3, SD=7.2 vs 65.5, SD=22.7, t= 3.11, p=0.01), and levels of self-reported anxiety (M=56.8, SD=4.9 vs. 47.6, SD=16.0, t=3.23, p=0.004).
Baseline Characteristics
Comparisons between groups at baseline are provided in the supplementary materials, Table 1. The ASAP group demonstrated greater sleep disturbance on the Pittsburgh Sleep Quality Index (39) (ASAP: M=12.4, SD=3.7; Treatment as Usual: M=10.1, SD=3.6; t=2.47, p=0.02).
Site differences included age (M=15.7, SD=1.1 vs. M=14.6, SD=1.7, t=3.04, p=0.004), annual income bracket (M=3.0, SD=1.5 vs. M=3.9, SD=1.3, z=−2.31, p=0.02), living with both biological parents (5 (17.2%) vs. 19 (52.8%), χ21=8.71, p=0.003), lifetime suicidal ideation with plan and intent (29 (100.0%) vs. 30 (83.3%), χ21=5.32, p=0.03), and weeks hospitalized (M=3.5, SD=2.8 vs. M=1.1, SD=0.2, z=6.53, p<0.001).
ASAP Intervention and Bridging Calls
The median total duration of the inpatient intervention was 2.7 hours (inter-quartile range [IQR] =2.8 hours), delivered over a median of 3 sessions (IQR=1), averaging 53 minutes per session. Two participants (5.9%) had 2 sessions; 18 participants (52.9%) had 3, 12 participants (35.3%) had 4, and 2 participants (5.9%) had 5.
Of the 34 participants who received ASAP, 10 had family sessions, with median duration of 23 minutes (IQR=15 minutes). Post-discharge, 26/34 participants received at least one bridging phone call (median=1.5, IQR=2), with median total duration of 17.5 minutes (IQR=35).
Follow-up Assessments
Suicidal behavior
There were no significant differences in the rates of post-discharge suicide attempts, although the findings were in the hypothesized direction (ASAP + TAU, n=5, 16.1% vs. TAU n=9, 31%;χ21=1.86, p=0.17; g=−0.36) (Table 2), as were the findings for time to attempt in ASAP vs. TAU (Wilcoxon: χ21=0.76, p=0.38; Log-Rank: χ21=1.74, p=0.19; hazard ratio=0.49, 95% CI:0.16, 1.47, z=−1.27, p=0.20).
Table 2.
Suicidality and Self-Harm during Follow-Up- Data Aggregated from Week 4, 12, and 24 Interviews
| Treatment as Usual (n = 29) | ASAP + Treatment as Usual (n = 31) | Test | P value | Effect Size | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Attempt | 9 | 31.0 | 5 | 16.1 | χ21=186 | 0.17 | g =−0.36 | −0.87, 0.15 |
| Ideation | 22 | 75.9 | 21 | 67.7 | χ21=0.49 | 0.49 | g =−0.18 | −0.69, 0.33 |
| Ideation/Attempt | 23 | 79.3 | 21 | 67.7 | χ21=1.03 | 0.31 | g =−0.26 | −0.77, 0.24 |
| Suicide Related Behavior | 3 | 10.3 | 4 | 12.9 | FET | >0.99 | g =0.08 | −0.43, 0.59 |
| Non-Suicidal Self Injury | 13 | 44.8 | 14 | 45.2 | χ21=0.001 | 0.98 | g =0.01 | −0.50, 0.51 |
| Attempt/Suicide Related Behavior | 9 | 31.0 | 7 | 22.6 | χ21=0.55 | 0.46 | g =−0.19 | −0.70, 0.32 |
| Attempt/Suicide Related Behavior/Non-Suicidal Self Injury | 14 | 48.3 | 15 | 48.4 | χ21=0.0001 | 0.99 | g =0.002 | −0.50, 0.51 |
|
| ||||||||
| Mean | SD | Mean | SD | Test | P value | Effect Size | 95% CI | |
|
| ||||||||
| Number of Attempts | 0.7 | 1.4 | 0.9 | 3.6 | z=1.30 | 0.19 | g =0.07 | −0.44, 0.57 |
A previous history of a suicide attempt moderated treatment outcome (hazard ratio=0.07, 95% CI: 0.01, 0.79, z=−2.15, p=0.03), with a stronger, albeit non-significant effect of ASAP in those with a history of a suicide attempt (hazard ratio=0.23, 95% CI: 0.05, 1.09, z=−1.85, p=0.06).
