Abstract
OBJECTIVE:
Pilot study examining toll-like receptor 9 (TLR9) expression by sinonasal epithelial cells in allergic rhinitis (AR) subjects with and without a history of recurrent acute rhinosinusitis (RARS).
STUDY DESIGN:
Cohort study.
SETTING:
Outpatient clinic.
SUBJECTS AND METHODS:
Adult subjects were eligible for study if skin tested positive for inhalant allergens, and positive allergens were in season at time of study. Subjects were considered to have AR+RARS if they had four symptomatic episodes with major/minor factors in 12 months and CT evidence of sinusitis. Eight AR-only subjects and 13 AR+RARS subjects underwent endoscopic-guided cell brushing from the middle meatus. Flow cytometry for TLR9 expression was performed on collected fresh sinonasal epithelial cells.
RESULTS:
The AR+RARS group was found to have a significant increase in TLR9 expression in the sinonasal epithelium (66% ± 30%) compared with that of AR-only patients (32% ± 21%; P = 0.011).
CONCLUSION:
The significant difference in expression of TLR9 in allergic sinusitis patients compared with allergy-only patients in this study may indicate a difference in the role of innate immunity in these groups. The results suggest that expression of innate immune markers such as TLR9 may be upregulated in response to repeated microbial insults in AR+RARS. Further research is necessary to determine whether an initial impairment of innate immune gene expression may predispose some AR patients to subsequent development of RARS.
The incidence of allergic disease has increased signifycantly over the past 40 years.1 Allergic rhinitis (AR) is a very common health problem associated with considerable decrease in quality of life, and affects five to 22 percent of Americans.2 Although it is typically not a life-threatening disease, medical management costs are substantial. Over one billion dollars are spent on management of AR every year in the United States.3
Allergic rhinitis is an inflammatory disease of the upper airway mucosa, specifically the respiratory epithelium. The nose serves as a primary entryway for allergens. Therefore, the nasal mucosa is often the first to be exposed to allergens, leading to interactions with allergen-specific IgE on the surface of mediator cells.2 Complaints associated with AR include nasal obstruction, congestion, rhinorrhea, postnasal drip, nasal/ocular/palatal pruritus, anosmia, sneezing, and headache.
Allergic rhinitis is often associated with other diseases including asthma and otitis media. Chronic inflammation due to allergies can eventually lead to obstruction and subsequent rhinosinusitis (RS), such that AR is often considered a major predisposing factor of the development of rhinosinusitis.4
The close relationship between AR and sinusitis5 has been demonstrated and discussed previously in the scientific literature. However, not all individuals with AR develop frequent RS or mucosal inflammation visible on CT imaging. The potential differences in these two subgroups have not been well studied or categorized. Possible differences in innate immune expression and inflammation may exist between the two groups. These differences may contribute to the development of recurrent infections or exacerbations in one group over another.
Innate immunity provides first-line defense against pathogens and has long been thought to play a more nonselective role in the cascade of immune responses to allergens. However, recent evidence suggests that innate immunity also operates on a more specific level using pattern-recognition-receptors (PRRs) targeted to pathogen-associated-molecular-patterns (PAMPs). In the sinonasal tract, respiratory epithelial cells at the boundary between the mucosa and the external world express PRRs such as TLRs that can trigger local innate immune defenses while alerting the host immune system to the presence of specific microbes.6
All known TLRs are expressed by sinonasal epithelial cells,7 and their levels may be modulated in disease states such as allergy and chronic rhinosinusitis. There exists evidence for the role of TLRs in allergic airway inflammation. For example, TLR2, -3, and -4 have been shown to be upregulated in allergic sinonasal epithelial cells.8 TLR9 is a PRR that recognizes cytosine-phosphate-guanosine (CpG) dinucleotides found in bacterial and viral DNA. This receptor is of particular interest because its activation induces an antimicrobial response while suppressing allergic Th2 responses. CpG dinucleotides, presumably acting through TLR9, serve as potent adjuvants for allergen desensitization therapy.9 Immunostimulatory oligonucleotide sequences containing unmethylated CpG dinucleotides have a long-lasting Th1 effect in laboratory animals.10 Recent studies have demonstrated a decreased level of TLR9 protein in sinonasal epithelial cells from patients with chronic RS with nasal polyps, a condition characterized by Th2 inflammation and abundant eosinophils.11 Although there have been prior descriptions of innate immunity markers in chronic RS, we chose to focus on recurrent acute sinusitis, which may represent a distinct disease process in pathogenesis from chronic RS.
