Abstract
The major histocompatibility complex (MHC) Class I-related receptor, FcRn, serves multiple roles ranging from the regulation of levels of IgG isotype antibodies and albumin throughout the body to delivery of antigen into antigen loading compartments in specialized antigen presenting cells. In parallel with studies directed towards understanding FcRn at the molecular and cellular levels, there has been an enormous expansion in the development of engineering strategies involving FcRn to modulate the dynamic behavior of antibodies, antigens and albumin. In the current review, we focus on a discussion of FcRn-targeted approaches that have resulted in the production of novel antibody-based platforms with considerable potential for use in the clinic.
Keywords: Antibody engineering, FcRn, pharmacokinetics, FcRn-targeted therapeutics
The motivation behind modulating antibody dynamics in the body
The efficacy of current antibody-based therapeutics largely depends on homeostatic regulation of antibody levels at the appropriate site in the body. For example, for maximum efficacy of a therapeutic antibody in the treatment of cancer, it is necessary for sufficient antibody levels to be maintained over extended time periods at the tumor site [1]. By contrast, however, for antibody-mediated autoimmune diseases such as myasthenia gravis or idiopathic thrombocytopenic purpura, the levels of pathogenic antibodies are too high and approaches to reduce antibody concentrations are desirable [2]. Similarly, during diagnostic or theranostic imaging (see Glossary) with (radio)labeled antibodies, the background signal can be problematic due to the prolonged persistence of the antibody in the circulation, leading to poor contrast and possibly non-specific organ damage [3,4]. A fundamental question is therefore what are the molecular and cellular processes that control antibody levels, particularly those of the immunoglobin G (IgG) class that are typically used as therapeutics? Further, how can this knowledge be used to tune the levels and distribution of therapeutic or endogenous antibodies?
The neonatal crystallizable fragment receptor (FcRn) is a major histocompatibility complex (MHC) Class I-related receptor that interacts with antibodies of the IgG class as a heterodimer of a heavy (α)-chain and β2-microglobulin and binds to the constant or fragment crystallizable (Fc) region of IgG (Figure 1A). FcRn is a key player in regulating the dynamic behavior, including distribution, transport and persistence, of IgG antibodies throughout the body [5–7]. The identification of these activities extends the role of this Fc receptor well beyond its original identification as the transporter of IgG from mother to young (hence the name n, for neonatal), prompting extensive analyses of the molecular and cellular properties of FcRn. In parallel, the relevance of FcRn to antibody-based therapeutics has motivated the development of technologies to modulate the dynamic properties of these biologics and, in some cases, their cognate antigens in vivo. Given the enormous expansion of the use of antibodies in therapy over the past decade, the current review will cover a brief overview of FcRn biology, followed by a discussion of engineering approaches to generate improved, new generation therapeutics. Due to space limitations, we will not discuss the role of FcRn in regulating albumin levels and the related implications for therapy in this Review (see Text Box 1 for a brief overview of the topic).
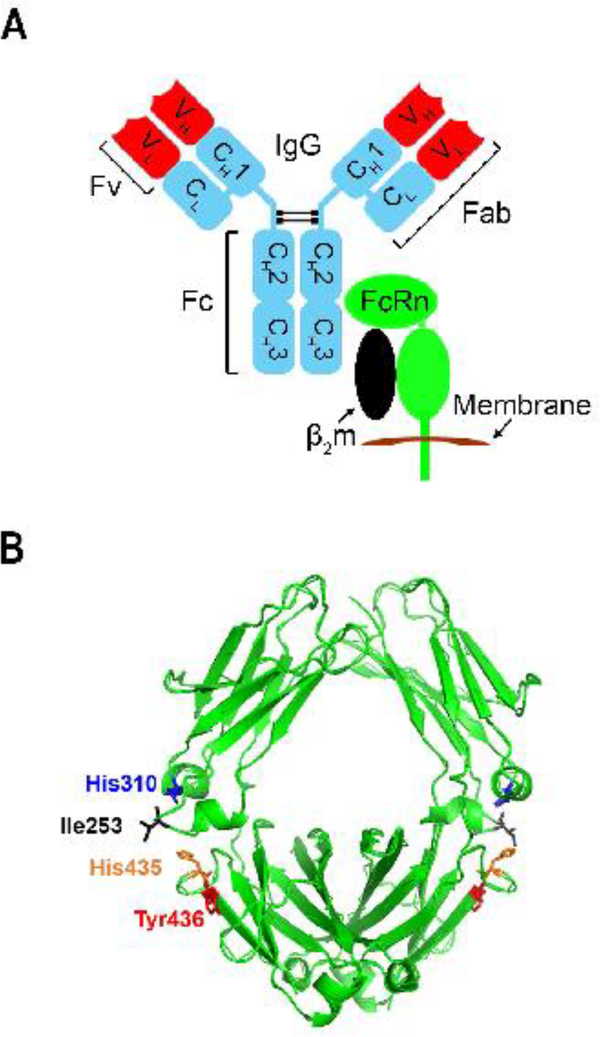
Figure 1.
The molecular nature of the IgG-FcRn interaction.
A. Schematic representation of the IgG-FcRn interaction. The constant regions and variable domains (Fv) of the antibody IgG are colored blue and red, respectively. FcRn is a heterodimer of an α-chain (green) and β2-microglobulin (β2m, black) and is shown in its transmembrane form. B. The location of the residues (Ile253, His310, His435 and Tyr436) at the CH2-CH3 domain interface of the Fc region of human IgG1. These residues play a central role in binding to FcRn. The figure was drawn using the x-ray crystallographic structure of human IgG1-derived Fc fragment [100] and Pymol (PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC).
TEXT BOX 1.
Beyond the IgG-related functions of FcRn, the identification of this receptor as a transporter of albumin has resulted in the engineering of albumin-FcRn interactions, or albumin binding proteins, to generate therapeutics [6,96,97], in addition to the recent use of FcRn inhibitors to accelerate the clearance of toxic drug-albumin complexes [98]. Through the ability of FcRn to bind and recycle albumin away from lysosomal degradation, this receptor can also regulate nutrient supply to cells by controlling the intracellular supply of albumin-derived amino acids [99].
The molecular details of FcRn-IgG interactions
IgG residues that are critical for the binding of mouse or human IgGs to FcRn include residues isoleucine (Ile)253, histidine (His)310, His435, His436 (mouse) or tyrosine (Tyr)436 (human) that are located on the exposed loops at the CH2-CH3 domain interface of IgG1 [5,8–10] (Figure 1B). In general, these amino acids are well-conserved across IgG isotypes and species, although amino acids such as residues 435 and 436 show higher variability than Ile253 and His310 [11].