Because of the intent of the intervention to reduce attempts post-discharge, we re-analyzed the data excluding three participants who were still in the hospital at the time of attempt. The differences in rates of attempts between the groups was not significant, but in the hypothesized direction (10.3% [n=3] vs. 28.6%, [n=8], χ21=3.04, p=0.08, g=−0.47), as was the difference in time to attempt (Wilcoxon: χ21=1.66, p=0.20; Log-Rank: χ21=3.02, p=0.08, hazard ratio=0.33, 95% CI:0.09, 1.26, z=−1.62, p=0.11). After adjusting for significant covariates related to time to attempt (age), the ASAP group had a longer time to attempt (hazard ratio=0.19, 95% CI:0.04, 0.85, z=−2.18, p=0.03).
Mixed effects regression showed an effect of time on suicidal ideation for the entire sample (β=−0.57, 95% CI: −0.84, −0.30, z=−4.09, p<0.001), but not for group or for a group by time interaction, indicating a similar decrease in suicidal ideation over time between the two groups.
Mixed effects regression indicated an increase in social support over time for the ASAP group as compared to TAU (group by time: β=0.32, 95% CI: 0.08, 0.56, z=2.60, p=0.01). No other treatment targets showed an effect for group over time.
Within ASAP, participants whose families attended one or more treatment sessions had lower ideation over time (treatment by time interaction, β=−0.61, 95% CI: −1.12, −0.11, z=−2.38, p=0.02), although there was no impact on time to attempt.
Phone app (Table 3)
Table 3.
Use of the Phone App
| N | % | |||
|---|---|---|---|---|
| Viewed (Yes/No) | 24 | 70.6 | ||
| Added Content (Yes/No) | 18 | 75.0 | ||
| Removed Content (Yes/No) | 10 | 41.7 | ||
|
| ||||
| Mean | SD | Range | Median | |
|
| ||||
| # Times added content | 14.6 | 10.7 | 2-41 | 10 |
| # Times removed content | 9.5 | 5.9 | 2-25 | 8.5 |
| # Times entered mood rating | 28.7 | 29.6 | 1-119 | 19 |
| # Times viewed crisis contacts | 0.7 | 1.7 | 0-7 | 0 |
Most ASAP participants (70.6%) used the app at least once. Participants rated their mood a median of 19 times (IQR=54), 75.0% added content (number of times content added: median=10, IQR=17), and 41.7% removed content (number of times content removed: median=8.5, IQR=1). Nearly half (45.5%) activated contacts as part of their safety plan at least once; the median number of times participants accessed their contacts was 21 (IQR=34). We did not find a relationship between the frequency of app use and risk for suicide attempt (hazard ratio=1.01, 95% CI: 0.98, 1.04, z=0.54, p=0.59) or decline in suicidal ideation (spearman ρ =−0.24, p=0.23), although the relationship between frequency of mood ratings and increase in reasons for living was in the hypothesized direction (ρ=0.37, p=0.08).
Participant Satisfaction
The scores on the app using the CSUQ indicated a generally high level of satisfaction (lower scores indicate more satisfaction; range 10 to 70) for week 4: M = 17.6, SD = 7.1; week 12: M = 18.6, SD = 10.4; week 24: M = 18.4, SD = 8.0.
There was no difference in client satisfaction at the end of the intervention, as measured with the Computer Systems Usability Questionnaire (40), although results were in the hypothesized direction (ASAP plus treatment as usual group: mean=26.6 [SD=3.8]; treatment as usual group: mean=24.1 [SD=5.2]; z=1.64, p=0.10, g=0.58).
Service use/Medications
There were no differences between the groups for duration of hospital stay for the index episode (ASAP: mean=2.1 weeks, SD=2.3; TAU: mean=2.3 weeks, SD=2.3; z=−0.71, p=0.48). Nearly all participants engaged in some type of treatment after discharge from hospital (ASAP vs. TAU: 96.7% (n=29) vs 96.6% (n=28)). Unexpectedly, patients in the ASAP plus treatment as usual group were less likely than patients in the treatment as usual group to participate in outpatient therapy (60.0% [N=18] compared with 89.7% [N=26]; c2=6.84, df=1, p=0.01, g=–0.73). However, they had higher, albeit non-statistically significant, rates of use of more intensive interventions (e.g., intensive outpatient treatment, partial hospitalization, and residential treatment) (ASAP plus treatment as usual group: 73.1% [N=19]; treatment as usual group: 53.6%[N=15]; x2=2.20, df=1, p=0.14, g=0.41). The two groups showed similar rates of emergency department visits (ASAP plus treatment as usual group: 13.3% [N=4]; treatment as usual group: 10.3% [N=3]; Fisher’s exact test, p.0.99, g=0.09). Stratified contrasts found similar trends between ASAP and TAU with regard to rates and time to attempt for those participants in more intensive programs, ASAP+TAU vs. TAU (10.5% vs. 26.7%; hazard ratio=0.42, 95% CI: 0.08, 2.37, z=−0.98, p=0.33), and for those in outpatient programs (ASAP+TAU: 14.3% vs. TAU: 38.5%; hazard ratio=0.23, 95% CI: 0.03, 2.02, z=−1.33, p=0.18).
