Abstract
Seabuckthorn is a medicinal plant that is used to prevent cold. It was tested for its metabolic content followed by activity against cancer and virus. The metabolic distribution of different polarity solvent extractions from the leaves was analyzed by LC–MS/MS. Flavonol glycoside contents in EA and Bu extracts were higher than MeOH and DW was observed. MeOH and EA extracts recorded high activity against influenza A/PR virus with IC50 of 7.2 μg/mL and 10.3 μg/mL compared with known drug Oseltamivir of 60.3 μg/mL. A similar trend showed in influenza A/Victoria virus. In case of influenza B viruses such as B/Lee and B/Maryland, EA extract (2.87 μg/mL and 4.5 μg/mL of IC50) emerged strongest among other extracts and Oseltamivir (103.73 μg/mL and 71.6 μg/mL). Each extract showed potent anticancer activities. Interestingly, Bu extract showed stronger anticancer activity against human cancer cells such as NCL-H1299, HeLa, SKOV and Caski (8.2 μg/mL, 8.6 μg/mL, 18.2 μg/mL and 9.2 μg/mL of IC50) respectively. Correlation study reveals that aglycones and flavonol mono-glycosides highly correlated with anti-influenza activities but not correlated with anticancer activities. Reversely, di-glycosides and tri-glycosides have a high correlation with cytotoxic effect with both normal and cancer cells. Therefore, this study provides significant information concerning Seabuckthorn for further medicinal drug development.
Keywords: Seabuckthorn leaf, Influenza, Cancer, SRB assay, Metabolite correlation
1. Introduction
Nowadays, many diseases as inflammation, cancer and cardiovascular in developed countries come out from stresses, reason of living and working conditions including high population and high competition in life (Esch et al., 2002, Black and Garbutt, 2002). Appearance of cancer risk is increasing significantly in modern society in 21st century. Hence many anticancer methods were discovered such as radiation therapy and chemotherapy, unfortunately these anticancer methods have high side effects to the patient (Lee et al., 2012).
Additionally, virus infections such as influenza and their seasonal breakouts happen frequently and have a dangerous impact on elders and children. Until now few drugs have been developed, unfortunately drug sensitiveness is reducing day by day (Choi et al., 2012, Choi et al., 2009). Vaccination is the key inhibitor against influenza virus infection, but because of high rate of genetic drift and shift of influenza virus, people should take vaccine once or more a year (Stephan et al., 2009). Hence, scientists need to develop more treatments and scientific proof against influenza virus and cancer.
Plants contain many different primary and secondary metabolites. In secondary metabolites such as phenolic compounds, terpenoids and flavonoids play a crucial role in the enhancement of medicinal properties against human and animal diseases (Williams et al., 2004). Previous studies reported that plant secondary metabolites are helping to prevent many cancers, coronary heart and kidney diseases, viral infection, oxidation and many other age-related diseases (Havsteen, 2002, Hertog et al., 1993, Potter et al., 1998).
Some plant derived extracts and quercetin glycosides are showing high activity against influenza viruses in in vivo and in vitro (Choi et al., 2012, Choi et al., 2009, Patil et al., 2013). Interestingly, our daily use of green tea derived catechin showed antiviral activity (Song et al., 2005). Moreover, polyphenol rich plant extracts exerted influenza virus activity without showing side effects on cultured cells (Ehrhardt et al., 2007).
Hippophae rhamnoides L., commonly called Seabuckthorn, is a fruit plant in the family of Elaeagnaceae, native to the Asian parts of Mongolia, China, and Europe (Rousi, 1971). Berries of Seabuckthorn were used in ancient Tibetian, Chinese, Russian and Mongolian traditional medicine (Yang et al., 2000, Xu et al., 1994). In ancient Mongolian history, Seabuckthorn branches and leaves were administrated to stocks and humans to heal their intestinal pain (Kim et al., 2011). Nowadays, many countries are cultivating Seabuckthorn for nutritional value and medicinal activity (Xu et al., 1994, Suryakumar and Gupta, 2011). Also, Seabuckthorn berry juice is the most familiar drink among Mongolians nowadays.
Whole plant (fruit, leaf, and tree) of Seabuckthorn is known to be a great origin of a broad range of active biological substances contained in berries and leaves, are very plentiful in a diversity of vitamins and other biologically active materials like phenols, carotenoids, flavonoids, tocopherols and sterols, which have potent medicinal properties and high nutrients (Beveridge et al., 1999, Pop et al., 2013). Mainly, the berry of Seabuckthorn has been recorded to be a prosperous source of carotenoids and vitamin C, E (Zhong, 1989, Ganju et al., 2005).
In 2013, some researchers reported that the Seabuckthorn berry and leaf are rich in flavonol glycosides of isorhamnetin and quercetin derivatives. The flavonol glycosides content on leaves 917 mg/100 g and leaves contented flavonol glycosides higher than berries, 1118 mg/100 g on average (Pop et al., 2013). Seabuckthorn recorded a broad range of biological activities as an antioxidant (Geetha et al., 2008, Narayanan et al., 2005), anti-inflammatory (Padwad et al., 2006, Kwon et al., 2011), anticancer (Grey et al., 2010, Hibasami et al., 2005), antiviral (Jain et al., 2008) and antimicrobial (Chauhan et al., 2007, Upadhyay et al., 2010). The Recent clinical test shows that berries of Seabuckthorn did not prohibit common cold or digestive track infections however, reducing serum C-reactive protein, a marker of inflammation, and a reason for the cardiovascular disorder (Larmo et al., 2008).
In this study, we purposed to investigate the diversity and correlation between metabolites and biological activities of Seabuckthorn leaf extracts against influenza viral infections and cancers.
2. Materials and methods
2.1. Plant material
The leaf of Seabuckthorn was collected in April 2014 from Seabackthorn Farm (Anna’s Farm Co Ltd.,) located in Gyeonggi-do, South Korea. Collected plant material was dried under shade place for two weeks.
2.2. Chemicals
Butanol, ethyl acetate, methanol, and water were obtained from Duk-san Chemical Co. Korea. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2 diphenyl-2-picrylhydrazyl (DPPH) and Sulforhodamine B were purchased from Sigma Chemicals Co. St. Louis, MO, USA. The commercial drug Oseltamivir (Tamiflu® F. Hofmann-La Racho Ltd., Switzerland) was purchased from a pharmacy in Korea following doctor prescription.
