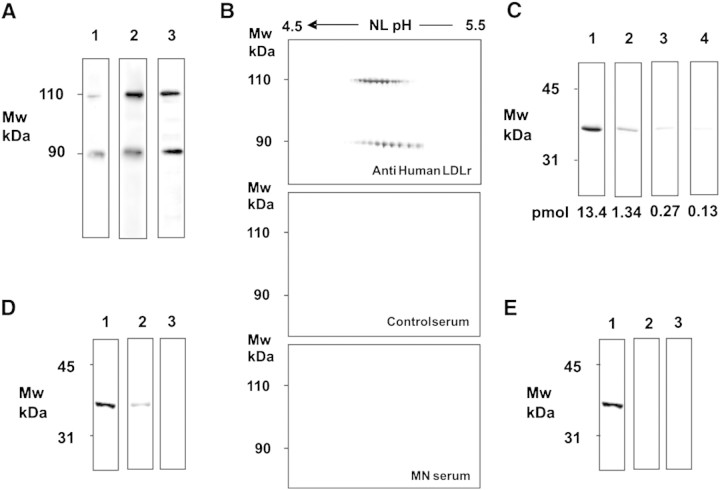
Primary membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults. We know very little about the mechanism for the sub-epithelial immune deposition typical of MN [1], with the notable exception of a rare condition recently described by Debiec et al. [2] in which antibodies against neutral peptidase (NEP) are raised for alloimmunization during a first pregnancy and transferred to a second foetus determining neonatal MN. Most of our knowledge on immune deposition is derived instead from the characterization of Heymann nephritis (HN), an experimental rat model described in the early fifties [3] that is mediated by the deposition of anti-megalin antibodies in rat glomeruli where they co-localize with C5–9 and clusterin, a natural ligand of megalin [4]. Since megalin is not present in human glomeruli, the findings obtained in rats cannot be completely exported to human MN and represent the missing point between HN and human MN. A recent work identified LDL receptor (LDL-r) as a megalin homologue in human glomeruli [5] raising the possibility that anti-LDL-r antibodies may be present in sera of patients with MN. The presence of LDL-r was then confirmed in primary podocytes, human/murine podocyte cell lines and murine liver with specific antibodies and by MALDI-TOF (Figure 1A). Based on this background, we have screened sera of 38 patients (15 collected at the time of diagnosis, before the start of any therapy) for circulating anti-LDL-r antibodies utilizing two-dimensional electrophoresis (2D-E) and western blot with liver extracts and with recombinant receptor-associated protein (RAP) domain as immobilized antigens. In particular, the reason to utilize the RAP domain is that it is made up by a short sequence of 71 amino acids lacking glycosylation sites and actually represents the accessible site for the binding of auto-antibodies. Both the intact protein in 2D-E and recombinant RAP (fusion complex with GST) in monodimensional-E were recognized by specific polyclonal antibodies at different dilutions, which rules out the possibility that chemical manipulation alters the binding site of the protein (Figure 1A–C). MN sera were utilized at different dilutions (from 1:10 to 1:100) to exclude problems linked to sensitivity of the assay. The sensitivity of the western blot technique to detect RAP was up to the pmole level (Figure 1C). Moreover, the assay was also tested by varying the dilution of anti-LDL-r antibodies (Figure 1D) in the presence of a constant amount of rRAP (13.4 pmol); the sensitivity of the assay was close to 1.2 mg/ml of the specific antibody under the condition used. In no case could circulating anti-LDL-r antibodies be detected in patients with MN (Figure 1B), and the same negative results were obtained utilizing recombinant RAP domain as immobilized antigen (Figure 1E). In this case, several combinations of antigen and serum quantities were utilized to avoid any interference of the antibody/antigen ratio on the sensitivity of the assay. It seems relevant to stress that patients were enrolled both before (n = 15) and after (n = 23) any therapy and therefore, even though this is a cross-sectional study, it gives insights unrelated to therapy.
Fig. 1.