Since AR is a Th2-dominated, eosinophilic disease, it is reasonable to postulate that innate antimicrobial immunity may be inhibited in the sinonasal mucosa, thus promoting bacterial colonization and recurrent symptomatic RS. The present study measures TLR9 expression between AR-only and subjects with AR and recurrent acute RS (AR+RARS), as a marker of overall innate immune gene expression. A finding of decreased TLR9 expression in the RARS group may suggest that ongoing innate immune dysfunction contributes to the development of recurrent sinus infections in susceptible allergic individuals.
Materials and Methods
Endoscopically guided brush biopsies of the middle meatus sinonasal mucosa in 21 adult patients from the Johns Hopkins Otolaryngology–Head and Neck Surgery clinic were collected. Samples were divided into AR-only (n = 8) and AR+RARS (n = 13). This research protocol was approved through the Johns Hopkins Institutional Review Board (NA_00008593), and informed consent was acquired from each research subject prior to enrollment into study.
Inclusion criteria for AR were positive skin-prick test (SPT), with appropriate symptoms of corresponding seasonal or perennial allergies. SPT was considered positive when the resulting wheal was greater than or equal to 3 mm compared with negative controls after 15 minutes. Controls used for SPT included glycerin (negative) and histamine (positive). Subjects with sensitivity to seasonal allergens alone (positive to seasonal allergens without sensitivity to any perennial allergens) were studied during a period when their allergens were “in-season.” Inclusion criteria for AR+RARS group were to meet the AR criteria listed above; in addition, these subjects had American Academy of Otolaryngology’s major and minor symptom complex factors.12 Specifically, AR+RARS subjects had greater than or equal to four infections in 12 months based on clinical major/minor symptoms; they also had additionally mucosal thickening with or without air-fluid levels on CT scan. Subjects were excluded when there was history of systemic corticosteroid use within the past two weeks. One AR+RARS subject had history of prior functional endoscopic sinus surgery.
Study subjects were recruited from the Johns Hopkins otolaryngology clinic, where a medical history with special attention to medications, previous surgeries, number of sinusitis episodes per year, sinusitis symptoms, and treatment was performed. Study subjects underwent rigid nasal endoscopy after topical nasal oxymetazoline and pontocaine were applied. A cytology brush was introduced in the middle meatus to collect sinonasal epithelial cells. Brushed cells were immediately placed in normal saline. Within four hours, the collected cells were then stained with immunofluorescent markers and analyzed by flow cytometry the day of collection. Cells were transferred into microfuge tubes at the concentration of 200,000 cells per tube. The cells were then incubated with anti-TLR9 antibody conjugated to phycoerythrin (PE) (eBioscience, San Diego, CA) and antiepithelial cell antigen conjugated to fluorescein isothiocyanate (FITC) (Dako, Carpenteria, CA). Cells were centrifuged, washed, and fixed. Analysis was performed on a FACScalibur flow cytometer (BD Biosciences, San Jose, CA). Each subject’s cell sample was divided into unstained, rat IgG1 isotype control (eBioscience), single-stain controls and double-stained samples, each of which underwent flow cytometric analysis. The cell surface proteins were expressed as the increase in mean fluorescence over background intensity.
Data were represented by means and their corresponding SDs. Raw data were entered into a spreadsheet (Excel; Microsoft, Redmond, WA). Statistical analysis was performed with the Mann-Whitney U test assuming nonparametric data owing to small sample size.