2.3. Cell lines and virus
Influenza A and B viruses (strain A/Puerto Rico/8/1934 – H1N1, A/Victoria/3/75 – H3N2, B/Lee/40 and B/Maryland/1/59) and MDCK cell line were purchased from ATCC (Manassas, VA, USA). Lung cancer cell (NCL-H1299), human ovary cancer cells (HeLa and SKOV) purchased from ATCC (Manassas, VA, USA) and cervix cancer cell (CaSki) were from KCLB (Korean Cell line Bank, Seoul, South Korea). RPMI-1640, DMEM medium (Dulbecco’s Modified Eagle’s Medium), fetal bovine serum (FBS), antibiotic-antimytotic and trypsin-EDTA was supplied by Gibco BRL (Grand Island, NY, USA). The cell culture dish and 96 well plates were purchased from Falcon (BD Bioscience, Franklin Lakes, NJ, USA).
2.4. Extraction of Seabuckthorn leaf
The Seabuckthorn leaves were dried under shade conditions for two weeks followed by grinding into powder using a commercial grinder (Hanil Co. Seoul, South Korea). 500 g of dried powder was extracted twice with 2 L of methanol 100% and twice with 80% methanol using a shaker for 10 h at room temperature followed by sonication for 10 min. The extracts were purified using a cotton plug followed by Whatman No1 filter paper. The collected methanol extract was dried using a rotary evaporator (EYELA N1001S-WD, NY, USA) and powdered. 10 g of methanol extracts was dissolved again in water followed by partitioning with ethyl acetate and butanol. The collected EA and Bu extracts were further condensed with a rotary evaporator and powdered. The remaining water extracts were condensed using a freeze drier to make it powder.
2.5. Total flavonoid content
Total flavonoid content of Seabuckthorn extracts was measured using the methods of Maria John et al. (2014). In brief, each extract of 20 μL was added to180 μL of 90% diethylene glycol and 20 μL of 1 M NaOH. The absorbance was measured at 515 nm, after 15 min of incubation at room temperature. Flavonoid content was expressed as milligram of catechin equivalents per gram of dried Seabuckthorn extracts.
2.6. Total polyphenol content
20 μL of different extracts were added to the 96 well plates followed by 100 μL of 0.2 M Folin–Ciocalteu’s phenol reagent. After 5 min of incubation, 80 μL of saturated sodium carbonate was added to the mixture and incubated for 1 h. Using a SpectraMax® Plus 384 – spectrophotometer, absorbance was measured at 750 nm. The results were expressed in milligram of gallic acid equivalent per gram of dried Seabuckthorn extracts.
2.7. LC–MS
Bruker EVOQ LC–MS system equipped with Bruker, CTC PAL-xt autosampler, and YMC packed C18 column was used for LC–MS analyzes of Seabuckthorn leaf. The flow rate was 200 μL/min; injection volume was 5 μL and mobile phase of A: 0.1% formic acid in water and B: 0.1% formic acid in acetonitrile. The gradient flow was 0% of mobile phase B during the 0–0.5 min, increase up to 50% in 5 min and maintained 50% of mobile phase B until 15 min. At 16 min, mobile phase B was reduced to 0% and maintained through analysis. The mass spectrometric identification used parameters as spray mode as HESI and voltage were positive mode 4000 V. The ESI was performed with positive (+) ion mode with the range of 100–1000 m/z, and cone temperature was 350 °C.
2.8. DPPH radical scavenging activity
The free radical scavenging activity of Seabuckthorn leaf extracts against DPPH radical was studied by following the methods of Maria John et al. (2014). Briefly, different extracts of Seabuckthorn leaf were dissolved in methanol. 20 μL of each extract and 180 μL of 0.1 mM concentrated DPPH solution were added to a 96 well plate. After 20 min of incubation at room temperature in a dark place, the absorbance was measured at 517 nm using a SpectraMax® Plus384 – spectrophotometer. 20 μL methanol was used as a control, and the radical scavenging activity was calculated based on the following formula:
| (1) |
where Acontrol: absorbance of control and Asample: absorbance of the sample.
2.9. ABTS radical scavenging activity
20 μL of the extracts was added to 180 μL of 7.4 mM ABTS solution containing 2.6 mM potassium persulfate. Samples were incubated for 15 min at room temperature. Then absorbance were measured at 734 nm using a SpectraMax® Plus384 – spectrophotometer. Methanol was used as a control and based on the OD differences the scavenging activity was calculated.
2.10. Cell culture
NCL-H1299, HeLa, SKOV and Caski cells were maintained at 10% of FBS and 1% of Antibiotic-Antimycotic solution mixed in the RPMI-1640 medium. MDCK cell was maintained in DMEM medium. Cells’ grown condition was 37 °C with 5% of CO2 in the humidified cell culture incubator.
2.11. Antiviral activity assay
MDCK cells (2 × 104) were seeded in 96 well plates and maintained for 24 h. Then, media were removed and washed twice with PBS (phosphate buffered saline). MDCK cells were infected with influenza viruses A/Victoria, A/PR, B/Lee and B/Maryland by ID50 (Infective dose 50%). The antiviral activity of dried extracts of Seabuckthorn leaf was determined based on different concentrations such as 0.1, 1, 10 and 100 μg/mL in triplicate. The plates were incubated at 37 °C, CO2 incubator during 48 h. After incubation of 48 h, the medium was removed and washed twice with PBS. The plates fixed with 70% of acetone during 1 h under −4 °C. Then 70% of acetone was removed, and plates were dried in a dry oven maintained at 60 °C. Continually used SRB assay was used for antiviral development.
2.12. Anticancer activity assay
NCL-H1299, HeLa, Caski and SKOV cells (2 × 104) were seeded in 96 well plates and maintained at 37 °C, 5% of CO2 and humidified incubator. After 24 h of incubation, the media were removed and washed 2 times with PBS solution. Prepared extracts in different concentrations as 0.1 μg/mL, 1 μg/mL, 10 μg/mL and 100 μg/mL in triplicate were added onto wells after new media were added to the wells. After 48 h of incubation with extract, the medium was removed and washed twice with PBS. Washed plates were fixed with 70% of Acetone for 1 h at −4 °C. Then acetone was removed, and plates were dried in drying oven at 60 °C. After drying, SRB assay was performed for anticancer activity.