The His residues of IgG interact with acidic residues of FcRn [9,12]. The interaction of the protonated imidazole side chain of His (pKa ~6) with these acidic residues at pH ~6 confers the characteristic pH dependent binding (relatively high affinity at acidic pH, with very weak to negligible binding at pH 7.3–7.4) that is observed for the majority of IgGs [13,14]. Consistent with this, isotypes such as mouse IgG2b and a human IgG3 allotype that contain Tyr and arginine (Arg), respectively, do not go through protonation/deprotonation cycles between pH 6.0–7.4 and have significantly higher binding affinity for FcRn at near neutral pH compared with other isotypes or IgG3 allotypes containing His at position 435 of IgG [13–15]. This gain of binding at pH ~7.3–7.4 has marked functional consequences that are discussed in detail below.
A further confounding factor related to FcRn-IgG interactions relates to the cross-species differences for FcRn-IgG interactions, for which the molecular basis has been defined [16,17]. These observations have led to the conclusion that mouse models have limitations for the preclinical pharmacokinetic analyses of therapeutic antibodies, particularly if they have been engineered to increase their affinity for FcRn [18]. This issue has motivated the development of mice that transgenically express human FcRn instead of mouse FcRn [19,20]. The use of such mice provides valuable insight into the pharmacokinetic behavior of engineered antibodies that can be translated to non-human primates and humans.
The subcellular trafficking behavior of FcRn and IgG
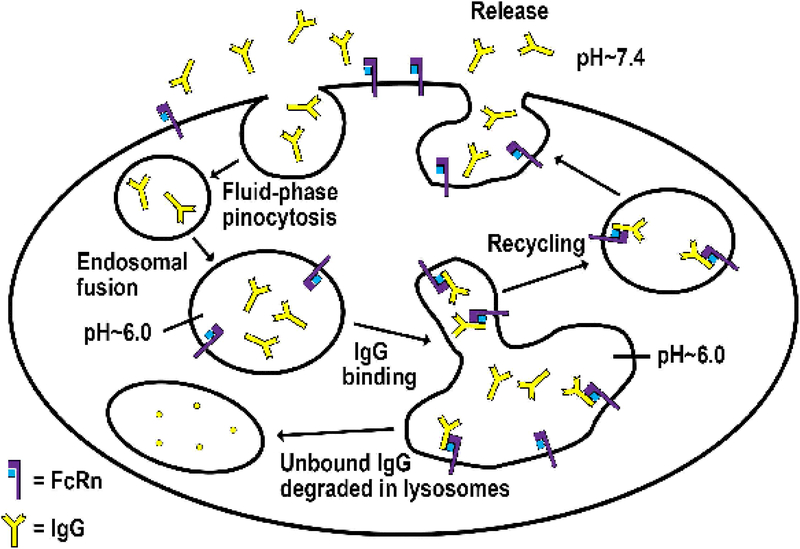
Understanding the subcellular trafficking behavior of FcRn is important for understanding the mechanisms that maintain IgG homeostasis, in addition to providing insight into the design of engineered antibodies with altered dynamic properties in the human body. This has led to multiple studies directed towards elucidating the cell biology of FcRn [21–29]. FcRn is ubiquitously expressed in both parenchymal (endothelial, epithelial) and hematopoietic cells [30–32], and the majority of cellular trafficking studies of FcRn and its IgG ligand have been carried out in endothelial or epithelial cells [21–29]. Live cell imaging of endothelial cells following transfection with FcRn tagged with green fluorescent protein (GFP; to track FcRn in live cells) demonstrates that FcRn-IgG complexes are internalized primarily by fluid phase pinocytosis (due to the very low affinity of FcRn for most IgG subclasses at extracellular, near neutral pH), sorted in early (sorting) endosomes away from lysosomal degradation and recycled back to the cell surface for exocytic release [24–26] (Figure 2). The acidic pH in early endosomes (pH ~6.0) is permissive for FcRn-IgG interactions, whereas the extracellular, near-neutral pH (pH ~7.4) for most cell types enables efficient dissociation of IgG from FcRn during fusion of exocytic compartments with the plasma membrane. The primary pathway for internalization of IgG into cells involves fluid phase pinocytosis, although receptor (FcRn)-mediated uptake becomes dominant for IgG molecules that naturally bind, or are engineered to bind, to FcRn at near neutral pH [33,34]. In contrast to IgGs that interact with FcRn, those that are either mutated to ablate binding, or naturally do not bind due to cross-species differences (e.g. mouse IgG1 to human FcRn [16]), fail to be sorted intracellularly and enter lysosomes [25]. These endosomal sorting processes provide a framework for the cellular mechanisms through which FcRn regulates the levels of IgGs in the body. For example, when the intracellular levels of IgG increase, all FcRn molecules are in the bound state and the excess free antibodies are degraded in lysosomes. Further, IgG recycling assays using transiently transfected, FcRn-GFP expressing endothelial cells can serve as a predictor of in vivo dynamics [18], and have been followed by the development of a stably transfected endothelial cell system to obtain correlates with behavior in mice transgenically expressing human FcRn [35].
Figure 2.
Model for the activity of FcRn as an IgG homeostat.
IgG enters cells by fluid-phase pinocytosis in small tubulovesicular transport carriers that fuse with larger, FcRn-positive early (sorting), acidic endosomes in which binding to FcRn can occur. Bound IgG molecules are recycled and released by exocytosis involving fusion of recycling compartments with the plasma membrane. By contrast, IgG that does not bind to FcRn in sorting endosomes enters lysosomes and is degraded.
FcRn can be distinguished from many other receptors through its ability to undergo both recycling and transcytosis. [21]. The complex intracellular processes involved in FcRn trafficking along with their molecular coordinators such as the Rab GTPases [36,37], have been investigated in both endothelial and polarized epithelial cells [28,38]. Live cell imaging of endothelial cells has been used to track highly motile FcRn+ tubulovesicular transport carriers (TCs), and reveals a highly dynamic network of TCs that can be identified by the presence of different complements of Rab GTPases [23]. These TCs mediate the transport of vesicles between different subcellular compartments or the plasma membrane [23], thereby playing a central role in determining the intracellular fate of internalized IgG. Analyses in endothelial cells have revealed that the Rab GTPase, Rab11A is associated with recycling compartments that lead to exocytosis of FcRn-IgG complexes, indicating that Rab11A plays a central role in IgG salvage and recycling [38]. Interestingly, although Rab11A is involved in exocytosis in polarized epithelial cells, it is not required for transcytosis [28]. Instead, Rab25 and the microtubular motor protein, Myosin (Myo) Vb, regulate the bidirectional transcytosis of FcRn [28]. Recent studies have resulted in the identification of multiple effectors that differentiate the transcytotic and recycling pathways in epithelial cells [39,40]. Combined with electron microscopy studies in rat intestinal epithelial cells, the subcellular trafficking analyses demonstrate that early (sorting) and recycling endosomes are structurally diverse, forming a network that is connected via small, highly motile TCs [41,42].
