Abstract
This study was carried out to determine the median lethal concentrations (LC50) of Zinc nanoparticles (ZnNPs) on Oreochromis niloticus and Tilapia zillii. The biochemical and molecular potential effects of ZnNPs (500 and 2000 μg L−1) on the antioxidant system in the brain tissue of O. niloticus and T. zillii were investigated. Four hundred fish were used for acute and sub-acute studies. ZnNP LC50 concentrations were investigated in O. niloticus and T. zillii. The effect of 500 and 2000 μg L−1 ZnNPs on brain antioxidants of O. niloticus and T. zillii was investigated. The result indicated that 69 h LC50 was 5.5 ± 0.6 and 5.6 ± 0.4 for O. nilotica and T. zillii, respectively. Fish exposed to 500 μg L−1 ZnNPs showed a significant increase in reduced glutathione (GSH), total glutathione (tGSH) levels, superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) activity and gene expression. On the contrary, malondialdehyde (MDA) levels significantly decreased. Meanwhile, fish exposed to 2000 μg L−1 ZnNPs showed a significant decrease of GSH, tGSH levels, SOD, CAT, GR, GPx and GST activity and gene expression. On the contrary, MDA levels significantly increased. It was concluded that, the 96 h LC50 of ZnNPs was 5.5 ± 0.6 and 5.6 ± 0.4 for O. nilotica and T. zillii, respectively. ZnNPs in exposure concentrations of 2000 μg/L induced a deleterious effect on the brain antioxidant system of O. nilotica and T. zillii. In contrast, ZnNPs in exposure concentrations of 500 μg L−1 produced an inductive effect on the brain antioxidant system of O. nilotica and T. zillii.
Keywords: ZnNPs, LC50, Antioxidants, O. nilotica, T. zillii
1. Introduction
The development of nanotechnology produces many nanoparticles (NPs) that are important in medicine, agriculture and industry (Grażyna et al., 2014). Nowadays, nanoparticles of metals are widely used in many sectors such as medicine, agriculture and industry; however, few studies were done on their environmental impact and fate (Paresh et al., 2009). Metal NPs may leak into natural bodies of water in their life cycles (production, storage, transportation, consumption, disposal, or reproduction). There is lack ofinformation regarding the magnitude of NPs released into the aquatic system and their impact on living organisms. Therefore, there is an urgent need for information on the ecological risks of metal NPs (Alkaladi et al., 2015). Recently, researchers are interested to investigate the toxicity of NPs. Some studies have proved the toxicity of NPs, such as metal oxides to bacteria, human cells, rodents and aquatics (Lin and Xing, 2007). NP toxicity mechanisms are complicated (Warheit et al., 2006). It may stimulate the reactive oxidative species (ROS) generation through disruption of intracellular metabolism (Long et al., 2006) or damage the antioxidant defense system (Brown et al., 2004), resulting in protein, lipids, DNA and carbohydrate damage (Kelly et al., 1998). Brain is the most liable organ in the body for the adverse effects of oxidative damage because it contains a high level of unsaturated fatty acids, which are easily peroxidizable, its disproportional large consumption of oxygen per unit weight and its not particularly generous antioxidant defense (Nikolaos et al., 2006, Afifi et al., 2010). Transcript level alterations are the earliest sensitive bio-indicators for biological responses to stress. Thus, genes with expression levels, that are altered in response to environmental stresses can be used for diagnosis and quantify of the effects of these stresses on the organisms (Dondero et al., 2006). The ecotoxicological data on ZnNPs are just emerging and scanty. Our previous work proved the toxic effects of Zinc oxide nanoparticles (ZnONPs) on the liver and gills of freshwater fish Oreochromis niloticus at low exposure level for LC5096h was 3.1 ± 0.4 mg L−1. Also, we indicated that the toxicity of ZnONPs occurred through the induction of lipid peroxide (LPO) synthesis (Alkaladi et al., 2014, Alkaladi et al., 2015). The toxicity of ZONPs in juvenile carp was documented and manifested by the inhibition of superoxide dismutase (SOD), catalase (CAT), and GPx activity and reduced GSH content as well as increase in the level of LPO (Linhua and Lei, 2012). Oberdo¨rster (2004) reported that, uncoated fullerenes produced an oxidative stress and lipid peroxidation in fish brain tissue, this proved the bad impact of NPs on aquatic health.
No studies have investigated the toxic effects of ZnNPs on expressions of oxidative stress-related genes in the brain of O. niloticus and Tilapia zillii. In the current study, we aimed to assess the changes of gene expression and activity of antioxidant enzymes in the brain tissue of both O. niloticus and T. zillii exposed to different levels of ZnNPs.
2. Materials and methods
2.1. Nanoparticles
Zn nanoparticle was purchased from Sigma–Aldrich Co. LLC. GmbH, Steinheim, Germany. Zn nanoparticle was in the form of nanopowder, 35 nm avg. part. Size, ⩾99% trace metals basis. Zn nanoparticle surface area was determined using the multi-point Brunauere Emmette Teller (BET) method. The measured surface areas were 40 m2/g that did not differ from the manufacturer’s values.