2.13. SRB assay
After 96 well plates had been fully dried in drying oven, each well was filled with 100 μL of SRB solution (0.4% w/v) and incubated overnight at room temperature. After SRB solution was discarded, plates were washed 5 times with 1% of acetic acid and then dried in a drying oven. SRB stained 96 well plates were observed for the morphology of cells using reflected light microscope (20×) and added 10 mM of Tris-base for overnight. After SRB completely dissolved in a Tris-base, the absorbance was read at 540 nm and inhibitory concentration of 50% (IC50), cytotoxic concentration of 50% (CC50) and therapeutic index (TI) were calculated.
2.14. Statistical analyses
The result of biological and analytical assay replicates was analyzed by Microsoft Excel and IBM SPSS Statistics 22 software (SPSS Inc., Chicago, IL, USA). Metabolite comparison and box plot analyzed by Statistica 7.1 (Statsoft, USA). LC–MS/MS spectrum and area of metabolites analyzed by MS workstation MS Data Review (Version 8.1.2; Bruker).
In case of correlation study between metabolites and biological activities, log10 value of metabolite areas collected and then Pearson’s correlation coefficient test performed by SPSS software. Results were illustrated using MeV software (version 4.9.0, http://tm4.org).
3. Result and discussion
3.1. TFC, TPC and metabolites content of extracts
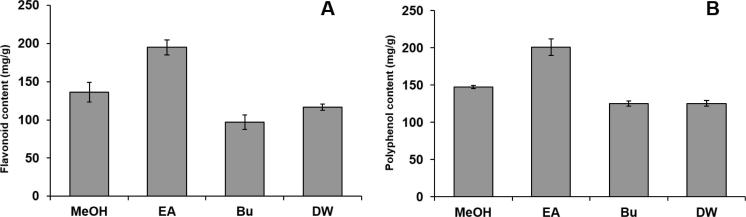
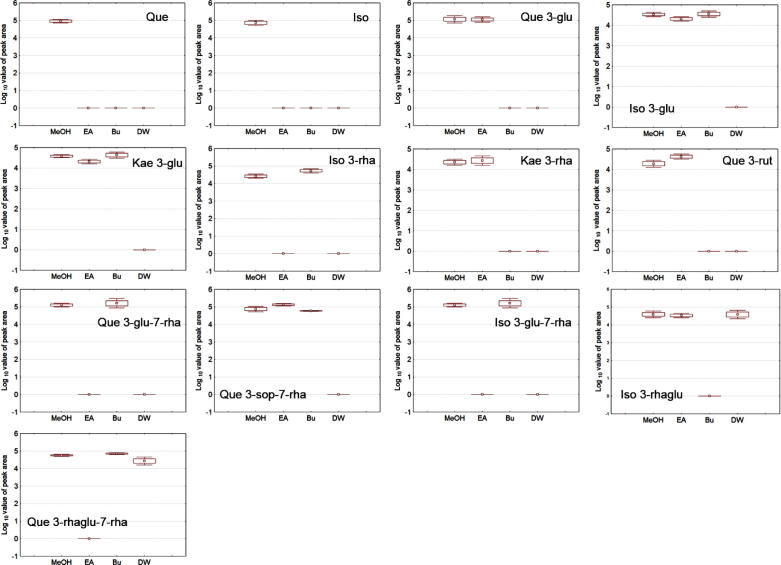
Seabuckthorn served as one of the most important natural medicinal plants in various countries was analyzed for its metabolic content along with antiviral and anticancer analysis. The crude methanol extracts from Seabuckthorn leaves were further purified using EA and Bu. From the solvent extracts and the remaining water extract, the TFC and TPC content were analyzed and presented in Fig. 1. The EA extracts revealed high TFC (195.12 mg/g) content followed by MeOH (136.25 mg/g), and DW (116.75 mg/g). Bu (96.78 mg/g) extracts showed poor content among the extracts analyzed. A similar trend was observed with the TPC where EA was 117.78 mg/g followed by MeOH, Bu and DW (88.22 mg/g, 76.05 mg/g and 76.18 mg/g) respectively. The metabolic distribution between the solvent extracts and the remaining water extracts were analyzed by LC–MS/MS and are listed in Table 1 and Fig. 3. Identified compounds from Seabuckthorn leaf extracts were derivatives of quercetin, isorhamnetin and kaempferol. Identification of compound was based on the retention time, UV spectra, positive ions [M+H]+ and reference data (Cuyckens et al., 2004, Cuyckens and Claeys, 2005, Kachlicki et al., 2008, Pop et al., 2013, Rösch et al., 2004). The 14 different flavonol glucosides were differently distributed in 3 extracts of Seabuckthorn leaf. Flavonol glucoside contents in EA and Bu extracts were higher than DW extract. Quercetin, isorhamnetin, quercetin 3-glucoside, quercetin 3-rutinoside, isorhamnetin 3-glucoside, kaempferol 3-glucoside, isorhamnetin 3-rhamnosylglucoside, quercetin 3-sophoroside-7-rhamnoside and kaempferol 3-rhamnoside were observed in EA extract. Quercetin 3-rhamnosylglucoside-7-rhamnoside, kaempferol 3-glucoside, quercetin 3-glucoside-7-rhamnoside, isorhamnetin 3-glucoside-7-rhamnoside, isorhamnetin 3-rhamnoside, isorhamnetin 3-glucoside, quercetin 3-rhamnosylglucoside-7-rhamnoside, quercetin 3-sophoroside-7-rhamnoside and isorhamnetin 3- sophoroside-7-rhamnoside were detected in Bu extract. Quercetin 3-glucoside-7-rhamnoside, isorhamnetin 3-rhamnosylglucoside, quercetin 3-rhamnoside-7-rhamnoside and isorhamnetin 3-sophoroside-7-rhamnoside were identified in DW extract. Mono-glycosides, di-glycosides and tri-glycosides were distributed differently in each extract since solvents used have different polarities. Coqueiro et al., reported, flavonol glycosides were altered biological activity depend on the number of glycosides with flavonoids (Coqueiro et al., 2013). The content of di-glycosides and tri-glycosides highly concentrated in the polar solvents such as Bu and DW. A low polar solvent such as EA presented mono-glucoside and aglycones. Because of these metabolic variations, the TFC, and TPC content of the extracts varied significantly. To find the influence of the different solvent extraction on radical damaging activity, the standard free radical assay such as ABTS and DPPH was tested.