The subcellular trafficking of multimeric IgG-antigen complexes
In contrast to monomeric IgG molecules that, with or without bound antigen, are typically recycled by FcRn, multimeric immune complexes (ICs) comprising antigen-antibody complexes have very short in vivo persistence due to internalization and trafficking into lysosomes [43]. This internalization is typically mediated by other Fc receptors, FcγRs, on FcγR-expressing cells that, in contrast to FcRn, can bind to ICs at near neutral pH [44]. The delivery of ICs to lysosomes has several important consequences for host defense. First, this pathway leads to the destruction of antibody-opsonized pathogens. Second, this may prevent the transcytosis of pathogens bound to antibody across epithelial barriers, although the delivery of ‘small’ ICs across the intestine can stimulate protective immunity [45]. Third, for hematopoietic cells, internalization of multimeric ICs by FcγRs in antigen-presenting cells is followed by ‘handover’ to FcRn (which is not typically involved in uptake due to the near neutral, extracellular pH) in early endosomes and antigen delivery into both the cross-presentation (involving MHC Class I) and MHC Class II presentation pathways to stimulate T cell mediated-immunity [46,47]. Consistent with a role for FcRn in antigen presentation, overexpression of this receptor in transgenic mice increases T cell responses [48]. The contribution of FcRn to antigen presentation pathways is not discussed further here, but for a comprehensive review of this topic, see [49].
FcRn as a regulator of the in vivo half-lives of IgG
The identification of FcRn as a global regulator of IgG levels over two decades ago has led to studies directed towards engineering antibodies for longer in vivo persistence [5,6]. This was initially achieved by mutagenesis of IgG residues in the vicinity of the FcRn interaction site to generate a library of engineered, Fc fragments derived from mouse IgG1. These mutated genes were used to produce a phage display library, from which Fc fragments with mutations that conferred higher affinity for FcRn at pH 6.0 (early endosomal pH) were selected [50]. Subsequently, similar approaches, or molecular modeling based on structural data, have been used to generate human IgG (IgG1 or IgG4) variants with increased affinity for human FcRn, resulting in 2–5-fold increases in half-life in monkeys and humans [51–58]. For example, the analysis of antibodies specific for Staphylococcal α-toxin with the ‘YTE’ half-life extending mutations (Met252 to Tyr, Ser254 to Thr, Thr256 to Glu that are all in proximity to the key residues of IgG that interact with FcRn (Figure 1B) [59]) during phase II clinical trials demonstrated half-lives ranging from 80–112 days, compared to around 20 days for wild type (WT) human IgG1 [52]. Importantly, half-life extended antibodies have been shown to have superior efficacy as therapeutics against tumor xenografts in mice and in the prophylaxis of simian-human immunodeficiency virus (HIV) in non-human primates [57,60].
A challenge associated with the engineering of antibodies for increased affinity for FcRn at acidic pH (pH ~6.0) is to retain sufficiently low affinity for FcRn at near neutral pH (pH 7.4) in parallel, to enable efficient exocytic release following recycling or transcytosis. The possibility of a negative effect of increased binding of IgG to FcRn at near neutral pH (pH 7.4), on the in vivo IgG persistence was first introduced in 1997 [50]. As the affinity of the FcRn-IgG interaction at pH 6.0 is enhanced, the binding at pH 7.4 tends to increase in parallel in most cases [56,61]. Further, although increased binding of an engineered antibody at near neutral pH can be offset by higher affinity at endosomal pH (pH ~ 6) to result in slower IgG clearance, analyses of mutated respiratory syncytial virus (RSV)-specific antibodies with a range of binding properties have revealed a requirement for the dissociation constant of IgG-FcRn interaction to be greater than around 400 nM at pH 7.4 for half-life extension to be achieved [61]. On the other hand, pharmacokinetic and modeling analyses of a panel of VEGF-specific antibodies demonstrated that as the dissociation constant of the IgG-FcRn interaction decreases below ~104 nM, further improvements in persistence are not observed [62]. This apparent discrepancy between the two studies is due to the consideration of different affinity thresholds, namely the threshold for a reduction in persistence below that of the WT parent, versus the threshold where the affinity starts to counteract the improved interaction at acidic pH (pH ~6.0) whilst still maintaining a longer half-life than the WT. Nevertheless, in both cases, at the cellular level the increased binding at near neutral pH (pH ~7.4) results in decreased exocytic release of IgG at the cell surface.
Although the nature of binding of the Fc region of the antibody with FcRn is a major determinant of its pharmacokinetic behavior, the antigen binding fragment (Fab) arms (Figure 1B) can also modulate IgG-FcRn interactions [63–66]. This effect can be attributed to electrostatic interactions of regions of high density of positively charged residues in the complementarity determining regions (CDRs) located in the variable fragment (Fv) of the antibody [65,66]. Such interactions can slow dissociation of the antibody from FcRn at near neutral pH which can be quantitated using pH-gradient FcRn affinity chromatography [64]. Clearly, this secondary, Fv-mediated interaction can also be modified by the binding of the antigen to the antibody. This regulation of IgG-FcRn interactions via antigen binding represents an additional pathway to alter antibody pharmacokinetics. In addition to this, other factors such as the isoelectric point (pI) of the antibody, that can also be related to the presence of charged residues in the CDRs, can affect internalization into cells [65–69], thereby contributing to the regulation of the dynamic behavior of antibodies.
Engineering antibodies for enhanced antigen clearance
Monoclonal antibodies are widely used to neutralize toxins or inflammatory molecules such as cytokines. However, these antibodies typically bind to one or two target molecules (due to the presence of two antigen binding sites per antibody molecule) and, unless the binding site on their target is repeated, delivery of these agents does not result in immune complex formation. Consequently, such antibodies can have the undesirable effect of acting as carriers for their targets, thereby prolonging rather than reducing the persistence of these unwanted molecules (Figure 3A) [70,71]. This effect can reduce the efficacy of antibodies that are delivered to neutralize such deleterious molecules. In addition, in some cases antigen binding to antibody can increase the clearance rate of the antibody, resulting in lower effective doses, through pathways that are not completely understood but can be determined by the clearance behavior of the target antigen following entry of the antibody-antigen complex into cells [72,73]. To circumvent these problems, several groups have used antibody engineering approaches to generate novel antibody platforms which are discussed below.