Results
Positivity on SPT included sensitivities to a wide range of allergens found in the regional Maryland area, including: American elm, Alternaria alternate, ash, Bermuda grass, box elder, candida, cat, cockroach, cottonwood, Dermatophagoides farinae, Dermatophagoides pteronyssinus, dog, English plantain, fusarium, hormodendrum, hickory, Johnson grass, mucor, penicillium, pigweed, ragweed, sycamore, Timothy grass, and white oak. All but one subject had at least two positive tests to allergens including sensitivities to common major allergens such as cat, dog, cockroach, dust mites, and local environmental allergens such as white oak, ragweed, and Timothy grass. The one subject (AR+RARS) found to be sensitive to only one antigen was positive to A. alternata. This mold has been found to be a common aeroallergen important in allergic rhinitis in the mid-Atlantic region.
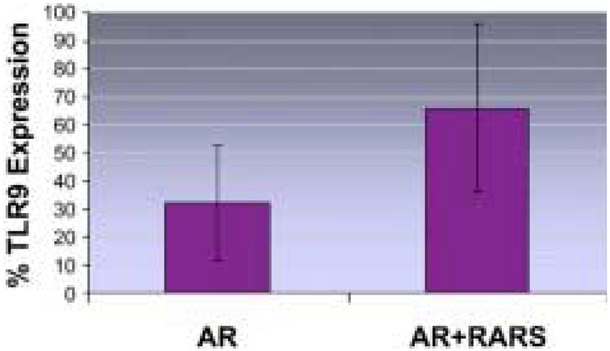
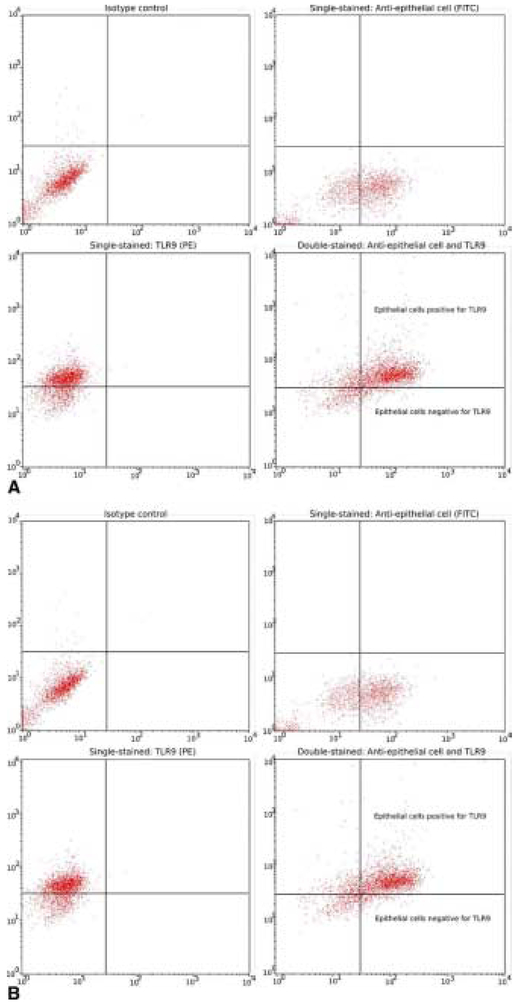
As seen in Figure 1, the AR+RARS group was found to have a significant increase in TLR9 expression in the sinonasal epithelium (66% ± 30%) compared with that of AR-only patients (32% ± 21%, P = 0.011). Figure 2A contains an example of flow cytometry dot plots from a subject with high TLR9 expression. In contrast, Figure 2B shows an example of dot plots from another subject with relatively low TLR9 expression.
Figure 1.
Percentage of sinonasal epithelial cells with positive toll-like receptor 9 (TLR9) expression in allergic rhinitis (AR) versus AR plus recurrent acute rhinosinusitis (AR+RARS) subjects. The bars indicate the means; the error bars represent SDs. The difference between the two groups was found to be statistically significant (P = 0.011).