Figure 1.
Total flavonoid and total polyphenol content of different extracts of Seabuckthorn leaf. (A) Total flavonoid content; (B) total polyphenol content. Each value was obtained from an average of three independent experiments’ standard deviation.
Table 1.
List of the Identified compounds using LC–MS Seabuckthorn leaf extracts.
| S. no | Rt | [M+H]+ | p-Value | MF | MS/MS | Name | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 12.69 | 303 | 1.29E−02 | C15H10O7 | 303, 285, 275, 247, 229, 201, 165 | Quercetin | Pop et al. (2013) |
| 2 | 14.22 | 317 | 1.32E−02 | C16H12O7 | 317, 302, 285, 274, 257, 165 | Isorhamnetin | Pop et al. (2013) |
| 3 | 7.03 | 465 | 2.35E−02 | C21H20O12 | 465, 303, 162 | Quercetin 3-glucoside | Rösch et al., 2004, Pop et al., 2013 |
| 4 | 7.25 | 479 | 4.09E−02 | C22H22O12 | 479, 317, 162 | Isorhamnetin 3-glucoside | Cuyckens et al., 2004, Pop et al., 2013 |
| 5 | 7.26 | 449 | 2.27E−02 | C21H20O11 | 449, 287, 162 | Kaempferol 3-glucoside | Cuyckens and Claeys (2005) |
| 6 | 6.75 | 463 | 1.38E−02 | C22H22O11 | 463, 317, 146 | Isorhamnetin 3-rhamnoside | Rösch et al., 2004, Pop et al., 2013 |
| 7 | 8.87 | 433 | 2.27E−02 | C21H19O10 | 433, 287, 146 | Kaempferol 3-rhamnoside | Cuyckens and Claeys (2005) |
| 8 | 7.11 | 611 | 1.38E−02 | C27H30O16 | 611, 465, 303, 308, 146 | Quercetin 3-rutinoside | Rösch et al., 2004, Pop et al., 2013 |
| 9 | 6.32 | 611 | 4.44E−02 | C27H30O16 | 611, 465, 449, 303, 162, 146 | Quercetin 3-glucoside-7-rhamnoside | Kachlicki et al., 2008, Pop et al., 2013 |
| 10 | 6.4 | 625 | 2.33E−02 | C28H32O16 | 625, 479, 465, 317, 162, 146 | Isorhamnetin 3-glucoside-7-rhamnoside | Rösch et al., 2004, Kachlicki et al., 2008, Pop et al., 2013 |
| 11 | 7.27 | 625 | 9.11E−02 | C28H32O16 | 625, 479, 463, 162, 317, 146, 162 | Isorhamnetin 3-rhamnosylglucoside | Pop et al., 2013, Kachlicki et al., 2008 |
| 12 | 7.57 | 757 | 1.73E−02 | C33H40O21 | 757, 611, 146, 465, 303, 162, 146 | Quercetin 3-rhamnosylglucoside-7-rhamnoside | Kachlicki et al., 2008, Pop et al., 2013 |
| 13 | 8.88 | 773 | 2.23E−02 | C33H40O21 | 773, 611, 449, 303, 162, 146 | Quercetin 3-sophoroside-7-rhamnoside | Rösch et al. (2004) |
| 14 | 9.13 | 787 | 1.21E−02 | C34H42O21 | 787, 625, 463, 317, 162 | Isorhamnetin 3-sophoroside-7-rhamnoside | Pop et al., 2013, Rösch et al., 2004 |
Figure 3.
Flavonol glycosides variation of Seabuckthorn leaf extracts. (MeOH) Methanol, (EA) ethyl acetate, (Bu) butanol and (DW) water extract.
3.2. ABTS and DPPH radical scavenging activities
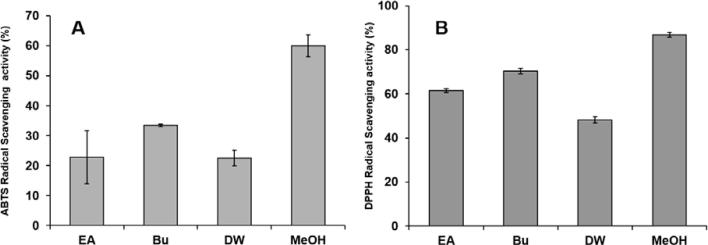
ABTS and DPPH radical scavenging activity of the different extracts were compared and presented in Fig. 2. All extracts showed strong antioxidant activity. Comparing the radical scavenging activity of extracts, the MeOH extract showed higher scavenging activity and DW extract showed less activity with both ABTS and DPPH radical scavenging activity. EA extract showed higher TFC and TPC, but its radical scavenging activity was not higher among other extracts. Previously report by Coqueiro et al., showed that, quercetin glycosides had two time’s higher activity than quercetin (aglycones) with both DPPH and ABTS radical scavenging activities (Coqueiro et al., 2013). Bu extract showed the higher content of di-glycosides and tri-glycosides than EA and DW extracts. When comparing radical scavenging activity of extracts, Bu extract showed stronger antioxidant activity than EA and DW extracts. Consequently, Seabuckthorn extracts showed their powerful radical scavenging activities.
Figure 2.
Free radical scavenging potential of different extractions of Seabuckthorn leaf. (A) ABTS radical scavenging activity, (B) DPPH radical scavenging activity. Each value was obtained from an average of three independent experiments’ standard deviation.
3.3. Antiviral activity against influenza viruses
Nowadays, Oseltamivir and Zanamivir are being used widely as a drug for the treatment of influenza viral infections. However, during the 2007–2008 season of influenza, different isolates of hemagglutinin and neuraminidase were resistant against Oseltamivir (Choi et al., 2009). Hence, new approaches to drug development to control the infections of influenza virus have to be discovered more. Seabuckthorn berries have been used in Eastern traditional medicine against infectious such as common cold, and it has immune modulatory properties (Larmo et al., 2008).