2.2. Preparation of Zn nanoparticle suspension
The Zn nanopowder was suspended directly in deionized water at concentrations of 500 and 2000 μg L−1. ZnNPs were dispersed using ultrasonic vibration (40 kHz) for 30 min to prevent NP aggregation. ZnNP suspension was daily prepared. Inductively coupled plasma mass spectrometry (ICP-MS) was used to determine Zn concentrations in the exposed water at zero, 12 and 24 h of exposure to verify that the exposure concentrations are the same as the prepared concentrations as indicated in Table 1. Zn shape and size were determined using the transmission electron microscope (TEM) (JEM-1011, JEOL, Japan). ZnNP nanoparticle was nearly spherical and very fit with the nano-scale, and the measured particle size was close to the manufacturer information as indicated in Fig. 1.
Table 1.
The actual ZnNP concentrations (mg L−1) in the exposed water.
| Concentrations | Time (h) |
||||
|---|---|---|---|---|---|
| Zero | 12 |
24 |
|||
| M ± SD | M ± SD | % of change | M ± SD | % of change | |
| Control | nd | nd | nd | nd | nd |
| 500 μg L−1 | 500 ± 30 | 470 ± 23 | −6 | 450 ± 33 | −10 |
| 2000 μg L−1 | 2000 ± 125 | 1920 ± 104 | −4 | 1890 ± 106 | −5.5 |
nd = not detected.
Figure 1.
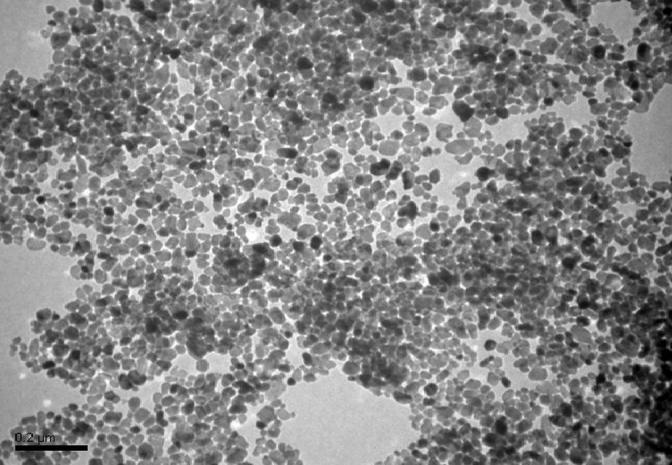
TEM photomicrograph of ZnNPs.
2.3. Fish preparation
The Ethics Committee of King Abdualaziz University approved the procedures of the current experiment. Four hundred males of both O. niloticus and T. zillii, weighting 90 ± 5 g and 15 ± 3 cm in length were used in this study. Fish were kept in 40 aquaria (n = 10 fish/aquarium). The aquarium water was changed daily. An aeration system (Eheim Liberty 150 Bio-Espumador cartridges) was used for water aeration. The temperature was maintained at 28 ± 2 °C and dissolved oxygen, at 7.0 ± 0.5 mg L−1. Fish were fed with a commercial fish diet. The daily feed amount was 10% of body weight and the fish were fed 3 times daily. Fish were acclimatized for 15 days before the beginning of the experiments.
2.4. Acute toxicity
A graded series of ZnNP suspension of 0, 1, 3, 5, 7 and 14 mg L−1 was used in triplicate for the lethal toxicity study. Ten fish each of O. niloticus and T. zillii were exposed to each concentration for 24, 48 and 96 h in a 30 L aquarium with 20 L of the NP treated water. The NP treated water was changed every 24 h to ensure a constant concentration of nanoparticles. Control fish was reared in distilled water free from NPs. During the experimental period, no food was supplied to fish for minimizing the absorption of NPs in food and the production of feces. The 24, 48 and 96 h sublethal concentrations were determined using the Environmental Protection Agency (EPA) probit analysis.
2.5. Experimental design
Fish were randomly divided into six groups, 30 fish in each group (3 replicates). The first and the fourth groups were left as a control for O. niloticus and T. zillii, respectively; the second and third groups were O. niloticus and were exposed to 500 and 2000 μg L−1 ZnNPs, respectively. The fifth and sixth groups were T. zillii and were exposed to 500 and 2000 μg L−1 ZnNPs, respectively. No food was supplied to fish during the experimental period for decreasing the absorption of ZnNPs by food and to maintain the water quality. After 15 days of the exposure, fish of each group were anesthetized on ice. The spinal cord transaction was applied to fish killing. The brain was quickly dissected, rinsed with saline, immersed in liquid nitrogen, and kept at −80 °C to be used. The brain tissue homogenates were prepared following the method described by Puerto et al. (2009), and the supernatant was used for biochemical analysis.