Figure 2.
Two sets of dot plots are presented to show examples of individuals with relatively (A) high toll-like receptor 9 (TLR9) expression and (B) low TLR9 expression. Each set contains four dot plots: isotype control identifying unstained and random binding cells (top left), single stain identifying epithelial cells only (top right), single stain identifying TLR9 only (bottom left), and double-stained epithelial cells positive for TLR9 (bottom right). The cross-hairs were placed according to the isotype control and single stain samples for each subject. Then the resulting epithelial cell positive for TLR9 population was extracted from the double-stained dot plots. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Discussion
Prior studies of innate immunity in allergic disease have focused on atopic versus non-atopic disease comparisons. For example, one study revealed a decreased rate of TLR9 expression in conjunctival epithelial cells in subjects with allergic disease.13 In a subsequent study, there was no significant difference in TLR9 expression from nasal lavage epithelial cells between allergic and non-allergic groups. Furthermore, this study revealed no difference in immunohistochemical TLR9 staining between allergic versus healthy inferior turbinate biopsies.8 From a Th1/Th2 paradigm standpoint, several studies support a Th2-domi-nant pathway when allergic patients with sinusitis and non-allergic sinusitis patients were compared.14 However, there is evidence of both Th1 and Th2 activity in allergic subjects with sinusitis, as measured by reverse transcriptase polymerase chain reaction quantifying messenger RNA content of interleukin-12 beta and CD30.15 Overall, the existing scientific literature appears to suggest that decreased TLR9 innate immunity is dominant in atopic subjects compared with non-atopic subjects.
To our knowledge, this is the first study to look at TLR9 expression in sinonasal epithelial cells of allergic patients with recurrent acute sinusitis as a unique subgroup of those with AR disease. Our results demonstrate a significant difference in TLR9 expression between AR subjects with and without RARS. We had hypothesized that deficient expression of innate immune genes, including TLR9, could predispose certain allergic individuals to RARS. The results indicate that, rather than being decreased, TLR9 expression was increased in those subjects with allergy and RARS. This suggests that there is no continuing defect in TLR9 expression in these subjects, but that TLR9 expression may be upregulated in the setting of some as yet undefined exacerbation of sinusitis symptoms. One speculates that one possible explanation could include an increase in TLR9 to microbial insults in repeated viral/bacterial infectious episodes. While this does not rule out an initially decreased expression of TLR9 that predisposes to development of RARS, the level of sinonasal epithelial TLR9 expression appears to become elevated once infections occur. It remains undetermined how long TLR9 expression remains upregulated after each episode of RS in allergic individuals. The fact that study subjects had active recurrent sinusitis symptoms in the six months preceding the study may suggest that this elevation can persist for several weeks to months. It is possible that with repeated microbial insults, the level of TLR9 expression never decreases to the baseline level normally associated with AR.
We speculate that the increased TLR9 expression may result from an elevation in the Th1-dominated adaptive immune response when an AR individual who is predominantly Th2-biased is faced with acute RS. This compensatory upregulation may contribute to the ability to recover from an acute episode of RS in the context of allergic disease, given that prior studies have shown that TLR9 is an important activation of antimicrobial responses. There has been a shift from Th2 toward Th1-type responses in upper respiratory tissue including RS described during TLR9 activation via CpG-ODN (cytosine-phosphate-guanosine-oligodeoxynucleotide) ligands.16 The duration of this Th1-biased state remains unknown, except that it may be shortlived, as evidenced by the commonality of recurrent RS disease in this study group. It cannot be said with great certainty that our findings are supported by an immunomodulated effect caused by the recurrent infections. The reverse cause-effect relationship cannot be completely excluded; that is, recurrent infections may be a direct result of relatively elevated TLR9 expression levels; however, that would counter what prior studies have supported. Additionally, it has been shown that TLR9 expression can vary significantly depending on the environmental hygiene and atopy of the individual.17 These factors are difficult to control for and thus highlight the need for further studies that would better elucidate the dynamic changes in TLR9 expression as well as its causes and effects.