Anti-influenza activity and cytotoxic against MDCK cells of various extracts of Seabuckthorn and standard drug Oseltamivir are presented in Table 2 and Fig. 4. Based on the divergent of IC50 and CC50 values, the anti-viral activities of extracts were compared. MDCK cells with and without influenza virus infection were used as positive and negative control, respectively.
Table 2.
Anti-influenza activities of different extracts of Seabuckthorn leaf.
| A/PR |
A/Victoria |
B/Lee |
B/Maryland |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CC50a | IC50b | TIc | IC50 | TI | IC50 | TI | IC50 | TI | |
| Methanol | 155.8 | 7.2 ± 0.2 | 21.7 | 8.39 ± 0.89 | 18.5 | 6.39 ± 0.89 | 24.3 | 9.29 ± 0.89 | 16.8 |
| Ethyl Acetate | 558.2 | 10.31 ± 0.3 | 54.4 | 8.86 ± 0.35 | 62.9 | 2.87 ± 0.35 | 194.5 | 4.52 ± 0.35 | 123.5 |
| Butanol | 62.1 | 14.3 ± 1.1 | 4.2 | 15.97 ± 0.15 | 3.9 | 5.97 ± 0.15 | 10.4 | 6.81 ± 0.15 | 9.1 |
| Water | 917.6 | 40.8 ± 4.9 | 22.5 | 58.87 ± 0.02 | 15.6 | 8.87 ± 0.02 | 103.4 | 21.7 ± 3.1 | 42.3 |
| Oseltamivir | 569.1 | 60.3 ± 5.6 | 9.5 | 37.2 ± 3.5 | 15.3 | 103.7 ± 8.75 | 5.5 | 71.6 ± 5.1 | 8.0 |
Each value was obtained from an average of three independent experiments’ standard deviation.
Concentration required to reduce MDCK cell growth by 50% (μg/mL).
Concentration required to inhibit virus infectivity by 50% (μg/mL).
Therapeutic index = CC50/IC50.
Figure 4.
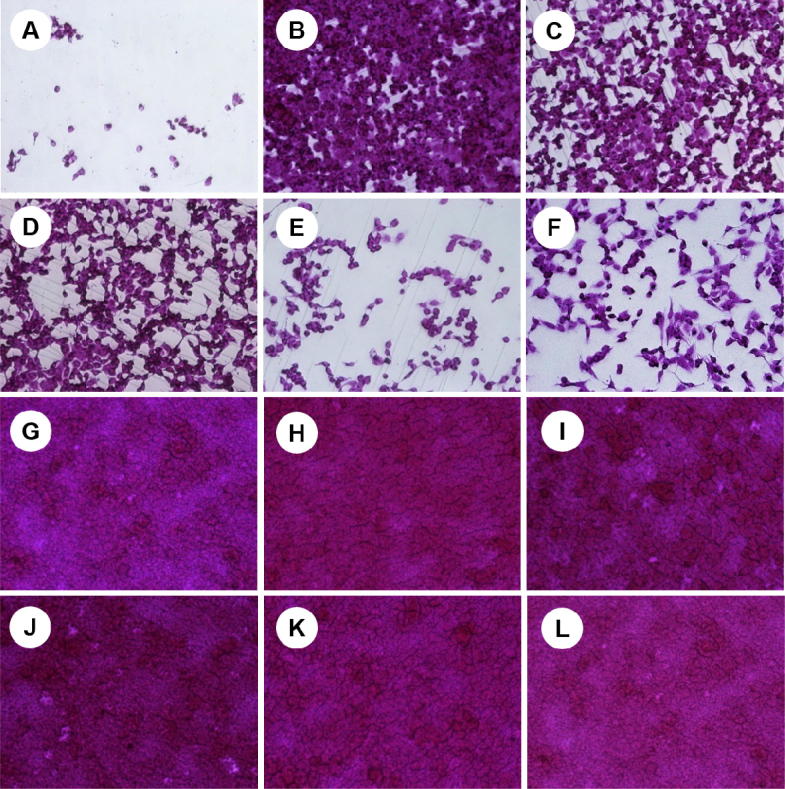
Effect of Seabuckthorn leaf extracts on influenza A/PR infected MDCK cells in a concentration of 10 μg/mL. (A) A/PR virus infected cells; (B) A/PR virus infected cells with MeOH extract; (C) A/PR virus infected cells with EA extract; (D) A/PR virus infected cells with Bu extract; (E) A/PR virus infected cells with DW extract; (F) A/PR virus infected cells with Oseltamivir; (G) non-infected cells; (H) non-infected cells with MeOH extract; (I) non-infected cells with EA extract; (J) non-infected cells with Bu extract; (K) non-infected cells with DW extract; (L) non-infected cells with Oseltamivir.
EA extract revealed the highest anti-influenza activity with therapeutic index (54.4, 62.3, 194.5 and 123.5) among other extracts against influenza A/PR, A/Victoria, B/Lee and B/Maryland viruses, respectively. Potential IC50 values against A/PR and A/Victory viruses observed in MeOH extract (7.2 μg/mL and 8.39 μg/mL) followed by Bu extract (14.3 μg/mL and 15.97 μg/mL) whereas it showed more cytotoxic among other extracts with 155.8 μg/mL (MeOH) and 62.1 μg/mL (Bu) of CC50, respectively. In terms of B/Lee and B/Maryland viruses, EA showed extreme inhibition activities with 2.87 μg/mL and 4.52 μg/mL of IC50 values followed by Bu extract (5.97 μg/mL and 6.81 μg/mL). Because of low cytotoxicity it was 917.6 μg/mL of CC50, DW extract showed higher TI values such as 22.5, 15.6, 103.4 and 42.3 against influenza A/PR, A/Victoria, B/Lee and B/Maryland viruses, respectively. TI value of Oseltamivir against influenza viruses (A/PR, A/Victoria, B/Lee and B/Maryland) were 9.5, 15.3, 5.5 and 8.0, respectively. Amazingly all extracts from Seabuckthorn leaf being inhibited influenza A and B virus infections more effectively than Oseltamivir, which is used as a control drug.