2.6. Brain Zn, MDA, GSH levels and antioxidant enzyme activity assays
Brain Zn concentrations were analyzed by using an inductively coupled plasma-atomic emission spectroscopy with an ULTIMA 2 apparatus (Horiba Jobin Yvon, France).
Brain tissue MDA was analyzed by measuring the production of thiobarbituric acid reactive substances (TBARS) using a TBARS assay kit (Cat. No. 10009055, Cayman, USA). NPSH, GST, GR, GPx, CAT and SOD activity were determined using the kits (Cat. No. NWK GSH01, NWK-GST01, NWK-GR01, NWK-GPx01, NWK-CAT01 and NWK-SOD01) purchased from Northwest Life Science Specialties (NWLSSTM), Vancouver, Canada.
2.7. Molecular assays and gene expressions
The expression of brain GST, GR, GPx, CAT and SOD genes was quantified using real time PCR. Total RNA was isolated from tissue samples using the RNeasy Mini Kit Qiagen (Cat. No. 74104). 0.5 μg of total RNA was used for the production of cDNA by Qiagen RT-PCR Kit, (Cat. No. 205920). Five μL of cDNA, 12.5 μL of 2× SYBR® Green PCR mix with ROX from BioRad were mixed with 10 pmol/μL of each primer for the quantified genes. The house keeping gene β-actin was used as a constitutive control for normalization. Primers were designed by using Primer3 software (http://bioinfo.ut.ee/primer3/) as per the published O. niloticus gene sequence in the NCBI database. GST, Forward 5′ TAATGGGAGAGGGAAGATGG3′, Reverse 5′ CTCTGCGATGTAATTCAGGA3′; GR, Forward 5′ CATTACCGAGACGCGGAGTT3′, Reverse 5′ CAGTTGGCTCAGGATCATTTGT3′; GPx, Forward 5′ CCAAGAGAACTGCAAGAACGA3′, Reverse 5′ CAGGACACGTCATTCCTACAC3′; CAT, Forward 5′ TCCTGAATGAGGAGGAGCGA3′, Reverse 5′ ATCTTAGATGAGGCGGTGATG3′; SOD, Forward 5′ GGTGCCCTGGAGCCCTA3′, Reverse 5′ ATGCGAAGTCTTCCACTGTC3′ and β-actin gene Forward 5′ CAATGAGAGGTTCCGTTGC3′, Reverse 5′ AGGATTCCATACCAAGGAAGG3′ (XM_003445184, EU234530, EF206801, JF801726.1, JF801727.1 and EU887951). Primers were synthesized and provided by Sigma Aldrich (Sigma–Aldrich Chemie GmbH, Steinheim, Germany). The conditions of PCR were initial denaturation at 95 °C, 2 min, then 28 cycles of 95 °C, 1 min; 60 °C, 1 min; 72 °C, 1 min. PCR reactions were carried out in an AbiPrism 7300 (Applied Biosystems, USA). Levels of RNA were quantified by the values of the threshold cycle (Ct). mRNA fold changes (mRNA relative expression) were expressed relative to the corresponding mRNA mean value found in the control group and was calculated using the 2DDCt method (Livak and Smittgen, 2001).
2.8. Statistical analysis
The statistical package for social science (SPSS Inc., Chicago, IL, version 20, USA) was used for processing of our data. The result was shown as mean ± SD. Student’s t-test was used to calculate the differences between groups.
3. Results
3.1. Lethal concentrations (LC) of ZnNPs on fish
There was no mortality observed in the control fish. ZnNP exposure produced some mortality that increased with the ZnNP concentration (Table 2). 69 h LC50 was 5.5 ± 0.6 and 5.6 ± 0.4 mg L−1 for O. niloticus and T. zillii, respectively. As in the control group, no mortality was observed in fish exposed to ZnNPs at a concentration of 1 mg/L. 14 mg L−1 ZnNP suspension caused 100% mortality with a calculated 96 h LC90 of 7.7 ± 1.2 and 7.94 ± 1.1 mg L−1 for O. niloticus and T. zillii, respectively.
Table 2.
Lethal concentrations (mg/L) of ZnNPs on O. niloticus and T. zillii after 24, 48 and 96 h of exposure.
| Time (h) | Fish | Toxicity (mg L−1) |
||
|---|---|---|---|---|
| LC10 | LC50 | LC90 | ||
| 24 | O. niloticus | 5.62 ± 0.9 | 7.48 ± 1 | 10.7 ± 1.3 |
| T. zillii | 5.81 ± 1 | 7.6 ± 0.8 | 10.99 ± 1.2 | |
| 48 | O. niloticus | 4.89 ± 0.6 | 6.94 ± 0.9 | 8.98 ± 1.4 |
| T. zillii | 5 ± 0.4 | 7 ± 1.1 | 9.2 ± 1.3 | |
| 96 | O. niloticus | 4 ± 0.7 | 5.5 ± 0.6 | 7.7 ± 1.2 |
| T. zillii | 4.14 ± 0.5 | 5.6 ± 0.4 | 7.94 ± 1.1 | |
3.2. The actual aqueous exposure to Zn nanoparticles
The actual ZnNP concentrations in the experimental water column were determined at zero, 12 and 24 h of exposure. As indicated in Table 1 a non significant time dependent loss of ZnNPs was seen. The losses may be due to nanoparticle aggregates despite continual aeration. The experimental water was changed every 24 h to keep the nanoparticle concentration and decrease aggregation and sedimentation of the particles to a certain extent.