Participants who had follow-up assessments were evaluated on medication use after hospital discharge. Participants assigned to ASAP plus treatment as usual were more likely to use a pharmacological sleep aid (ASAP plus treatment as usual group: N=19 [63.3%]; treatment as usual group: N=10 [34.5%]; x2=4.91, df=1, p=0.03), whereas participants in the treatment as usual group were more likely to receive antipsychotic medication (treatment as usual group: N=12 [41.4%]; ASAP plus treatment as usual group: N=4 [13.3%]; x2=5.87, df=1, p=0.02). Adjusting for differences in medication use did not alter our initial findings (see supplementary Tables 2–4).
Discussion
In this treatment development study, we demonstrated the acceptability and feasibility of the ASAP intervention and supporting BRITE app. The RCT was not large enough to detect even substantial clinical effects, but the rates of suicide attempt in those assigned to ASAP/BRITE were half of those in TAU, indicating that this intervention is promising and may have utility in the reduction of post-discharge suicide attempts in hospitalized, suicidal adolescents.
To our knowledge, this is the first inpatient intervention designed to reduce suicide attempts post-discharge, other than one study conducted 2 decades ago that sent caring letters post-discharge to adults at high risk for suicide who refused further outpatient care (41). ASAP is brief, focused, supported by an app, and showed its strongest effects in the most vulnerable subsample in this study, namely those who made a previous suicide attempt. Supporting the likelihood that this intervention has the potential to be widely disseminated, ASAP/BRITE were well accepted and a high proportion of eligible participants were recruited into the study. However, the sample size was small, with limited power, and was largely female and Caucasian, limiting our ability to generalize to other populations. We did not use structured diagnostic assessments, but instead relied on clinical diagnoses and a self-report diagnostic tool. Another limitation was the difficulty engaging families in the intervention during hospitalization. Finally, our design did not allow us to determine which components of the intervention or phone app were effective.
As hypothesized, those participants assigned to ASAP tended to have a lower hazard of suicide attempts post-discharge, with significant moderation in those with a history of a suicide attempt. Sensitivity analyses excluding 3 participants who made suicide attempts while still hospitalized continued to be in the hypothesized direction. While a larger sample will be required to be able to definitively assert that ASAP is effective, the findings are plausible because the focus of the intervention was on well-recognized intervention targets for suicidal behavior.
There were no main effects for the intervention on suicidal ideation, which is in keeping with our primary focus on reducing risk of acting on suicidal urges. Participants in the ASAP group showed a higher level of social support over time compared to those in the Treatment as Usual condition. Thus, the intervention appeared to impact social support, which may be related to the lower rate of attempts in this treatment group.
The majority of the ASAP participants used the phone app actively, modified content, frequently rated their level of distress, activated the personal contacts on their safety plan, and reported high satisfaction with the app. Future studies will be required to determine if the app adds to the ASAP intervention, and if so, which components are the most important in protecting youth from suicidal behavior.
While both treatment groups showed very high rates of participation in treatment, after discharge, the ASAP group was statistically less likely to be involved in outpatient care and while not statistically significant, had higher rates of involvement in higher intensity treatments. However, the impact of ASAP+TAU vs. TAU on subsequent suicide attempts was similar in participants who were in higher levels of care and those who were in outpatient treatment.
The low rate of family engagement in the ASAP intervention speaks to the rapid pace of inpatient care, during which parents may not have had the time or inclination to participate in research above and beyond visitation and inpatient therapeutic activities. Of note is that participants whose families received at least one ASAP session had a greater decline in suicidal ideation over time than those who did not receive the intervention (even after adjusting for baseline differences). Since future studies of ASAP on inpatient units will most likely be delivered by inpatient staff, they may be in a better position to promote and improve family engagement.
In summary, these findings indicate that ASAP and BRITE are acceptable, feasible, and promising interventions for hospitalized suicidal adolescents. Future studies are needed to determine which aspects of ASAP and BRITE are most active, and hence worth disseminating, and, whether the intervention can be effectively delivered on inpatient units by existing staff.