Previous research showed that some flavonoids such as quercetin derivatives had better inhibition activity against influenza A virus than a well-known drug (Choi et al., 2012). According to the TFC and LC/MS observation, Seabuckthorn extracts were highly concentrated with various derivatives of flavonol glycosides. Consequently, extreme anti-influenza activity was observed with these extracts. Flavonol glycosides do not directly interact with influenza viral particles, and they inhibit only initial stage of virus replication (Choi et al., 2012). That indicates flavonol glycosides concentrated in Seabuckthorn extracts have some vital role in cellular. The EA and Bu extract of the Seabuckthorn leaf could be an important source of potent antiviral activity that could be used to develop novel antiviral agents. Based on some cytotoxicity of these extracts, we checked their cytotoxic ability against cancer cell lines and further analysis was done to identify a correlation between flavonol glycosides and biological activities.
3.4. Anticancer activity
The lung cancer found to be one of the most dangerous cancer diseases among humans leading to 1.56 million deaths annually (Wild and Stewart, 2014). Moreover, some lung cancer induced cell contains partial genes to decrease expression of p53 protein. This p53 protein increases prevention of influenza virus infection in human lungs (Turpin et al., 2005). It showed that patients with lung cancer have a high possibility of infections, and it is easier for an influenza virus to replicate in cancer patients than healthy people. Also, some cancer cells (SKOV) are not easily controlled by known anticancer toxins and drugs such as Diphtheria toxin, Adriamycin and Cis-Platinum (Morimoto et al., 1993). Associated with previous reports, we tested Seabuckthorn extracts along with cancer cells.
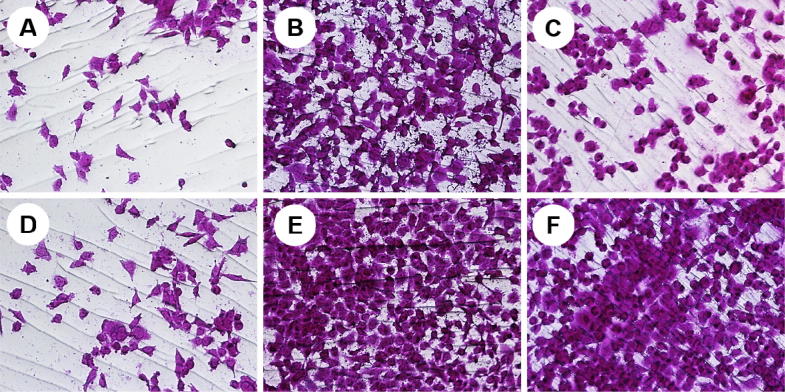
The extracts of Seabuckthorn leaf was tested against cancers, and the results are demonstrated in Table 3 and Fig. 5. Normal kidney epithelial cell (MDCK), lung cancer cell (NCL-H1299), human ovary cancer cells (HeLa and SKOV) and cervix cancer cell (Caski) were used to evaluate the anticancer activity of Seabuckthorn leaf extracts compared with berberine. Based on the IC50 values the anticancer activity of different solvent Seabuckthorn extract was compared. Bu extracts considered most strong toxic to the cancer cells than other extracts and berberine. IC50 values of Bu extract were 8.2 μg/mL, 8.6 μg/mL, 18.2 μg/mL and 9.2 μg/mL against NCL-H1299, HeLa, Caski and SKOV cell lines, respectively. MeOH and DW extract showed higher anticancer activity against lung cancer cell line as NCL-H1299 (9.6 μg/mL and 9.0 μg/mL of IC50) comparing with berberine (57.02 μg/mL of IC50). In case of SKOV cell line, Bu and EA extracts showed highest anticancer activity with 9.2 μg/mL and 9.4 μg/mL. MeOH and DW extracts showed weaker activities against SKOV cell line whereas these two extracts presented stronger toxicity in a NCL-H1299 cell line with 9.6 μg/mL and 9.0 μg/mL, respectively. Furthermore, Bu extract exhibited fascinatingly higher and a wider range of cytotoxic activities against all lung, ovary, and cervical cancer cells. Therefore, all extracts from Seabuckthorn are a possible candidate for cancer medicinal development.
Table 3.
Anticancer activity of different extracts of Seabuckthorn leaf.
| IC50a on cancer cell growth |
||||
|---|---|---|---|---|
| NCL | HeLa | Caski | SKOV | |
| Methanol | 9.6 ± 1.8 | 121.5 ± 23.8 | 67.7 ± 4.0 | 218.7 ± 23.9 |
| Ethyl acetate | 60.2 ± 5.6 | 9.5 ± 0.78 | 63.3 ± 0.5 | 9.4 ± 1.9 |
| Butanol | 8.2 ± 0.3 | 8.6 ± 0.78 | 18.2 ± 4.1 | 9.2 ± 1.3 |
| Water | 9.0 ± 1.4 | 128.4 ± 33.0 | 91.8 ± 3.7 | 2472.9 ± 185.8 |
| Berberine | 57.02 ± 0.49 | 9.1 ± 0.8 | 44.3 ± 5.1 | 52.1 ± 4.7 |
Concentration required to reduce cancer cell growth by 50% (μg/mL).
Figure 5.
Effect of Seabuckthorn leaf extracts on the NCL-H1299 lung cancer cell line. NCL-H1299 cells seeded onto 96 well plates and maintained at 37 °C, 5% of CO2 and humidified incubator. After 24 h incubation, media was removed and washed with PBS solution. Then MeOH, EA, Bu and DW extracts or Berberine concentrations such as 1, 10 and 100 μg/mL. After incubation 48 h, the morphology of cells was observed under microscope 20× magnification, and a photograph taken. (A) MeOH extract; (B) EA extract; (C) Bu extract; (D) DW extract; (E) Berberine; (F) control, non-treated NCL-H1299 cells.