Interestingly, although the subjects in the AR+RARS group had reported a history of frequent clinical symptoms of sinusitis, none of the study subjects had evidence of purulent drainage during their endoscopic examination and cytology brushing for this study. Therefore, no cultures were sent. All had abnormal CT findings however. The findings of our study suggest that frequent sinusitis episodes can increase TLR9 expression in allergic individuals, but the individual need not have active purulent rhinorrhea for this increase in TLR9 expression to be evident. Perhaps the increase in TLR9 expression occurs with a RS episode and persists for a period of yet to be determined time, or the level of expression in these individuals may be persistently elevated.
Prior studies have shown that in chronic sinusitis with nasal polyposis (CRSwNP), TLR9 expression was decreased compared with controls.11 Allergic rhinitis and CRSwNP groups share similar characteristic in that both groups typically share eosinophilic and Th2-type inflammation. It appears from the findings of previously published studies13 that both may also share a relative decrease in TLR9 sinonasal epithelial expression.
TLR9 activation has been shown to have therapeutic potential in the treatment of allergy and asthma.18 TLR9 activation results in Th1-biased cellular and humoral effector functions of innate and adaptive immunity. Prevention and therapy of infectious diseases caused by intracellular pathogens (e.g., category A agents, Listeria monocytogenes) have been demonstrated in mouse studies. Monotherapy, TLR9 activation alone, against AR has been shown to be effective in mouse models.18 Immunotherapy with ragweed-TLR9 agonist vaccine showed possible long-term clinical efficacy in the treatment of ragweed AR that lasted through two ragweed seasons after a six-week course of injections.19 These promising therapies could eventually be extended to treat specifically allergic individuals with RARS, given that the TLR9 profiles for this patient population differ from those of individuals with AR-only.
Future Studies
Our current study demonstrates that AR patients currently suffering from recurrent sinusitis have increased TLR9 expression compared with those who are allergic and do not have RS. Given the therapeutic advances in TLR9 agonists, TLR9 and innate immunity continue to grow as a research interest. Areas for future study would include studies with larger sample size to compare allergic patients with and without sinusitis to non-allergic patients with sinusitis and healthy control subjects. Longitudinal studies of TLR9 expression in allergic individuals may play an important role in explaining duration and fluctuations in TLR9 expression that may be triggered by active sinus infections or increasing sinusitis symptoms. Further studies of other markers of innate immunity may further clarify the relationship of innate immunity in allergic individuals with sinusitis. Limitations of the current study include the lack of specificity of the allergic subjects studied, in that the small number of subjects was sensitive to a broad and heterogeneous range of antigens. Studying a pure population with a robust response to a specific single antigen with confirmatory in vitro specific IgE would add strength to any future studies in this area.
Conclusion
In this pilot study, we found a significant increase in innate immunity of AR+RARS as evidenced by the difference between TLR9 expression in sinonasal epithelial cells compared with that of AR-only subjects. The results suggest that in the allergic group with sinusitis, there is no active defect in TLR9 expression increasing susceptibility to recurrent sinusitis; instead an increased relative expression of TLR9 may be a compensatory upregulation in response to repeated antimicrobial insults.
This finding suggests that antimicrobial inflammation in AR-RARS subjects modulates innate immune activity compared with AR-only subjects. Although the immunological kinetics of this finding is not known at this time, it does offer an explanation for the recurrent resolution of acute sinusitis seen in those with AR+RARS. However, the duration of antimicrobial effect from elevated TLR9 expression remains unclear. It may be short-lived, given the frequent recurrence of infection seen in AR+RARS.
Acknowledgments
We would like to thank Lee Blosker, BS, Thanh Doyle, BS, Ashley Lalekar, MHS, Murugappan Ramanathan, MD, and Steven Chang, MD, for help with running and analyzing flow cytometry data.