3.3. Effect of ZnNPs on Zn levels in brain tissue
Our result reported that, the level of Zn in the brain tissue of O. niloticus and T. zillii was increased. The increase was significant in fish exposed to 500 μg L−1 (P < 0.05) and highly significant in fish exposed to 2000 μg L−1 (P < 0.001) as compared to the control. Moreover, Zn level was highly significantly increased (P < 0.001) in fish exposed to 2000 μg L−1 as compared to 500 μg L−1 (P < 0.05) as indicated in Table 3, Table 4.
Table 3.
Effect of ZnNPs on Zn, MDA, GSH and tGSH levels in the brain of O. niloticus.
| Group | Zn (μg g−1) | MDA (nmol g−1 wt.w) | GSH (μmol g−1 wt.w) | tGSH (μmol g−1 wt.w) |
|---|---|---|---|---|
| Control | 59.4 ± 5 | 1.8 ± 0.3 | 42.2 ± 3 | 67 ± 4 |
| 500 μg L−1 | 70.6 ± 6∗ | 1.06 ± 0.2∗∗ | 59 ± 3.4∗∗∗ | 78 ± 6∗ |
| 2000 μg L−1 | 99 ± 14∗∗∗,### | 6.1 ± 0.8∗∗∗,### | 22.4 ± 3.8∗∗∗,### | 45.8 ± 3.8∗∗∗,### |
wt.w: wet weight tissue. ∗: P < 0.05, ∗∗: P < 0.01, ∗∗∗: P < 0.001, compared to the control group; #: P < 0.05, ##: P < 0.01, ###: P < 0.001, comparing to 500 μg L−1 of the ZnNP exposed group.
Table 4.
Effect of ZnNPs on Zn, MDA, GSH and tGSH levels in the brain of T. Zillii.
| Group | Zn (μg g−1) | MDA (nmol g−1 wt.w) | GSH (μmol g−1 wt.w) | tGSH (μmol g−1 wt.w) |
|---|---|---|---|---|
| Control | 62.4 ± 5.3 | 1.6 ± 0.3 | 44.6 ± 4.2 | 69.2 ± 2.8 |
| 500 μg L−1 | 72.8 ± 8.2∗ | 0.8 ± 0.13∗∗∗ | 64.8 ± 6.7∗∗∗ | 80.8 ± 2.3∗ |
| 2000 μg L−1 | 102 ± 15∗∗∗,### | 5.2 ± 0.9∗∗∗,### | 17.8 ± 3.8∗∗∗,### | 39 ± 2.6∗∗∗,### |
wt.w: wet weight tissue. ∗: P < 0.05, ∗∗∗: P < 0.001, compared to the control group; ##: P < 0.01, ###: P < 0.001, comparing to 500 μg L−1 of the ZnNP exposed group.
3.4. Effect of ZnNPs on MDA levels in brain tissue
Exposure of fish to ZnNPS 500 μg L−1 produced a highly significant decrease of MDA in brain tissue of O. niloticus and T. zillii as compared to the control. In contrast, exposure of fish to ZnNP concentration of 2000 μg L−1 resulted in a highly significant increase (P < 0.001) of MDA in brain tissue as compared to control and 500 μg L−1 exposed fish as shown in Table 3, Table 4.
3.5. Effect of ZnNPs on GSH and tGSH levels in brain tissue
Our result reported that, the level of GSH and tGSH in the brain tissue of O. niloticus and T. zillii was increased in fish exposed to 500 μg L−1 as compared to the control. The increase was highly significant (P < 0.001) for GSH and significant (P < 0.05) for tGSH. In contrast, GSH and tGSH levels were highly significantly decreased (P < 0.001) in fish exposed to 2000 μg L−1 as compared to the control and 500 μg L−1 exposed fish as indicated in Table 3, Table 4.
3.6. Effect of ZnNPs on antioxidant enzymes activity in brain tissue
Our result indicated that the exposure to ZnNPs caused a significant increase in the activity of CAT, SOD, GST, GR and GPx in the brain tissues of fish exposed to 500 μg L−1 as compared to the control. In contrast, CAT, SOD, GST, GR and GPx activity were highly significant decreased in fish exposed to 2000 μg L−1 as compared to the control and 500 μg L−1 exposed fish as indicated in Table 5, Table 6.
Table 5.