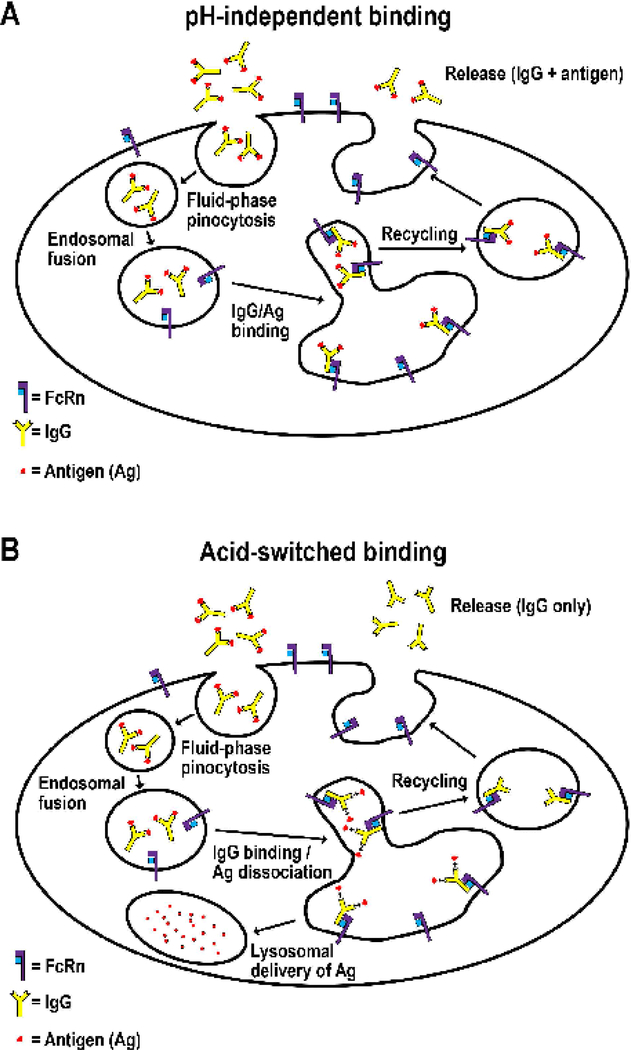
Figure 3.
Enhancement of antigen-clearance by acid-switching.
A. pH-independent binding. Antibodies that bind to antigen with similar affinity in the pH range 6.0–7.4 enter cells by fluid-phase pinocytosis in small tubulovesicular TCs that fuse with early (sorting) endosomes in which binding to FcRn can occur, followed by recycling of the IgG:antigen complex and exocytic release at the plasma membrane. This results in the buffering effect that prolongs the half-life of the targeted antigen.
B. Acid-switched binding. By contrast, pH-dependent or acid-switched antibodies have substantially higher affinity for antigen at near neutral pH relative to acidic, endosomal pH. Following internalization into cells by fluid-phase pinocytosis in small tubulovesicular TCs, the complexes enter acidic sorting endosomes in which antigen dissociates from antibody and enters the lysosomal pathway. The FcRn-bound antibody is recycled and exocytosed. This results in increased clearance of antigen combined with recycling of antibody for re-use.
Acid-switched antibodies
Acid-switched antibodies bind to their target with higher affinity at near neutral pH than at acidic pH. These antibodies dissociate from their antigen in acidic endosomes following internalization into cells [72,74,75] (Figure 3B). The consequence of acid-switching is that whilst the antibody is recycled by FcRn, the antigen is delivered to lysosomes and degraded. Each antibody therefore avoids lysosomal degradation and can be loaded multiple times with an antigen, with the advantage that the iterative use of such antibodies confers a need for sub-stoichiometric doses relative to antigen load. This strategy has been used to generate acid-switched antibodies specific for targets such as the interleukin (IL)-6 receptor (IL-6R), IL-6, proprotein convertase subtilisin kexin type 9 (PCSK9), complement component 5 (C5), C-X-C motif chemokine 10 (CXCL10) and connective tissue growth factor (CTGF) [72–77]. Most acid-switched antibodies have been generated for antigens that exist in soluble form, with the exception of the IL-6R which can be membrane-bound or soluble [74].
The anticipated effects of acid-switching on endosomal dissociation have been corroborated by cell biological analyses using fluorescence microscopy [73,75]. These studies show the appearance of antigen in the endosomal lumen followed by delivery to lysosomes, whereas the antigen-specific antibody remains on the FcRn-positive membrane of the endosome and is recycled. Based on analyses of antibodies with a range of target binding properties, an upper estimate of 20–70 seconds for the half-life of the antibody-antigen complex at acidic pH has been estimated to enable endosomal dissociation [76,78]. Collectively, these studies have led to the conclusion that the dissociation rate (‘off-rate’) at acidic endosomal pH is a dominant factor, rather than the relative ratios of dissociation constants at pH 6.0 to pH 7.4, in determining the efficiency of antigen clearance [78,79]. Nevertheless, for antibodies with very fast on-rates at acidic pH, rebinding may mitigate the effects of rapid dissociation. Interestingly, the analysis of the effect of combining acid-switching with half-life extension through enhancement for FcRn binding has met with mixed results, showing benefit for target antigen clearance over acid-switching alone in some, but not other, studies [73,74,76,78]. Importantly, for antigens that can contribute to antibody clearance due to endo-lysosomal trafficking of antibody-antigen-cognate receptor complexes, the interplay between antigen-receptor, antigen-antibody and antibody-FcRn interactions is complex, leading to different ‘rules’ for effective acid-switching in such systems.
Calcium-switched antibodies
An alternative approach to acid-switching has been described that exploits the substantially lower levels of calcium (Ca2+; around 2 μM) in endosomes relative to the extracellular space (around 2 mM) [80]. Specifically, Ca2+-switched antibodies can be engineered that bind to their antigen with higher affinity at millimolar (mM) Ca2+ concentrations relative to micromolar (μM) concentrations. Significantly, the 1,000-fold difference in Ca2+ concentration results in a larger ‘dynamic range’ for engineering endosomal dissociation relative to a pH change of around 1.5 pH units (i.e. ~30-fold difference in proton concentration).
Sweeping antibodies
The knowledge that the cellular uptake of proteins by fluid phase processes is substantially less efficient than receptor-mediated internalization has prompted the generation of antibodies that are both acid-switched and engineered at the sites on IgG interacting with FcRn. These engineered antibodies have increased binding to FcRn at near neutral pH (such mutations have been identified in earlier studies using phage display or other approaches; see section ‘FcRn as a regulator of the in vivo half-lives of IgG’ above) and are known as ‘sweeping’ antibodies [76,79,81].
Live cell imaging shows that FcRn is exposed at the cell surface during exocytic release, followed by internalization to maintain low steady state surface levels [24]. This behavior enables the rapid accumulation of sweeping antibodies within cells [33]. The increased internalization of these antibodies bound to cognate antigen into early endosomes results in more effective clearance of targeted antigen, due to increased endosomal delivery of antibody-antigen complexes [76,79,81]. However, as the binding affinity of an antibody for FcRn at near neutral pH increases, its half-life decreases due to enhanced accumulation and retention within cells, culminating in lysosomal delivery [82,83]. Thus, there is a trade-off between increasing the delivery of an antibody-antigen complex and decreasing the in vivo persistence of the (therapeutic) antibody. How these two parameters interplay to achieve the desired effect on target antigen will be dependent upon the dynamic behavior of the particular antigen and longevity of the desired pharmacodynamic effect.