Supplementary Material
Acknowledgments
Disclosures
Dr. Kennard receives research support from the National Institute of Mental Health and royalties from Guilford Press. Dr. Brent receives research support from NIMH, royalties from Guilford Press, royalties from the electronic self-rated version of the C-SSRS from ERT, Inc., royalties from performing duties as an UptoDate Psychiatry Section Editor, and consulting fees from Healthwise. Dr. Goldstein receives research support from the National Institute of Mental Health, the American Foundation for Suicide Prevention, and the Brain and Behavior Foundation and royalties from Guilford Press. Dr. Foxwell receives royalties from Guilford Press. Dr. Douaihy receives research support from Substance Abuse and Mental Health Services Administration, National Institute of Drug Abuse, NIMH, Health Resources and Services Administration, and Alkermes, and receives royalties from Oxford University Press and PESI Publishing and Media. Funding for this study was provided by NIMH R34 MH100375 (PI’s David Brent and Betsy Kennard).
The authors are grateful to the following contributors:
Evaluators: Jessica Jones, MA, Lindsey Jenkins, BA
DSMB: Boris Birmaher, MD, Graham Emslie, MD, Dara Sakolsky, PhD, MD
Consultants: Anthony Spirito, PhD, Shirley Yen, PhD, Judith Callan, PhD, Rasim Diler, MD, Clinical and administrative support: Andrew Dietrich, MD, Laura Stone, MD, Jane LeVieux, PhD, Jeanne Nightingale, RN, MS, Jennifer Hughes, PhD, Frank DePietro, MD, PhD Rasim Diler, MD, Garrett Sparks, MD, and Henry Patrick Driscoll, MD
Application design and development: Raelynn O’Leary and Ashley Deal of Dezudio and Derek Wahila of Wahila Creative
We also are thankful to all of the children and families who participated in this study.
Footnotes
The other authors report no conflict of interests or financial disclosures.
Clinical trial registration information: Brief Intervention for Suicide Risk Reduction in High Risk Adolescents (ASAP): http://clinicaltrials.gov/; ClinicalTrials.gov identifier (NCT number): NCT02272179
References
- 1.WISQARS Leading Causes of Death. Centers for Disease Control and Prevention. From http://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html Published June 24, 2015.
- 2.Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014. Hyattsville, MD: National Center for Health Statistics; 2016. (NCHS data brief, no 241). [Google Scholar]
- 3.Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931–1933. doi: 10.1001/jama.2017.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han B, Compton WM, Blanco C, Colpe L, Huang L, McKeon R. National trends in the prevalence of suicidal ideation and behavior among young adults and receipt of mental health care among suicidal young adults. J Am Acad Child Adolesc Psychiatry. 2018;57:20–27. doi: 10.1016/j.jaac.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Shain BN. American Academy of Pediatrics Committee on Adolescence: Suicide and suicide attempts in adolescents. Pediatrics. 2016;138(1):e20161420. [Google Scholar]
- 6.American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40:24S. doi: 10.1097/00004583-200107001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Goldston DB, Daniel SS, Reboussin DM, Reboussin BA, Frazier PH, Kelley AE. Suicide attempts among formerly hospitalized adolescents: a prospective naturalistic study of risk during the first five years after discharge. J Am Acad Child Adolesc Psychiatry. 1999;38:660–671. doi: 10.1097/00004583-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Chung DT, Ryan CJ, Hadji-Pavlovic D, et al. Suicide rates after discharge from psychiatric facilities: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(7):694–702. doi: 10.1001/jamapsychiatry.2017.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olfson M. Suicide risk after psychiatric hospital discharge. JAMA Psychiatry. 2017;74(7):669–670. doi: 10.1001/jamapsychiatry.2017.1043. [DOI] [PubMed] [Google Scholar]
- 10.Asarnow JR, Hughes JL, Babeva KN, Sugar CA. Cognitive-behavioral family treatment for suicide attempt prevention: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2017;56(6):506–514. doi: 10.1016/j.jaac.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond GS, Wintersteen MB, Brown GK, et al. Attachment-based family therapy for adolescents with suicidal ideation: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2010;49(2):122–131. doi: 10.1097/00004583-201002000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Mehlum L, Tormoen AJ, Ramberg M, et al. Dialectical behavior therapy for adolescents with repeated suicidal and self-harming behavior: a randomized trial. J Am Acad Child Adolesc Psychiatry. 2014;53:1082–1091. doi: 10.1016/j.jaac.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Stanley B, Brown G, Brent DA, et al. Cognitive-behavioral therapy for suicide prevention (CBT-SP): treatment model, feasibility, and acceptability. J Am Acad Child Adolesc Psychiatry. 