Effect of ZnNPs on antioxidant enzymes activity in the brain of O. niloticus.
| Group | CAT μMH2O2 decomposed/g tissue | SOD ug/mgP | GST nmol/mg P/min | GR U/mgP | GPx (μU min−1 mg−1 P) |
|---|---|---|---|---|---|
| Control | 38.6 ± 3 | 20 ± 3 | 250 ± 19 | 18 ± 3 | 127 ± 15 |
| 500 μg L−1 | 40 ± 6.2 | 30 ± 2.1∗∗ | 308 ± 24∗∗ | 28.4 ± 3∗∗ | 182 ± 12∗∗∗ |
| 2000 μg L−1 | 26 ± 4∗∗,## | 12.2 ± 2∗,### | 96 ± 8∗∗∗,### | 9.8 ± 2∗,### | 71 ± 6∗∗∗,### |
∗: P < 0.05, ∗∗: P < 0.01, ∗∗∗: P < 0.001, compared to the control group; #: P < 0.05, ##: P < 0.01, ###: P < 0.001, comparing to 500 μg L−1 of the ZnNP exposed group.
Table 6.
Effect of ZnNPs on antioxidant enzyme activity in the brain of T. Zillii.
| Group | CAT μMH2O2 decomposed/g tissue | SOD ug/mgP | GST nmol/mg P/min | GR U/mgP | GPx (μU min−1 mg−1 P) |
|---|---|---|---|---|---|
| Control | 36.6 ± 3 | 18.4 ± 2.7 | 233 ± 16 | 17.4 ± 2.7 | 127.4 ± 28 |
| 500 μg L−1 | 43.6 ± 2.6∗∗ | 27.8 ± 2∗∗∗ | 304 ± 10∗∗∗ | 23 ± 2.4∗ | 180 ± 10∗∗ |
| 2000 μg L−1 | 13 ± 3∗∗∗,### | 9.4 ± 1∗∗∗,### | 102 ± 12∗∗∗,### | 6 ± 1∗∗∗,### | 81 ± 5∗∗∗,### |
wt.w: wet weight tissue. ∗: P < 0.05, ∗∗: P < 0.01, ∗∗∗: P < 0.001, compared to the control group; #: P < 0.05, ##: P < 0.01, ###: P < 0.001, comparing to 500 μg L−1 of the ZnNP exposed group.
3.7. Effects of ZONPs on the relative gene expression of antioxidant enzymes in fish tissues
The relative gene expression of antioxidant enzymes in the brain tissues of O. niloticus and T. zillii are shown in Table 7, Table 8. The exposure to ZnNPs caused a significant induction of CAT, SOD, GST, GR and GPx gene expression of fish exposed to 500 μg L−1 as compared to the control. In contrast, CAT, SOD, GST, GR and GPx gene expression were highly significantly decreased in fish exposed to 2000 μg L−1 as compared to the control and 500 μg L−1 exposed fish.
Table 7.
Effect of ZnNPs on mRNA expression (relative expression to β-actin) of antioxidant genes in the brain of O. niloticus.
| Group | CAT | SOD | GST | GR | GPx |
|---|---|---|---|---|---|
| Control | 23.4 ± 2.9 | 7.4 ± 0.6 | 172 ± 11 | 1.4 ± 0.19 | 42 ± 8 |
| 500 μg L−1 | 34.2 ± 3.1∗∗ | 12.5 ± 1.8∗∗∗ | 221 ± 9∗∗∗ | 2.7 ± 0.2∗∗∗ | 52 ± 12∗∗ |
| 2000 μg L−1 | 12.6 ± 2∗∗∗,### | 4.8 ± 0.6∗∗∗,### | 115 ± 15∗∗∗,### | 0.76 ± 0.18∗∗,### | 29 ± 6∗∗,### |
∗: P < 0.05, ∗∗: P < 0.01, ∗∗∗: P < 0.001, compared to the control group; #: P < 0.05, ##: P < 0.01, ###: P < 0.001, comparing to 500 μg L−1 of the ZnNP exposed group.
Table 8.
Effect of ZnNPs on mRNA expression (relative expression to β-actin) of antioxidant genes in the brain of T. Zillii.
| Group | CAT | SOD | GST | GR | GPx |
|---|---|---|---|---|---|
| Control | 30 ± 2.5 | 7.8 ± 1 | 166 ± 8 | 1.66 ± 0.17 | 37.8 ± 10 |
| 500 μg L−1 | 34.8 ± 2.9∗ | 14.1 ± 3∗∗∗ | 208 ± 20∗∗∗ | 2.8 ± 0.16∗∗∗ | 47.4 ± 8∗ |
| 2000 μg L−1 | 13.2 ± 3∗∗∗,### | 4 ± 1∗∗∗,### | 97.4 ± 6∗∗∗,### | 0.58 ± 0.03∗∗∗,### | 26 ± 4∗∗,### |
∗: P < 0.05, ∗∗: P < 0.01, ∗∗∗: P < 0.001, compared to the control group; #: P < 0.05, ##: P < 0.01, ###: P < 0.001, comparing to 500 μg L−1 of the ZnNP exposed group.