Targeting FcRn to modulate IgG levels
The role of FcRn as a global regulator of IgG provides opportunities for the use of FcRn inhibitors to reduce the levels of antibodies that cause symptoms in diseases such as autoimmune disorders [33,84,85]. The FcRn inhibitors bind to FcRn with higher affinity than naturally occurring IgGs in the pH range 6.0–7.4 [33,84–89]. Consequently, they compete with endogenous antibodies for FcRn binding, driving such antibodies into degradative lysosomes [33]. Significantly, clinical studies have demonstrated that greater than 50% reductions in pathogenic antibody levels in several autoimmune diseases can result in therapeutic benefit [90,91], indicating that even partial reduction by FcRn inhibitors could be effective. A further application area is to use FcRn inhibitors to decrease background and improve contrast during diagnostic/theranostic imaging with (radio)labeled antibodies [89]. FcRn inhibition also offers an alternative to current strategies to reduce pathogenic antibody levels, such as plasmapheresis, intravenous immunoglobulin (IVIG) delivery or B cell depletion, all of which can have undesirable side effects.
To date, FcRn inhibitors ranging from peptide/small protein to antibody-based blockers have been generated [33,84–89,92], and several of these are in various stages of clinical trials following preclinical analyses in animal models. One class of antibody-based inhibitors involves the use of Fc engineering to generate competitive IgG molecules with substantially increased affinity for FcRn at both near neutral and acidic pH [33] and are called Abdegs (for antibodies that enhance IgG degradation) (Figure 4A). Furthermore, several antibody-based FcRn inhibitors have been generated that induce similar effects on endogenous IgGs by binding to FcRn through their variable domains [86,87].
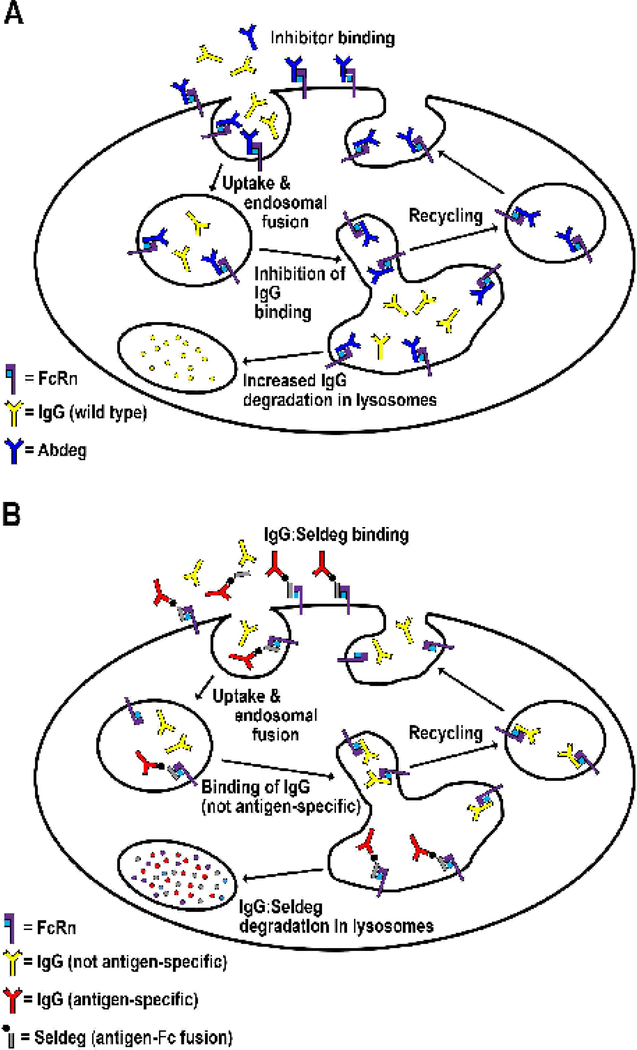
Figure 4.
Antibody engineering strategies to reduce endogenous IgG levels.
A. In the presence of an inhibitor of FcRn such as an Abdeg that binds to FcRn with increased affinity at both near neutral and acidic pH, the inhibitor is internalized by receptor (FcRn)-mediated processes and competes with endogenous IgGs for FcRn binding in acidic endosomes. Consequently, endogenous IgG molecules are driven into lysosomes and are degraded. The inhibitor shown binds through its Fc region to FcRn, whereas other classes of inhibitors can be peptide-based inhibitors or antibodies that bind through their variable domains [33,84–89,92]. B. A specific class of engineered proteins comprising antibody Fc regions fused to an antigen (one antigen molecule per Fc fragment) can be used to deplete antigen-specific antibodies. This fusion protein, named a Seldeg (for selective degradation of antigen-specific antibodies), is engineered to bind to FcRn via its Fc region with increased affinity at both near neutral and acidic pH. Seldeg-antibody complexes are internalized into FcRn-expressing cells by receptor-mediated uptake, and subsequently follow the constitutive degradation pathway of FcRn into lysosomes [83], leading to the breakdown of the targeted antibody. Due to their different mechanism of action, Seldegs can be used at lower doses than Abdegs and do not affect the levels of antibodies that are not specific for antigen.
The competitive advantage of Abdegs and variable domain-based FcRn blockers over WT IgG occurs at several levels. First, they accumulate in FcRn-expressing cells via receptor-mediated, rather than fluid-phase pinocytosis processes [33]. Second, they have higher affinity for FcRn at acidic, endosomal pH. In this context, Abdegs also have higher affinity at endosomal, acidic pH compared with extracellular, near neutral pH, for binding to FcRn, due to the use of the ‘natural’ interaction sites on FcRn and IgG that confer pH-dependence (see section ‘The molecular details of FcRn-IgG interactions’ above). By contrast, antibodies that bind through their variable domains typically involve distinct sets of interacting residues and do not have this property [93]. These variable domain-binding antibodies interact with FcRn with affinities in the nanomolar range across pH 6.0–7.4 and can also bind with four functional sites per antibody (two in the variable domain, two in the Fc fragment). Based on earlier studies, these differences are expected to result in distinct pharmacokinetic and pharmacodynamic behavior [18,82]. Specifically, variable domain-based agents will induce a more rapid inhibitory effect and have shorter intrinsic half-lives due to reduced exocytic release from FcRn-expressing cells. Interestingly, however, studies in non-human primates have revealed that despite very rapid clearance, a prolonged reduction in serum IgG levels that extended beyond that based on the pharmacokinetic behavior of the inhibitor was observed [87]. This may be due, in part at least, to the assays used to assess remaining levels of inhibitor: typically, serum levels are analyzed, which does not yield insight into intracellular load. The rapid internalization of FcRn blockers into FcRn-expressing cells is expected to result in substantially higher intracellular vs. circulating concentrations [33,86].