(A) Primary human podocytes, murine podocytes and liver extracts express LDL-r. Extracts from cells and liver were run in a gradient electrophoresis gel and were then incubated with anti-human LDL-R antibodies. Proteins recognized by antibodies were recovered from the gel and analysed by MALDI-TOF, which confirmed in all cases the identity as LDL-r. The two bands at 110 and 90 kDa were recognized as the two subunits of LDL-R. Electrophoresis conditions were SDS-PAGE T%8–16 and Laemmli buffer with 5% v/v β mercaptoethanol. (B) MN patients lack circulating auto-antibodies against LDL-r. Murine liver extracts were incubated with anti-human LDL-R antibodies and with sera from all 38 patients with primary MN (15 patients were enrolled prior to any therapy) and from 10 healthy controls. The upper gel shows reactivity with LDL-r antibodies of the two micro-heterogeneous subunits of LDL-r (2 μg/ml); the middle and the lower panels show representative sera obtained from a normal control and a patient with MN, respectively (dilution 1:10 in both cases). No autoimmunity was detected in any tested sera. Detection of anti-HDL-r utilized western blot and two-dimensional electrophoresis in soft gels. After separation, protein extracts were trans-blotted to nitrocellulose membranes Protean BA (Schleicher & Schuell, Dassel Germany) with a Novablot semidry system utilizing a continuous buffer system with 2-amino 2-idroxymethyl 1.3-propanediol tris 38 mM, glycine 39 mM, sodium dodecyl sulphate (SDS) 0.035% and methanol 20%. Five hundred to 10 μl of serum (diluted in TBS 1.10 to 1:500) were incubated overnight at room temperature with trans-blotted membranes, rinsed with TBS-T 0.15% and incubated with HRP-conjugated anti-human IgG (Dako, Glostrup, Denmark—2 h, 1:5000) for immune detection. Chemioluminescence was used for immune detection. Images were digitalized by means of VersaDoc 4000 (Bio-Rad, Hercules—CA, USA) and analysed with QuantityOne software (Bio-Rad). Sera of healthy donors were used as control. (C) Sensitivity of western blot analysis with rRAP dilutions. Western blot of recombinant human RAP domain (AA 105-206) fused with GST (AA 234) (Abnova Corp., Taipej, Taiwan) and incubated with anti-LDL-r antibodies. The rRAP was utilized at several dilutions 13.4–0.13 pmol. Anti-LDL-r antibodies were utilized at a constant 2 μg/ml concentration. After gradient SDS-PAGE, the protein was transferred to nitrocellulose and then incubated with a constant amount of anti-LDL antibodies. Results indicated sensitivity of the assay up to pmoles of protein. (D) Sensitivity of western blot analysis with anti-LDL-r antibodies at different dilutions. For the assay, a constant amount of 13.4 pmol of rRAP was utilized. Lanes 1–3 show reactivity of anti-LDL-r antibodies at various dilutions from 2 μg/ml (lane 1) to 0.2 μg/ml (lane 2) and 0.02 μg/ml (lane 3). (E) MN patients lack circulating auto-antibodies against RAP domain. The same western blot analysis was repeated with sera from MN patients maintained by using a constant amount of 13.4 pmol of rRAP. Lane 1 shows reactivity anti-LDL-r antibodies (2 μg/ml); lane 2 shows a representative serum obtained from a patient with MN at 1:10 dilution; lane 3 shows the same sample at 1:100 dilution. Western blot analysis was repeated with sera from all 38 patients with MN and from 10 healthy donors. No autoimmunity was detected in any tested sera.
A final experiment tested with immunofluorescence the binding of a few sera to podocyte cell lines. In this case, MN sera indeed recognized that surface proteins on podocytes did not correspond to LDL-r. They have been characterized by proteomics and are currently under investigation (Ghiggeri, personal observation).
Overall, our results suggest that LDL-r is not a target of an autoimmune response in human MN. Even though negativity of anti-LDL-r serum Ab was shown by two independent techniques (western blot and immunofluorescence) and by two different antigens (intact LDL-r and recombinant RAP) at different dilutions, the problem of sensitivity of the assay cannot be completely ruled out. Other technological approaches should be considered. In consideration of the negative results presented here, also the extension of the analysis of autoimmunity to other podocyte components must be considered.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Collins AB, Andres GA, McCluskey RT. Lack of evidence for a role of renal tubular antigen in human membranous glomerulonephritis. Nephron. 1981;27:297–301. doi: 10.1159/000182074. [DOI] [PubMed] [Google Scholar]
- 2.Debiec H, Guigonis V, Mougenot B, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–2060. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 3.Heymann W, Hackel DB, Harwood S, et al. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959;100:660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- 4.Kerjaschki D, Horvat R, Binder S, et al. Identification of a 400-kd protein in the brush borders of human kidney tubules that is similar to gp330, the nephritogenic antigen of rat Heymann nephritis. Am J Pathol. 1987;129:183–191. [PMC free article] [PubMed] [Google Scholar]
- 5.Rastaldi MP, Candiano G, Musante L, et al. Glomerular clusterin is associated with PKC-alpha/beta regulation and good outcome of membranous glomerulonephritis in humans. Kidney Int. 2006;70:477–485. doi: 10.1038/sj.ki.5001563. [DOI] [PubMed] [Google Scholar]