4. Discussion
This study is the first trial that tends to estimate, the median lethal concentration (LC50) of ZnNPs on O. niloticus and T. zillii. Aqueous exposure to 1 and 3 mg L−1 of ZnNPs suspension did not cause any fish mortality, while, 14 mg L−1 resulted in 100% fish mortality. The 69 h LC50 was 5.5 ± 0.6 and 5.6 ± 0.4 mg/L for O. nilotica and T. zillii, respectively this indicating the toxic potential of ZnNPs on fish. O. nilotica and T. zillii nearly have the same sensitivity to ZnNPs. The LC50 of ZnONPs on O. niloticus was previously determined by our study (Alkaladi et al., 2015) it was 3.1 ± 0.4 mg L−1 this indicating that ZnNPs were less toxic on O. niloticus than ZnONPs. Although there were no data on LC50 of ZnNPs on fish, there were some reports that were determined at 69 h LC50 of ZnONPs in the common carp (Cyprinus carpio) 4.897 mg L−1 and in zebra fish 4.92 mg/L (Asghar et al., 2015).
In the present study, Zn particles were increased significantly in the brain tissues of ZnNP exposed fish. The increase was significant in fish exposed to 500 μg L−1 and highly significant in fish exposed to 2000 μg L−1. NPs were taken up by the gills and digestive tract and absorbed into the circulation. Long circulation of nanoparticles leads to an increase in the chance of their passage into tissues, and hence higher cellular uptake (Li and Huang, 2008). Once taken up in cells, particles encounter an increasingly acidic environment as they move from early to late endosomes and finally to lysosomes, resulting in their dissolution (Nel et al., 2009). Such phenomena may have contributed to the observed increases of Zn levels in the brain tissue in our study. This result goes on the same lines as we found previously in rat (Afifi and Abdelazim, 2015). Zn possesses antioxidant ability at normal levels, but becomes toxic at high and moderate concentrations (Trevisan et al., 2014). The antioxidant defense systems include a series of antioxidative enzymes and low-molecular non-enzymatic antioxidants. They protect the biological systems from environmental stress at molecular level (Van der Oost et al., 2003, Pandey et al., 2003).The present study, therefore, assessed the activity and gene expression of main enzymatic antioxidants such as CAT, GST, GR, SOD and GPx and content of antioxidant substances as GSH in response to a 15-day exposure to ZnNPs at 500 and 2000 μg L−1. Our results indicated that the antioxidative enzyme activity, gene expression and non-enzymatic antioxidant levels in the brain tissue increased at lower ZnNP (500 μg L−1) exposure concentrations and were inhibited at higher (2000 μg L−1) concentrations. The induction of the antioxidant defense systems of fish exposed to lower concentrations of ZnNPs indicate an obvious adaptive threshold. This is in contrast to the inhibition of all measured enzymes activity, gene expression and nonenzymatic antioxidant (GSH and tGSH) produced in fish exposed to higher ZnNP concentrations. That indicated the over accumulation of free radicals which, exceeded the scavenging ability of antioxidant defense systems and the impact of ZnNPs on the balance of antioxidant defense system and oxidative stress in vivo under this exposure concentration. Our results enforced by the results of Hao and Chen (2012), who reported that ZnONPs possessed a dual effect on the enzymatic and non-enzymatic antioxidant defense system of juvenile carp, it activated the antioxidant defense system at lower concentrations of 0.5 and 5.0 mg L−1 and inhibited the antioxidant defense system at a higher concentration of 50.0 mg/L. In the present study, SOD and CAT activity and gene expression fluctuated with the concentrations of ZnNPs. SOD activity and gene expression were increased at lower ZnNPs concentrations and reduced at the highest concentrations. SOD activity was inhibited in the liver, gills and brain of carp exposed to 100 and 200 mg L−1 nano-TiO2 (Hao et al., 2009). The same result was reported by Xiong et al. (2011) who proved the inhibitory effect of ZnONPs on liver SOD in fish exposed to 5 mg/L ZnONPs. Similarly, our previous study (Alkaladi et al., 2014) reported that ZnONPs inhibited the activity and reduced the gene expression of SOD and CAT in gills and liver of O. nilotica exposed to 1 and 2 mg L−1 ZnONPs. Also, the activity of three important antioxidant enzymes GR, GPx and GST was determined. As SOD and CAT the activity and gene expression of GST, GR and GPx were induced at lower ZnNP concentrations and induced at higher concentrations. Decreased GR activity and gene expression were a major effect of ZnNPs. The inhibitory effect of soluble Zn on GR enzyme activity was previously proved in many organisms, including fish, mussels and rats, suggesting an important role for GR in Zn toxicity (Trevisan et al., 2014). Moreover, GPx and GST activity were inhibited, the inhibition was dose-dependent. Ionic Zn that was produced from ZnNP dissolution may possess a direct inhibitory effect on these two enzymes. This explanation was enforced by that of Shi et al. (2012) who reported that the over expression of GST failed to protect the MCF-7 cell line from the toxic effect of ZnONPs, this may be explained by the direct inhibition of GST by Zn2+. Ali and Ali (2015) documented that copper oxide nanoparticles (CuONP) induced the oxidative stress in the digestive gland of freshwater snail Lymnea luteola L. through inhibition of GPx and GST activity and decreasing the levels of GSH. The expression of GR, GPx and GST gene was repressed in fish exposed to the highest ZnNP concentrations. The repression effect of ZnNPs on the antioxidant genes may be explained by the deleterious effect of metal on DNA. The expression of antioxidant genes and the activity of enzymatic antioxidant were modulated in fish, bivalves and protozoa that were exposed to the metal (Trevisan et al., 2014).