To date, the results from studies in non-human primates and clinical trials for several FcRn-based inhibitors indicate that they induce significant and sustained decreases in endogenous IgG levels in healthy volunteers and also have beneficial effects in the autoimmune disease, myasthenia gravis [86,87] (www.argenx.com). Although such FcRn inhibitors have considerable potential in the clinic for the management of autoimmunity, they induce a global reduction in IgG levels. This has motivated the development of an approach to selectively deplete antibodies of a particular binding specificity, whilst not affecting the clearance of antibodies of distinct specificities, using engineered antigen-Fc fusions called Seldegs (for selective degradation of antigen-specific antibodies) [94] (Figure 4B). Seldegs comprise an antigen molecule fused to an engineered Fc fragment that, through knobs-into-holes mutations that are designed to insert protrusions and cavities in the Fc chains [95], heterodimerizes with a second Fc fragment with no antigen (Figure 4B). Heterodimer formation results in monomeric display, and the Fc fragment is engineered to bind to FcRn with increased affinity in the pH range 6.0–7.4. As such, Seldegs bind to, and are rapidly internalized by surface-exposed FcRn followed by entry into the lysosomal pathway. Consequently, antibodies that bind to the antigen-Fc fusion protein are co-opted into this degradative pathway, resulting in their destruction. To date, Seldegs have been shown to specifically clear antigen-specific antibodies without affecting the levels of antibodies of irrelevant specificities. The mechanism of action of Seldegs requires only a subset of FcRn molecules to be targeted and consequently, relatively low doses that do not lower total IgG levels are sufficient for efficacy [94]. This contrasts with FcRn inhibitors that are used at higher doses to achieve quantitative binding to functional FcRn in the body, resulting in effective competition with endogenous IgG.
Concluding remarks and future directions
The identification of FcRn as a major player in the regulation of IgG pharmacokinetics has prompted several waves of antibody engineering to produce novel antibody-based platforms. Half-life extension technology has been implemented to result in longer-lived antibodies. The development of antibodies with prolonged in vivo persistence has been followed by technologies to inhibit/block the salvage function of FcRn and thereby reduce the levels of endogenous IgG. More recently, strategies to expedite the clearance of deleterious antigens and antigen-specific antibodies have been developed through acid-switching and/or sweeping approaches. The ongoing clinical trials implementing several of these strategies present an exciting era for FcRn-based therapeutics. Despite these developments, there are multiple outstanding questions such as: how does the molecular nature of FcRn-IgG and antigen-IgG interactions correlate with the dynamic behavior of IgG or antigen at the whole-body level? Further, how do the FcRn-interaction properties of different FcRn inhibitors affect their therapeutic efficacy in distinct disease settings? Addressing these questions using a combination of molecular and cellular approaches is expected to lead to important insight into these issues, which in turn could result in improved therapeutics for human disease.
Outstanding questions.
What is the maximum increase in half-life that is achievable for an IgG?
How can we predict the optimal binding properties of acid-switched/sweeping antibodies for clearance of antigens that differ in the rates at which they are generated (biosynthesized), their concentrations in the body etc.?
How does the binding and subcellular trafficking behavior of an FcRn inhibitor affect its activity as a therapeutic to treat autoimmune disease?
Highlights.
Improved knowledge of FcRn at both molecular and cellular levels can be used towards better designing of novel classes of therapeutic antibodies.
Engineering antibodies to modulate their interaction with FcRn has resulted in several strategies to alter their dynamic behavior.
Half-life extended, therapeutic antibodies with substantial increases in persistence in the body have been developed and are currently in clinical trials.
Antibodies that bind to their target antigen in a pH-dependent way (‘acid-switched’ antibodies) have been shown to be superior agents for the clearance of inflammatory cytokines, regulators or cholesterol levels etc. from the body.
Engineered inhibitors that block the binding of antibodies to FcRn could provide a new generation of therapeutics to reduce the levels of pathogenic antibodies.
Acknowledgements
We thank our current and past laboratory members and collaborators who have contributed to our FcRn-related studies. We apologize that, due to space limitations, we have not been able to cite many relevant publications. We are grateful to Sungyong You and JunHaeng Cho for providing expert assistance with the figures. The research of the authors described herein was supported by grants from the NIH (RO1 AI039167, RO1 AR056478), CPRIT (RP110070, RP110441, RP140141, RP160051), National Multiple Sclerosis Society (RG 4308), MedImmune (Scientific Research Agreement) and argenx (Scientific Research Agreement) to E.S.W. and from the NIH (RO1 GM071048 and RO1 GM085575) and CPRIT (RP110441) to R.J.O.
Glossary
- Allotype
A polymorphic variant of an antibody gene. Antibody alleles frequently vary between individuals within a species, giving rise to allotypic variation
- Cross-presentation
The loading of peptides derived from exogenous proteins onto major histocompatibility complex class I molecules following the internalization of the protein into the antigen presenting cell
- Fluid phase pinocytosis
The internalization of molecules into cells via the pinching off of vesicles that contain extracellular fluid. Internalization is not mediated by binding of the molecules to receptors, but instead is dependent on the extracellular concentration of the molecule of interest
- Intravenous immunoglobulin (IVIG)
A preparation of IgG prepared from plasma pooled from multiple human donors. This preparation is used to treat autoimmune and immunodeficiency diseases in the clinic
- Isoelectric point (pI)
The pH at which a protein (or other molecule) has no net positive or negative charge
- Major Histocompatibility Complex (MHC)
A group of proteins (class I or class II) that is recognized by immune cells called T cells and are involved in inducing an immune response to destroy pathogens, or in the case of autoimmunity, to cause tissue damage. MHC proteins can vary from one person to the next and, as a consequence, are major players in transplant rejection if the donor and recipient are not matched. A minority of MHC Class I-related molecules, such as FcRn, do not play a role in activating T cells, but instead serve other functions
- Off-rate
The rate at which two proteins or other molecules dissociate from each other
- On-rate
The rate with which two proteins or other molecules associate with each other. The ratio of the off-rate to on-rate yields the dissociation constant of the interaction
- Phage display library
The generation of bacteriophage particles, using molecular biology methods, that bear a ‘library’ (i.e. large number) of different recombinant proteins such as antibody variable domains or Fv/Fab fragments. The phage bearing antibody fragments or domains that bind to target antigen can be selected, with the selected phage harboring the genes encoding the displayed fragment or domain
- Plasmapheresis
Treatment of the blood of a patient to remove plasma components such as autoreactive (pathogenic) antibodies. This procedure involves the separation of plasma from blood cells, followed by the replacement of the plasma with saline, albumin and/or specially prepared plasma
- Rab GTPase
A class of G-coupled proteins that are involved in the regulation of intracellular trafficking pathways, such as the movement of vesicles along microtubules or in membrane fusion. Typically, different cellular compartments have specific Rab GTPases associated with them, allowing Rab GTPases to be used as markers of early endosomes, late endosomes etc
- Theranostic imaging
The use of imaging to identify patients that are likely to respond to treatment due to the presence of the molecular target of a specific therapeutic agent
- Transcytosis