Footnotes
Disclosures
Competing interests: None.
Sponsorships: American Academy of Otolaryngic Allergy provided funding and approved original grant.
References
- 1.Howarth PH. Is allergy increasing?—early life influences. Clin Exp Allergy 1998;28(suppl 6):2–7. [DOI] [PubMed] [Google Scholar]
- 2.Bellanti JA, Wallerstedt DB. Allergic rhinitis update: epidemiology and natural history. Allergy Asthma Proc 2000;21:367–70. [DOI] [PubMed] [Google Scholar]
- 3.Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc 2007;28:3–9. [DOI] [PubMed] [Google Scholar]
- 4.Kirtsreesakul V, Naclerio RM. Role of allergy in rhinosinusitis. Curr Opin Allergy Clin Immunol 2004;4:17–23. [DOI] [PubMed] [Google Scholar]
- 5.Dykewicz MS. Rhinitis and sinusitis. J Allergy Clin Immunol 2003; 111(2 suppl):S520–9. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DR. Toll-like receptors and other links between innate and acquired alloimmunity. Curr Opin Immunol 2004;16:538–44. [DOI] [PubMed] [Google Scholar]
- 7.Vandermeer J, Sha Q, Lane AP, et al. Innate immunity of the sinonasal cavity: expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg 2004;130:1374–80. [DOI] [PubMed] [Google Scholar]
- 8.Fransson M, Adner A, Erjefalt J, et al. Up-regulation of Toll-like receptors 2, 3, and 4 in allergic rhinitis. Respir Res 2005;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangloff SC, Guenounou M. Toll-like receptors and immune response in allergic disease. Clin Rev Allergy Immunol 2004;26:115–25. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Horner AA, Martin-Orozco E, et al. Pre-priming: a novel approach to DNA-based vaccination and immunomodulation. Springer Semin Immunopathol 2000;22:85–96. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan M, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol 2007;21:373–7. [DOI] [PubMed] [Google Scholar]
- 12.Lanza D, Kennedy D. Adult rhinosinsuitis defined. Otolaryngol Head Neck Surg 1997;117(suppl):S1–S7. [DOI] [PubMed] [Google Scholar]
- 13.Bonini S, Micera A, Iovieno A, et al. Expression of toll-like receptors in healthy and allergic conjunctiva. Ophthalmology 2005;112:1528. [DOI] [PubMed] [Google Scholar]
- 14.Elhini A, Abdelwahab S, Ikeda K. Th1 and Th2 cell population in chronic ethmoidal rhinosinusitis: a chemokine receptor assay. Laryngoscope 2005;115:1272–7. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, Goto S, Ikeda K, et al. IL-12 recetor beta2 and CD30 expression in paranasal sinusmucosa of patients with chronic sinusitis. Eur Respir J 1999;13:1008–13. [DOI] [PubMed] [Google Scholar]
- 16.Tan L, Rogers TJ, Hatzirodos N, et al. Immunomodulatory effect of cytosine-phosphate-guanosine (CpG)-oligonucleotides in nonasthmatic chronic rhinosinusitis: an explant model. Am J Rhinol Allergy 2009;23:123–9. [DOI] [PubMed] [Google Scholar]
- 17.Majak P, Brzozowska A, Bobrowska-Korzeniowska M, et al. Early exposure to unhygienic conditions and infections is associated with expression of different Toll-like receptors. Investig Allergol Clin Immunol 2009;19:260–5. [PubMed] [Google Scholar]
- 18.Krieg A Therapeutic potential of toll-like receptor 9 activation. Nat Rev Drug Discov 2006;5;471–84. [DOI] [PubMed] [Google Scholar]
- 19.Creticos P, Schroeder J, Hamilton R, et al. Immunotherapy with a ragweed-toll-like-receptor 9 agonist vaccine for allergic rhinitis. N Eng J Med 2006;355:1445–55. [DOI] [PubMed] [Google Scholar]