In this study, GSH levels varied with ZnNP concentrations. Exposure to 500 μg L−1 ZnNP produced a significant increase in GSH contents in the brain tissue, while exposure to 2000 μg L−1 produced a significant decrease in GSH contents. It was consistent with the generation of excessive ROS that reacted with and neutralized GSH. Similarly, Hao and Chen (2012) found that GSH increased in the liver, gills and brain of carp exposed to 0.5 and 5 mg L−1 of ZnONPs and decreased in all tissues of fish exposed to 50 mg/L ZnONPs. Also Xiong et al. (2011) reported GSH inhibition exposed to 50.0 mg L−1 nano-iron for 14 days in adult Medaka and 5.0 mg/L nano-ZnO for 96 h in adult zerafish, respectively.
MDA is an important biomarker for monitoring lipid peroxidation and the health condition of biological membranes that are rich in polyunsaturated fatty acids. In the present investigation, brain of the fish exposed to lower ZnNP concentrations exhibited a decreased MDA level, while that exposed to the highest concentration exhibited elevated MDA levels. Decreased levels of MDA at lower ZnNP concentration indicated the antioxidant effect of ZnNPs. At low levels ZnNPs induced the activity of enzymatic and non-enzymatic antioxidants, increasing the scavenging of free radicals and decreasing MDA production. The increase of MDA in fish exposed to the highest ZnNPs could be explained by the depletion of the antioxidant system that was proved in this study. Also, ionic Zn that was produced from ZnNPs in the brain tissue is incriminated in the distraction of the cell and mitochondrial membranes with over oxidation of the polyunsaturated fatty acids producing MDA. There are many mechanisms that may explain Zn toxicity, the important one is the induction of ROS synthesis. This mechanism was documented in many cells such as neutrophils, macrophages, epithelial cells, liver cells, algae, bacteria, fungi and clams (Trevisan et al., 2014). Our previous study (Alkaladi et al., 2014) showed that, ZnONPs accumulated in gills and liver of O. niloticus producing a disturbance in mitochondrial and cell membranes resulting in the over production of ROS, causing destruction of mitochondria followed by the death of the cell. ZnNP toxicity may be produced from the ionic dissociation (Zn2+) in water. Increased Zn2+ was incriminated in the activation of ROS production through interaction with membrane lipids damaging the cell membrane, DNA and proteins (Ma et al., 2013). In the same line, Ciacci et al. (2012) reported that ZnNP toxicity occurred in blue mussel hemocytes through the induction of ROS production. Also, many reports using in vitro systems indicated that the mechanism of ZnO toxicity involves the generation of ROS (Adamcakova-Dodd et al., 2014).