The transport of proteins or other macromolecules across cellular barriers, such as polarized epithelial or endothelial cells. For some receptors such as FcRn, this process can occur in both directions across the cells and is therefore bidirectional
Footnotes
Conflict of interest disclosure
E.S.W. is a (co-)inventor on licensed patents related to half-life extension and Abdeg technology and has a financial interest in argenx. The licensed patents are owned by UT Southwestern Medical Center.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Scott AM et al. (2012) Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 [DOI] [PubMed] [Google Scholar]
- 2.Ludwig RJ et al. (2017) Mechanisms of autoantibody-induced pathology. Front. Immunol 8, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein R et al. (2013) Developments in single photon emission computed tomography and PET-based HER2 molecular imaging for breast cancer. Expert Rev. Anticancer Ther 13, 359373. [DOI] [PubMed] [Google Scholar]
- 4.Wu AM (2009) Antibodies and antimatter: the resurgence of immuno-PET. J. Nucl. Med 50, 2–5 [DOI] [PubMed] [Google Scholar]
- 5.Challa DK et al. (2014) FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr. Top. Microbiol. Immunol 382, 249–272 [DOI] [PubMed] [Google Scholar]
- 6.Pyzik M et al. (2015) FcRn: The architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol 194, 4595–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghetie V et al. (1996) Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol 26, 690–696 [DOI] [PubMed] [Google Scholar]
- 8.Kim JK et al. (1994) Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur. J. Immunol 24, 2429–2434 [DOI] [PubMed] [Google Scholar]
- 9.Martin WL et al. (2001) Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: mechanism of pH dependent binding. Mol. Cell 7, 867–877 [DOI] [PubMed] [Google Scholar]
- 10.Kim JK et al. (1999) Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol 29, 2819–2825 [DOI] [PubMed] [Google Scholar]
- 11.Kabat EA et al. (1991) Sequences of proteins of immunological interest, U.S. Dept. of Health and Human Services. [Google Scholar]
- 12.Vaughn DE et al. (1997) Identification of critical IgG binding epitopes on the neonatal Fc receptor. J. Mol. Biol 274, 597–607 [DOI] [PubMed] [Google Scholar]
- 13.Raghavan M et al. (1995) Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 34, 14649–14657 [DOI] [PubMed] [Google Scholar]
- 14.Zhou J et al. (2003) Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J. Mol. Biol 332, 901–913 [DOI] [PubMed] [Google Scholar]
- 15.Stapleton NM et al. (2011) Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun 2, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ober RJ et al. (2001) Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol 13, 1551–1559 [DOI] [PubMed] [Google Scholar]
- 17.Zhou J et al. (2005) Conferring the binding properties of the mouse MHC class I-related receptor, FcRn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J. Mol. Biol 345, 1071–1081 [DOI] [PubMed] [Google Scholar]
- 18.Vaccaro C et al. (2006) Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc. Natl. Acad. Sci. USA 103, 18709–18714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proetzel G et al. (2014) Genetically engineered humanized mouse models for preclinical antibody studies. BioDrugs 28, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery LB et al. (2016) Utility of a human FcRn transgenic mouse model in drug discovery for early assessment and prediction of human pharmacokinetics of monoclonal antibodies. MAbs 8, 1064–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claypool SM et al. (2004) Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fc-γ receptor. Mol. Biol. Cell 15, 1746–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claypool SM et al. (2002) Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human β2-microglobulin. J. Biol. Chem 277, 28038–28050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan Z et al. (2013) Using multifocal plane microscopy to reveal novel trafficking processes in the recycling pathway. J. Cell. Sci 126, 1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ober RJ et al. (2004) Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc. Natl. Acad. Sci. USA 101, 11076–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ober RJ et al. (2004) Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J. Immunol 172, 2021–2029 [DOI] [PubMed] [Google Scholar]
- 26.Prabhat P et al. (2007) Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc. Natl. Acad. Sci. USA 104, 5889–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesar DB et al. (2006) Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic 7, 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzaban S et al. (2009) The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J. Cell Biol 185, 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weflen AW et al. (2013) Multivalent immune complexes divert FcRn to lysosomes by exclusion from recycling sorting tubules. Mol. Biol. Cell 24, 2398–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X et al. (2001) MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol 166, 3266–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akilesh S et al. (2007) Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J. Immunol 179, 4580–4588 [DOI] [PubMed] [Google Scholar]
- 32.Perez-Montoyo H et al. (2009) Conditional deletion of the MHC Class I-related receptor, FcRn, reveals the sites of IgG homeostasis in mice. Proc. Natl. Acad. Sci. USA 106, 2788–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaccaro C et al. (2005) Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat. Biotechnol 23, 1283–1288 [DOI] [PubMed] [Google Scholar]
- 34.Ram S et al. (2008) High accuracy 3D quantum dot tracking with multifocal plane microscopy for the study of fast intracellular dynamics in live cells. Biophys. J 95, 6025–6043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grevys A et al. (2018) A human endothelial cell-based recycling assay for screening of FcRn targeted molecules. Nat. Commun 9, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wandinger-Ness A and Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect Biol 6, a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeffer SR (2017) Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell 28, 712–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward ES et al. (2005) From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol. Biol. Cell 16, 2028–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelms B et al. (2017) A targeted RNAi screen identifies factors affecting diverse stages of receptor-mediated transcytosis. J. Cell Biol 216, 511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sand KM et al. (2014) Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front. Immunol 5, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rink J et al. (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 [DOI] [PubMed] [Google Scholar]
- 42.He W et al. (2008) FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature 455, 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amigorena S and Bonnerot C (1999) Fc receptor signaling and trafficking: a connection for antigen processing. Immunol. Rev 172, 279–284 [DOI] [PubMed] [Google Scholar]
- 44.Baker K et al. (2011) Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl. Acad. Sci. USA 108, 9927–9932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida M et al. (2004) Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20, 769–783 [DOI] [PubMed] [Google Scholar]
- 46.Baker K et al. (2013) Cross-presentation of IgG-containing immune complexes. Cell. Mol. Life Sci 70, 1319–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao SW et al. (2008) Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl. Acad. Sci. USA 105, 9337–9342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vegh A et al. (2012) FcRn overexpression in transgenic mice results in augmented APC activity and robust immune response with increased diversity of induced antibodies. PLoS. One 7, e36286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker K et al. (2014) The role of FcRn in antigen presentation. Front. Immunol 5, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghetie V et al. (1997) Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat. Biotechnol 15, 637–640 [DOI] [PubMed] [Google Scholar]