3.5. Correlation between metabolites and biological activities
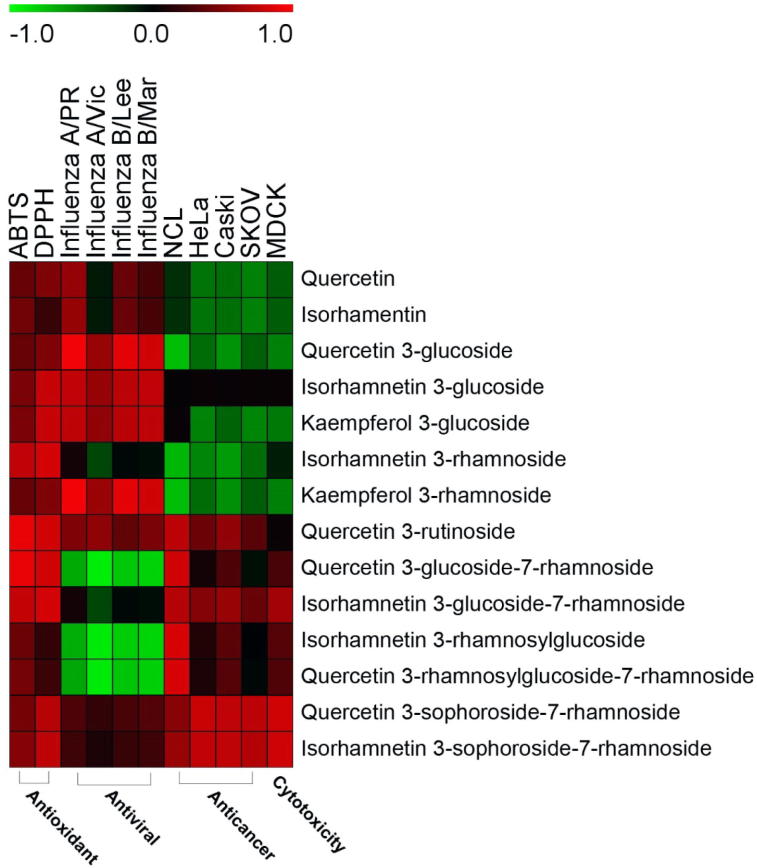
14 different flavonol glycosides were identified in Seabuckthorn extracts such as two aglycones, five mono-glycosides, four di-glycosides and three tri-glycosides (Table 1 and Fig. 3). Correlation study showed that the number of glycosides has a high impact on the biological activities such as antioxidant, antiviral and anticancer (Fig. 6). Previously, the antioxidant activity of quercetin glycosides was higher than quercetin without glycosides (Coqueiro et al., 2013). In our observation, aglycones and mono-glycosides have a better inhibition activity to influenza virus infections but not cytotoxic against cancer and normal cells as compared with the di- and tri-glycosides. Di-glycosides and tri-glycosides have the cytotoxic effect to the normal cell (MDCK) and extremely more toxic to the human cancer cells.
Figure 6.
Correlation heat map between flavonol glycosides and biological activities such as antioxidant, antiviral, anticancer and cytotoxicity.
4. Conclusion
Seabuckthorn has various and a rich content of flavonoid and polyphenol compounds. Presently, Seabuckthorn is used in drink and food as additional product in Mongolia, Korea, China and European countries. It is a rich content of potential metabolites as flavonol glycosides checked by LC–MS, results into strong antioxidant, antiviral and anticancer activities. EA and MeOH extracts showed stronger antiviral activities against influenza viruses compared with the well-known anti-influenza agent Oseltamivir. Also, Bu extract recorded high toxicity against human cancer cells. Depending on the correlation between metabolites and biological activities, aglycones and mono-glycosides are better targets to influenza viral drug development, di- and tri-glycosides are acceptable to inhibit the growth of cancer cells. The observation advises to further cancer drug developments. Using Seabuckthorn leaf extracts is helpful in preventing and healing the diseases such as both viral infections and cancer.
Acknowledgement
This paper work was supported by KU Research Professor Program of Konkuk University, Seoul, South Korea.
Footnotes
Peer review under responsibility of King Saud University.
References
- Beveridge T., Li T.S., Oomah B.D., Smith A. Seabuckthorn products: manufacture and composition. J. Agric. Food Chem. 1999;47:3480–3488. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- Black P.H., Garbutt L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002;52(1):1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Chauhan A.S., Negi P.S., Ramteke R.S. Antioxidant and antibacterial activities of aqueous extract of Sea buckthorn (Hippophae rhamnoides) seeds. Fitoterapia. 2007;78:590–592. doi: 10.1016/j.fitote.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Song J.H., Kwon D.H. Quercetin 3-rhamnoside exerts antiinfluenza a virus activity in mice. Phytother. Res. 2012;26:462–464. doi: 10.1002/ptr.3529. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Song J.H., Park K.S., Kwon D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Coqueiro A., Regasini L.O., Skrzek S.C., Queiroz M.M., Silva D.H., Silva Bolzani V. Free radical scavenging activity of Kielmeyera variabilis (Clusiaceae) Molecules. 2013;18(2):2376–2385. doi: 10.3390/molecules18022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuyckens F., Claeys M. Mass spectrometry in the structural analysisof flavonoids. J. Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- Cuyckens F., Claeys M. Determination of the glycosylation site in flavonoidmono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. J. Mass Spectrom. 2005;40:364–372. doi: 10.1002/jms.794. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C., Hrincius E.R., Korte V., Mazur I., Droebner K., Poetter A., Dreschers S., Schmolke M., Planz O., Ludwig S. A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res. 2007;76(1):38–47. doi: 10.1016/j.antiviral.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Esch T., Stefano G.B., Fricchione G.L., Benson H. Stress in cardiovascular diseases. Med. Sci. Monit. 2002;8(5):93–101. [PubMed] [Google Scholar]
- Ganju L., Padwad Y., Singh R., Karan R., Chanda S., Chopra M.K. Antiinflammatory activity of Seabuckthorn (Hippophae rhamnoides) leaves. Int. Immunopharmacol. 2005;5:1675–1684. doi: 10.1016/j.intimp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Geetha S., Jayamurthy P., Pal K., Pandey S., Sawhney R.C. Hepatoprotective activity of Sea buckthorn (Hippophae rhamnoides L.) against carbon tetrachloride induced hepatic damage in rats. J. Sci. Food Agric. 2008;88:1592–1597. [Google Scholar]
- Grey C., Widen C., Adlercreutz P., Rumpunen K., Duan R.D. Antiproliferative effects of sea buckthorn (Hippophae rhamnoides L.) extracts on human colon and liver cancer cell lines. Food Chem. 2010;120:1004–1010. [Google Scholar]
- Havsteen B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Mitani A., Katsuzaki H., Imai K., Yoshioka K., Komiya T. Isolation of five types of flavonol from seabuckthorn (Hippophae rhamnoides) and induction of apoptosis by some of the flavonols in human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005;15:805–809. [PubMed] [Google Scholar]