5. Conclusion
The 96 h LC50 of ZnNPs was 5.5 ± 0.6 and 5.6 ± 0.4 mg L−1 for O. nilotica and T. zillii, respectively. ZnNPs in an exposure concentration of 2000 μg L−1 induced a deleterious effect on the brain antioxidant system of O. nilotica and T. zillii. In contrast, ZnNPs in an exposure concentration of 500 μg L−1 produced an inductive effect on the brain antioxidant system of O. nilotica and T. zillii.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant no. (28-130-36-RG). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adamcakova-Dodd A., Stebounova L.V., Kim J.S., Vorrink S.U., Ault A.P., O’Shaughnessy P.T., Grassian V.H., Thorne P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part. Fibre Toxicol. 2014;11:15–22. doi: 10.1186/1743-8977-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi M., Alkaladi A., Mohamed M.A. Effect of n-acetyl-l-cysteine, vitamin c and vitamin e on heat stress induced disturbances in broiler chickens. EJBMB. 2010:45–62. (special issue) [Google Scholar]
- Afifi M., Abdelazim A.M. Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac. J. Trop. Biomed. 2015;5(10):832–834. [Google Scholar]
- Ali D., Ali H. Susceptibility of the freshwater pulmonate snail Lymnea luteola L. to copper oxide nanoparticle. Toxicol. Environ. Chem. 2015;97(5):1–26. [Google Scholar]
- Alkaladi A., Afifi M., Mosleh Y., AbuZinada O. Hestopathological effects of zinc oxide nanoparticles on the liver and gills of Oreochromis niloticus, protective effect of vitamins C and E. J. Pure Appl. Microbiol. 2014;8(6):4549–4558. [Google Scholar]
- Alkaladi A., El-Deen N., Afifi M., Abu Zinadah O. Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J. Biol. Sci. 2015;22:556–563. doi: 10.1016/j.sjbs.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M.S., Qureshi N.A., Jabeen F., Khan M.S., Shakeel M., Noureen A. Toxicity of zinc nanoparticles in fish: a critical review. J. Biol. Environ. Sci. 2015;7(1):431–439. [Google Scholar]
- Brown D., Donaldson K., Borm P., Schins R., Dehnhardt M., Gilmour P. Calcium and ROS-mediated activation of transcription factors and TNF-¦Á cytokine gene expression in macrophages exposed to ultrafine particles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:344–353. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- Ciacci C., Canonico B., Bilanicov D., Fabbri R., Cortese K., Gallo G., Marcomini A., Pojana G., Canesi L. Immunomodulation by different types of N-oxides in the hemocytes of the marine bivalve Mytilus galloprovincialis. PLoS One. 2012;7:e36937. doi: 10.1371/journal.pone.0036937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondero F., Dagnino A., Jonsson H., Capri F., Gastaldi L., Viarengo A. Assessing the occurrence of a stress syndrome in mussels (Mytilus edulis) using a combined biomarker/gene expression approach. Aquat. Toxicol. 2006;78:S13–S24. doi: 10.1016/j.aquatox.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Grażyna A.P., Joanna C., Ibrahim M.B. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int. J. Mol. Sci. 2014;15(8):13720–13737. doi: 10.3390/ijms150813720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Chen L. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012;80:103–110. doi: 10.1016/j.ecoenv.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Hao L.H., Wang Z.Y., Xing B.S. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio) J. Environ. Sci. 2009;21:1459–1466. doi: 10.1016/s1001-0742(08)62440-7. [DOI] [PubMed] [Google Scholar]
- Kelly K., Havrilla C., Brady T., Abramo K., Levin E. Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ. Health Perspect. 1998;106:375–384. doi: 10.1289/ehp.98106375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.D., Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharmacol. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- Lin D., Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Linhua H., Lei C. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012;80:103–110. doi: 10.1016/j.ecoenv.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Smittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long T.C., Saleh N., Tilton R.D., Lowry G.V., Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006;40:4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- Ma H., Williams P.L., Diamond S.A. Ecotoxicity of manufactured ZnO nanoparticles a review. Environ. Pollut. 2013;1987(172):76–85. doi: 10.1016/j.envpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M., Somasundaran P. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Nikolaos K., Nikolaos P., Elisabeth C., Dimitris K., Stelios F.A., Christos G. Effect of N-acetylcysteine, allopurinol and vitamin E on jaundice-induced brain oxidative stress in rats. Brain Res. 2006;1111(1):203–212. doi: 10.1016/j.brainres.2006.06.088. [DOI] [PubMed] [Google Scholar]
- Oberdo¨rster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile large mouth bass. Environ. Health Perspect. 2004;112:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Parvez S., Sayeedl I., Haque R., Bin-Hafeez B., Raisuddin S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.) Sci. Total Environ. 2003;309:105–115. doi: 10.1016/S0048-9697(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Paresh C.R., Hongtao Y., Peter P.F. Toxicity and environmental risks of nanomaterials: challenges and future needs. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009;27(1):1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerto M., Prieto A.I., Pichardo S., Moreno I., Jos A., Moyano R., Camean A.M. Effects of dietary N-acetylcysteine (NAC) on the oxidative stress induced in tilapia (Oreochromis niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Environ. Toxicol. Chem. 2009;28:1679–1686. doi: 10.1897/08-520.1. [DOI] [PubMed] [Google Scholar]
- Shi J., Karlsson H.L., Johansson K., Gogvadze V., Xiao L., Li J., Burks T., Garcia-Bennett A., Uheida A., Muhammed M., Mathur S., Morgenstern R., Kagan V., Fadeel B. Microsomal glutathione transferase 1 protects against toxicity induced by silica nanoparticles but not by zinc oxide nanoparticles. ACS Nano. 2012;6(3):1925–1938. doi: 10.1021/nn2021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan R., Flesch S., Mattos J., Milani M.R., Bainy A.C., Dafre A.L. Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp. Biochem. Physiol. C. 2014;159(2014):22–30. doi: 10.1016/j.cbpc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Van der Oost R., Beyer J., Vermeulen N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Warheit D., Webb T., Sayes C., Colvin V., Reed K. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol. Sci. 2006;91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- Xiong D.W., Fang T., Yu L.P., Sima X.F., Zhu W.T. Effects of nano-scale TiO2, ZnO and their bulk counter parts on zebra fish: acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011;409:1444–1452. doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]