- 51.Robbie GJ et al. (2013) A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 57, 6147–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu XQ et al. (2017) Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended-half-life, anti-Staphylococcus aureus alpha-toxin human monoclonal antibody, in healthy adults. Antimicrob. Agents Chemother 61, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinton PR et al. (2004) Engineered human IgG antibodies with longer serum half-lives in primates. J. Biol. Chem. 279, 6213–6216 [DOI] [PubMed] [Google Scholar]
- 54.Hinton PR et al. (2006) An engineered human IgG1 antibody with longer serum half-life. J. Immunol. 176, 346–356 [DOI] [PubMed] [Google Scholar]
- 55.Dall’Acqua WF et al. (2006) Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem 281, 23514–23524 [DOI] [PubMed] [Google Scholar]
- 56.Yeung YA et al. (2009) Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol 182, 7663–7671 [DOI] [PubMed] [Google Scholar]
- 57.Zalevsky J et al. (2010) Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol 28, 157–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nnane IP et al. (2017) Modification of the Fc region of a human anti-oncostatin M monoclonal antibody for higher affinity to FcRn receptor and extension of half-life in Cynomolgus monkeys. Basic Clin. Pharmacol. Toxicol 121, 13–21 [DOI] [PubMed] [Google Scholar]
- 59.Oganesyan V et al. (2014) Structural insights into neonatal Fc receptor-based recycling mechanisms. J. Biol. Chem 289, 7812–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko SY et al. (2014) Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514, 642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borrok MJ et al. (2014) pH-dependent binding engineering reveals an FcRn affinity threshold which governs IgG recycling. J. Biol. Chem 290, 4282–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng CM et al. (2016) Mechanism-based competitive binding model to investigate the effect of neonatal Fc receptor binding affinity on the pharmacokinetic of humanized anti-VEGF monoclonal IgG1 antibody in Cynomolgus monkey. AAPS J. 18, 948–959 [DOI] [PubMed] [Google Scholar]
- 63.Wang W et al. (2011) Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug. Metab. Dispos 39, 1469–1477 [DOI] [PubMed] [Google Scholar]
- 64.Schlothauer T et al. (2013) Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. MAbs 5, 576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoch A et al. (2015) Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proc. Natl. Acad. Sci. USA 112, 5997–6002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piche-Nicholas NM et al. (2018) Changes in complementarity-determining regions significantly alter IgG binding to the neonatal Fc receptor (FcRn) and pharmacokinetics. MAbs 10, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Datta-Mannan A et al. (2015) Balancing charge in the complementarity-determining regions of humanized mAbs without affecting pI reduces non-specific binding and improves the pharmacokinetics. MAbs 7, 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Igawa T et al. (2010) Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng. Des. Sel 23, 385–392 [DOI] [PubMed] [Google Scholar]
- 69.Li B et al. (2014) Framework selection can influence pharmacokinetics of a humanized therapeutic antibody through differences in molecule charge. MAbs 6, 1255–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finkelman FD et al. (1993) Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol 151, 1235–1244 [PubMed] [Google Scholar]
- 71.O’Hear CE and Foote J (2005) Antibody buffering of a ligand in vivo. Proc. Natl. Acad. Sci. USA 102, 40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaparro-Riggers J et al. (2012) Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J. Biol. Chem 287, 11090–11097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukuzawa T et al. (2017) Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement-mediated diseases. Sci. Rep 7, 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Igawa T et al. (2010) Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol 28, 1203–1207 [DOI] [PubMed] [Google Scholar]
- 75.Devanaboyina SC et al. (2013) The effect of pH dependence of antibody-antigen interactions on subcellular trafficking dynamics. MAbs 5, 851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang D et al. (2017) Maximizing in vivo target clearance by design of pH-dependent target binding antibodies with altered affinity to FcRn. MAbs 9, 1105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonvin P et al. (2015) De novo isolation of antibodies with pH-dependent binding properties. MAbs 7, 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henne KR et al. (2015) Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement. J. Pharmacol. Exp. Ther 353, 119–131 [DOI] [PubMed] [Google Scholar]
- 79.Igawa T et al. (2016) Sweeping antibody as a novel therapeutic antibody modality capable of eliminating soluble antigens from circulation. Immunol. Rev 270, 132–151 [DOI] [PubMed] [Google Scholar]
- 80.Hironiwa N et al. (2016) Calcium-dependent antigen binding as a novel modality for antibody recycling by endosomal antigen dissociation. MAbs 8, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igawa T et al. (2013) Engineered monoclonal antibody with novel antigen-sweeping activity in vivo. PLoS. One 8, e63236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dall’Acqua W et al. (2002) Increasing the affinity of a human IgG1 to the neonatal Fc receptor: biological consequences. J. Immunol 169, 5171–5180 [DOI] [PubMed] [Google Scholar]
- 83.Gan Z et al. (2009) Analyses of the recycling receptor, FcRn, in live cells reveal novel pathways for lysosomal delivery. Traffic 10, 600–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L et al. (2007) Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J. Immunol 178, 5390–5398 [DOI] [PubMed] [Google Scholar]
- 85.Low SC and Mezo AR (2009) Inhibitors of the FcRn:IgG protein-protein interaction. AAPS J. 11, 432–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nixon AE et al. (2015) Fully human monoclonal antibody inhibitors of the neonatal Fc receptor reduce circulating IgG in non-human primates. Front. Immunol. 6, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kiessling P et al. (2017) The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci. Transl. Med 9, eaan1208 [DOI] [PubMed] [Google Scholar]
- 88.Patel DA et al. (2011) Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model. J. Immunol 187, 1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swiercz R et al. (2014) Use of Fc-engineered antibodies as clearing agents to increase contrast during PET. J. Nucl. Med 55, 1204–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khosroshahi A et al. (2010) Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 62, 1755–1762 [DOI] [PubMed] [Google Scholar]
- 91.Ahmed AR et al. (2006) Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N. Engl. J. Med 355, 1772–1779 [DOI] [PubMed] [Google Scholar]
- 92.Seijsing J et al. (2018) In vivo depletion of serum IgG by an affibody molecule binding the neonatal Fc receptor. Sci. Rep 8, 5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kenniston JA et al. (2017) Structural basis for pH-insensitive inhibition of immunoglobulin G recycling by an anti-neonatal Fc receptor antibody. J. Biol. Chem 292, 17449–17460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Devanaboyina SC et al. (2017) Engineered clearing agents for the selective depletion of antigen-specific antibodies. Nat. Commun 8, 15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Merchant AM et al. (1998) An efficient route to human bispecific IgG. Nat. Biotechnol 16, 677–681 [DOI] [PubMed] [Google Scholar]
- 96.Schmidt MM et al. (2013) Crystal structure of an HSA/FcRn complex reveals recycling by competitive mimicry of HSA ligands at a pH-dependent hydrophobic interface. Structure 21, 1966–1978 [DOI] [PubMed] [Google Scholar]
- 97.Andersen JT et al. (2014) Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J. Biol. Chem 289, 13492–13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pyzik M et al. (2017) Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury. Proc. Natl. Acad. Sci. U S A 114, E2862–E2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swiercz R et al. (2017) Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget 8, 3528–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deisenhofer J (1981) Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry 20, 2361–2370 [PubMed] [Google Scholar]