Figure 7.
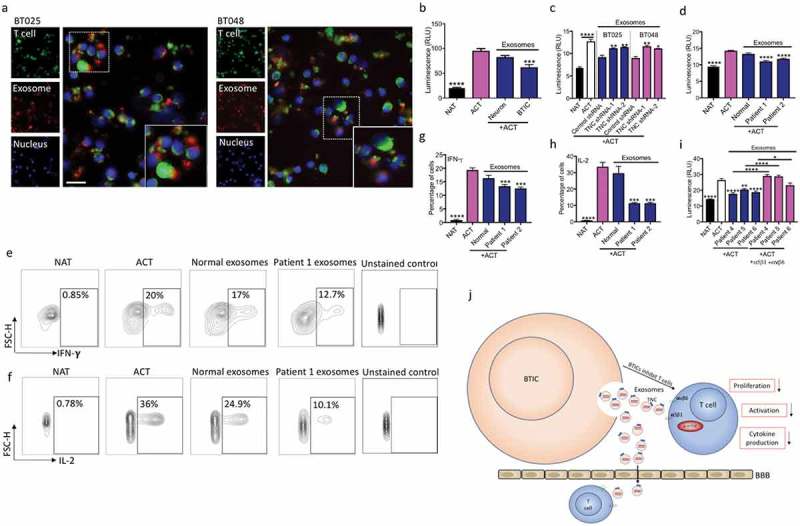
Exosomes from BTICs exert their inhibitory effects on T cells through TNC expression. (a) Exosomes isolated from human BTIC lines were labeled red with BODIPY TR Ceramide. T cells were purified from healthy individual and stained green with DiO. Representative images show exosome association with T cells. (b) In contrast to exosomes from neurons, BTIC exosomes suppressed T cell proliferation. (c) TNC-depleted exosomes had lower inhibitory effects on T cell proliferation compared to control shRNA exosomes. (d) Circulating exosomes from two glioblastoma patients induced a higher reduction in T cell proliferation than healthy control exosomes. (e-h) Exosomes from glioblastoma patients caused greater reduction of IFN-γ and IL-2 levels in activated T cells than normal exosomes (e, f: Representative flow plots). (i) Blocking both α5β1and αvβ6 receptors following treatment with plasma exosomes from three glioblastoma patients elevated T cell proliferation. (j) The postulated mechanism of T cell suppression by TNC expressed on exosomes. The cartoon depicts that BTICs release exosomes that carry TNC. TNC through interaction with integrin receptors α5β1 and αvβ6 attenuates the expression of p-mTOR in T cells. This results in the reduction of T cell proliferation, activation and cytokine production. Moreover, exosomes pass through the BBB in glioblastoma patients to alter systemic immunity. All bars are mean ± SEM of triplicate cultures. Data in all panels are analyzed by 1-way ANOVA with Tukey’s multiple comparisons post-hoc test. *P < 0.05, **P < 10–2, ***P < 10–3, ****P < 10–4 compared to ACT group unless otherwise displayed (p values in panel c are relative to control shRNA unless otherwise displayed). BTIC, Brain tumor initiating cell; ACT, activated T cell; NAT, non-activated T cell. Scale bar, 20 μm.