2009;48(10):1005–1013. doi: 10.1097/CHI.0b013e3181b5dbfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ougrin D, Tranah T, Stahl D, Moran P, Asarnow JR. Therapeutic interventions for suicide attempts and self-harm in adolescents: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2014;54(2):97–107. doi: 10.1016/j.jaac.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Brent DA, McMakin DL, Kennard BD, et al. Protecting adolescents from self-harm: a critical review of intervention studies. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1260–1271. doi: 10.1016/j.jaac.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brent DA, Greenhill LL, Compton S, et al. The Treatment of Adolescent Suicide Attempters Study (TASA): predictors of suicidal events in an open treatment trial. J Am Acad Child Adolesc Psychiatry. 2009;48(10):987–996. doi: 10.1097/CHI.0b013e3181b5dbe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, Vitiello B, Iyengar S, Birmaher B, Ryan ND, Zelazny J. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418–426. doi: 10.1176/appi.ajp.2008.08070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson LP, McCauley E, Grossman DC, et al. Evaluation of the Patient Health Questionnaire-9 item for detecting major depression among adolescents. Pediatrics. 2010;126(6):1117–1123. doi: 10.1542/peds.2010-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds W. Suicidal Ideation Questionnaire: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 20.Posner K, Brown GK, Stanley B. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achenbach TM. Manual for the Youth Self-Report and 1991 profile. University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 22.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36(1):81–90. [PubMed] [Google Scholar]
- 23.Knight JR, Shrier LA, Bravender TD, Farrell M, Vander Bilt J. A new brief screen for adolescent substance abuse. Arch Pediatr Adolesc Med. 2012;153:591–596. doi: 10.1001/archpedi.153.6.591. [DOI] [PubMed] [Google Scholar]
- 24.Miller WR, Rollnick S. Helping People Change. 3rd. New York: Gilford Press; 2013. Motivational interviewing. [Google Scholar]
- 25.McMakin DL, Siegle GJ, Shirk SR. Positive Affect Stimulation and Sustainment (PASS) module for depressed mood: a preliminary investigation of treatment-related effects. Cognit Ther Res. 2011;35(3):217–226. doi: 10.1007/s10608-010-9311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennard BD, Biernesser C, Wolfe KL. Developing a brief suicide prevention intervention and mobile phone application: a qualitative report. J Technol Hum Serv. 2016;33(4):345–357. doi: 10.1080/15228835.2015.1106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyers T, Martin T, Manual J, Miller W, Ernst D. Center on Alcoholism, Substance Use and Addictions. University of New Mexico; 2010. Revised global scales: Motivational Interviewing Treatment Integrity 3.1.1. Available at: https://casaa.unm.edu/download/MITI4_2.pdf. [Google Scholar]
- 28.Young JE, Beck AT. Cognitive Therapy Scale rating manual. Beck Institute for Cognitive Behavior Therapy; Philadelphia, PA: 1980. [Google Scholar]
- 29.Raghavan M, Golstein T, Poling K, Brent D. Safety Plan Rating Scale. 2008 Unpublished Instrument. [Google Scholar]
- 30.Birmaher B, Brent DA, Chiappetta L. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication stud. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Osman A, Downs WR, Kopper BA. The Reasons for Living Inventory for Adolescents (RFL-A): development and psychometric properties. J Clin Psychol. 1998;54(8):1063–1078. doi: 10.1002/(sici)1097-4679(199812)54:8<1063::aid-jclp6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Phillips KFV, Power MJ. A new self-report measure of emotion regulation in adolescents: The Regulation of Emotions Questionnaire. Clin Psychol Psychother. 2007;14:145–156. [Google Scholar]
- 33.Simons JS, Gaher RS. The Distress Tolerance Scale: development and validation of a self-report measure. Motiv Emot. 2005;29(2):83–102. [Google Scholar]
- 34.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess. 1988;52:30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 35.Ascher BH, Farmer EMZ, Burns BJ, Angold A. The Child and Adolescent Services Assessment (CASA): Description and psychometrics. J Emot Behav Disord. 1996;4:12–20. [Google Scholar]
- 36.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 1989;13(3):319–340. [Google Scholar]
- 37.Nguyen TD, Attkisson CC, Stegner BL. Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Eval Program Plann. 1983;6(3–4):299–313. doi: 10.1016/0149-7189(83)90010-1. [DOI] [PubMed] [Google Scholar]
- 38.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129–133. [Google Scholar]
- 39.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JR. IBM Computer Usability Satisfaction Questionnaires: psychometric evaluation and instructions for use. Int J Hum Comput Interact. 1995;7:57–78. [Google Scholar]
- 41.Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv. 2001;52(6):828–833. doi: 10.1176/appi.ps.52.6.828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