- Jain M., Ganju L., Katiyal A., Padwad Y., Mishra K.P., Chanda S. Effect of Hippophae rhamnoides leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomedicine. 2008;15(10):793–799. doi: 10.1016/j.phymed.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Kachlicki P., Einhorn J., Muth D., Kerhoas L., Stobiecki M. Evaluation of glycosylation and malonylation patternsin flavonoid glycosides during LC/MS/MS metaboliteprofiling. J. Mass Spectrom. 2008;43:572–586. doi: 10.1002/jms.1344. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kwon Y.S., Sa Y.J., Kim M.J. Isolation and identification of Sea Buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and a-glucosidase inhibitory effect. J. Agric. Food Chem. 2011;59:138–144. doi: 10.1021/jf103130a. [DOI] [PubMed] [Google Scholar]
- Kwon D.J., Bae Y.S., Ju S.M., Goh A.R., Choi S.Y., Park J. Casuarinin suppresses TNF-α-induced ICAM-1 expression via blockade of NF-κB activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2011;409:780–785. doi: 10.1016/j.bbrc.2011.05.088. [DOI] [PubMed] [Google Scholar]
- Larmo P., Alin J., Salminen E., Kallio H., Tahvonen R. Effects of sea buckthorn berries on infections and inflammation: a double-blind, randomized, placebo-controlled trial. Eur. J. Clin. Nutr. 2008;62:1123–1130. doi: 10.1038/sj.ejcn.1602831. [DOI] [PubMed] [Google Scholar]
- Lee V.H., Ng S.C., Leung T.W., Au G.K., Kwong D.L. Dosimetric predictors of radiation-induced acute nausea and vomiting in IMRT for nasopharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:176–182. doi: 10.1016/j.ijrobp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Maria John K.M., Enkhtaivan G., Kim J.J., Kim D.H. Metabolic variation and antioxidant potential of Malus prunifolia (wild apple) compared with high flavon-3-ol containing fruits (apple, grapes) and beverage (black tea) Food Chem. 2014;163:46–50. doi: 10.1016/j.foodchem.2014.04.074. [DOI] [PubMed] [Google Scholar]
- Morimoto H., Yonehara S., Bonavida B. Overcoming tumor necrosis factor and drug resistance of human tumor cell lines by combination treatment with anti-Fas antibody and drugs or toxins. Cancer Res. 1993;53(11):2591–2596. [PubMed] [Google Scholar]
- Narayanan S., Ruma D., Gitika B., Sharma S.K., Pauline T., Sai Ram M. Antioxidant activities of seabuckthorn (Hippophae rhamnoides) during hypoxia induced oxidative stress in glial cells. Mol. Cell. Biochem. 2005;278:9–14. doi: 10.1007/s11010-005-7636-2. [DOI] [PubMed] [Google Scholar]
- Padwad Y., Ganju L., Jain M., Chanda S., Karan D., Kumar R. Effect of leaf extract of Sea buckthorn on lipopolysac-charide induced inflammatory response in murine macrophages. Int. Immunopharmacol. 2006;6:46–52. doi: 10.1016/j.intimp.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Patil D., Roy S., Dahake R., Rajopadhye S., Kothari S., Deshmukh R., Chowdhary A. Evaluation of Jatropha curcas Linn. leaf extracts for its cytotoxicity and potential to inhibit hemagglutinin protein of influenza virus. Indian J. Virol. 2013;24(2):220–226. doi: 10.1007/s13337-013-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop R.M., Socaciu C., Pintea A., Buzoianu A.D., Sanders M.G., Gruppen H. UHPLC/PDA–ESI/MS analysis of the main berry and leaf flavonol glycosides from different carpathian Hippophaë rhamnoides L. varieties. Phytochemical Analysis. 2013;24:484–492. doi: 10.1002/pca.2460. [DOI] [PubMed] [Google Scholar]
- Potter S.M., Baum J.A., Teng H., Stillman R.J., Shay N.F., Erdman J.J. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998;68:1375–1379. doi: 10.1093/ajcn/68.6.1375S. [DOI] [PubMed] [Google Scholar]
- Rousi A. The genus Hippophae L. A taxonomic study. Ann. Bot. Fennici. 1971;8:177–227. [Google Scholar]
- Rösch D., Krumbein A., Mügge C., Kroh L.W. Structural investigations of flavonol glycosides from SeaBuckthorn (Hippophae2 rhamnoides) pomace by NMR spectroscopy and HPLC–ESI-MSn. Jo. Agric. Food Chem. 2004;52:4039–4046. doi: 10.1021/jf0306791. [DOI] [PubMed] [Google Scholar]
- Song J.M., Lee K.H., Seong B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Suryakumar G., Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.) J. Ethnopharmacol. 2011;138(2):268–278. doi: 10.1016/j.jep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Stephan P., Michael S., Roland S., James B.H. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus(H5N1, H7N7) and swine-origin H1N1 (S-OIV) Virol. J. 2009;6:197. doi: 10.1186/1743-422X-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin E., Luke K., Jones J., Tumpey T., Konan K., Schultz-Cherry S. Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J. Virol. 2005;79(14):8802–8811. doi: 10.1128/JVI.79.14.8802-8811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay N.K., Kumar M.S.Y., Gupta A. Antioxidant, cytoprotective and anti-bacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2010;48:3443–3448. doi: 10.1016/j.fct.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radical Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wild C.P., Stewart B. World Health Organization; 2014. World Cancer Report. Chap. 1.1. [Google Scholar]
- Xu M.Y., Sun X.X., Tong W.X. Medical research and development of seabuckthorn (J) Hippophae. 1994;7:32–40. [Google Scholar]
- Yang B., Kalimo K.O., Tahvonen R.L., Matilla L.M., Katajisto J.K., Kallio H.P. Effect of dietary supplementation with sea buckthorn (Hippophae¨ rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids of patients with atopic dermatitis. J. Nutr. Biochem. 2000;11(6):338–340. doi: 10.1016/s0955-2863(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Zhong F. Effects of the total flavonoids of Hippophae rhamnoides on nonspecific immunity in animals. Shaanxi Med. J. 1989;18:9–10. [Google Scholar]