ABSTRACT
Immunotherapy is reportedly effective in a subset of colorectal cancers (CRCs) with high microsatellite instability (MSI-H). Exploring the expression patterns and clinical values of immunologic molecules is critical in defining the specific responsive candidates. Here, we performed comprehensive molecular profiling of the B7 and TNFR family genes across 6 CRC datasets with over 1,000 patients’ details using cBioPortal TCGA data. About 20% of patients had B7 and TNFR family gene alterations. The frequency of B7 gene mutations (2.2%–5%) were similar to copy number alterations (0.53%–5.46%). TNFR amplifications were relatively more common (5.45–11.32%) than that of B7 (0.09–2.73%). B7 and TNFR gene mRNAs were upregulated in 26% of cases (102/379) and 16% of cases (61/379), respectively. The mRNA levels of B7 and TNFR genes were inversely correlated with promoter methylation status. Clinically, both B7-H3 and TNFSF7 mRNA overexpression were associated with unfavorable clinical outcomes, and the B7-H3 expression was increased gradually in cases with gene amplifications. Moreover, patients with MSI-H had significantly higher PD-L1 or PD-1 expression. Most importantly, in MSI-H group, patients with PD-L1 or PD-1 upregulation had poorer survivals than those with PD-L1/PD-1 downregulation. This is the first study drawing the immune landscapes of the co-stimulator B7 and TNFR families in CRC and showing that MSI-H patients with PD-1/PD-L1 upregulation are associated with poor clinical outcomes, providing potential markers to stratify patients responsive to immune checkpoint therapy.
Keywords: colorectal cancer, B7 family, microsatellite instable, checkpoint immunotherapy, TNFR superfamily
Introduction
Colorectal cancer (CRC) is one of the major causes of worldwide cancer-related mortality.1 The carcinogenesis of colorectum is driven by genetic and epigenetic alterations and also closely regulated by tumour-host interactions.2,3 Accumulating evidence has revealed immune microenvironment could help tumor cells to evade immune destruction by the infiltrating immune cells.4 Immunotherapy, especially checkpoint antibodies targeting the PDCD1 (programmed cell death 1, PD-1) and B7-H1 (programmed death-ligand 1 (PD-L1)) protein, has been emerged as a promising strategy to treat a number of cancer types.5 However, objective responses were observed in only 53% of patients with deficient mismatch repair (dMMR) solid tumors and in 31% of distant metastatic CRC patients with high microsatellite instability (MSI-H).6-8 The best candidates that benefit from immune checkpoint therapy are still unclear.
Nowadays, immunologic factors have shown promise as prognostic parameters, which could help to characterize the tumor response to immunotherapies.9 Previous studies have found that tumors with a high infiltrate of CD8+ cytotoxic T lymphocytes (CTL) and T helper 1 (Th1)-type cells had a more favorable prognosis than those with a low infiltrate.10,11 Recent publications have demonstrated the high level of tumour-infiltrating T lymphocytes (TIL) is associated with MSI-H features in CRC.12 Most significantly, CRC tumors with MSI creates a rich abundance of neoantigens responsible for the immune response.13 Meanwhile, these tumors have significant high level expressions of immune checkpoint proteins, including PD-1 and PD-L1, enabling them to survive from immune eradication.14,15 However, the TIL and tumor molecular features remains largely unknown in CRC. Therefore, understanding the expression and clinical patterns of immunologic molecules will be critical in characterizing and selecting patient populations to benefit from their application in CRC.
T cell plays an essential role in regulating immune responses and are activated by two classical signals: antigen recognition (signal one) and co-stimulation (signal two).16 The T cell co-stimulation molecules are key regulators of T-cell activation, tolerance, and exhaustion.17 Modification of these pathway alterations has been translated into effective strategies for cancer treatment. Presently, the co-stimulation pathways mainly involve two major families: the B7 family of immunoglobulins3 and the tumor necrosis factor receptor (TNFR) superfamily.18,19 The B7 family has 10 reported members, including CD80 (B7-1), CD86 (B7-2), PD-L1 (B7-H1), B7-H2, B7-H3, B7-DC, B7-H4, B7-H5, B7-H6 and B7-H7.18 These proteins have been proven to regulate both T cell co-stimulatory and co-inhibitory pathways.5,20 The TNFR superfamily proteins are expressed by antigen-presenting cells (APC) or tumor cells, functioning as important secondary signals: OX40L (TNFSF4), CD40 (TNFRSF5), CD70 (TNFSF7), CD137L (TNFSF9), HVEM (TNFRSF14), and GITRL (TNFSF18).19 Previous studies find that many of these molecules are dysregulated and associated with prognosis in different cancer types.21,22 Moreover, the well-known B7-1 or B7-2/CTLA-4 and PD-L1/PD-1 pathways are promising targets for tumor immune checkpoint therapy.5,23 However, the systemic alteration of these families has not been defined in CRC.
Currently, using TCGA data from cBioPortal, we elucidate comprehensive molecular profiling of the 10 B7 and 6 TNFR family genes across 6 CRC studies. We find gene alterations and mRNA dysregulations of B7 and TNFR family genes, and the abnormalities of mRNA expression is attributed to promoter DNA methylation. Both B7-H3 and TNFSF7 mRNA overexpression were associated with unfavorable survival. Moreover, the MSI status of CRC was correlated with both PD-L1 and PD-1 mRNA levels, and MSI-H patients with PD-1/PD-L1 pathway upregulation had poor clinical outcomes. Therefore, this study describes the systemic landscape of the B7 and TNFR families and highlights the clinical value of PD-1/PD-L1 expression in MSI-H patients, thereby aiding the development of rationales to guide immune checkpoint therapy in CRC.
Results
Gene alterations of B7 and TNFR family across colorectal cancer studies
To date, B7 and TNFR family molecules have been identified to play important roles in immunoregulation between T cells and APC/tumor cells.18,19 However, the gene wide alterations of B7 and TNFR families remain largely unknown in colorectal cancer (CRC). Here, the frequency of B7 and TNFR gene alterations (including mutations, amplifications, and deletions) across 6 studies in colorectal cancer were shown in Figure 1. In two studies, about 20% of patients had B7 and TNFR gene alterations (Figure 1A). The frequency of B7 family gene mutations (2.2%–5%) were similar to B7 copy number alterations (CNA), including amplifications, deep deletions and fusions (a total of 0.53%–5.46%; Figure 1B). Moreover, the amplification rates (0.09%–2.73%) were as common as deletion rates (0.35%–2.73%) in B7 gene groups (Figure 1B). Meanwhile, TNFR amplifications were relatively more common (5.45–11.32%) than that of B7 (0.09–2.73%) (Figure 1B and C).
Figure 1.
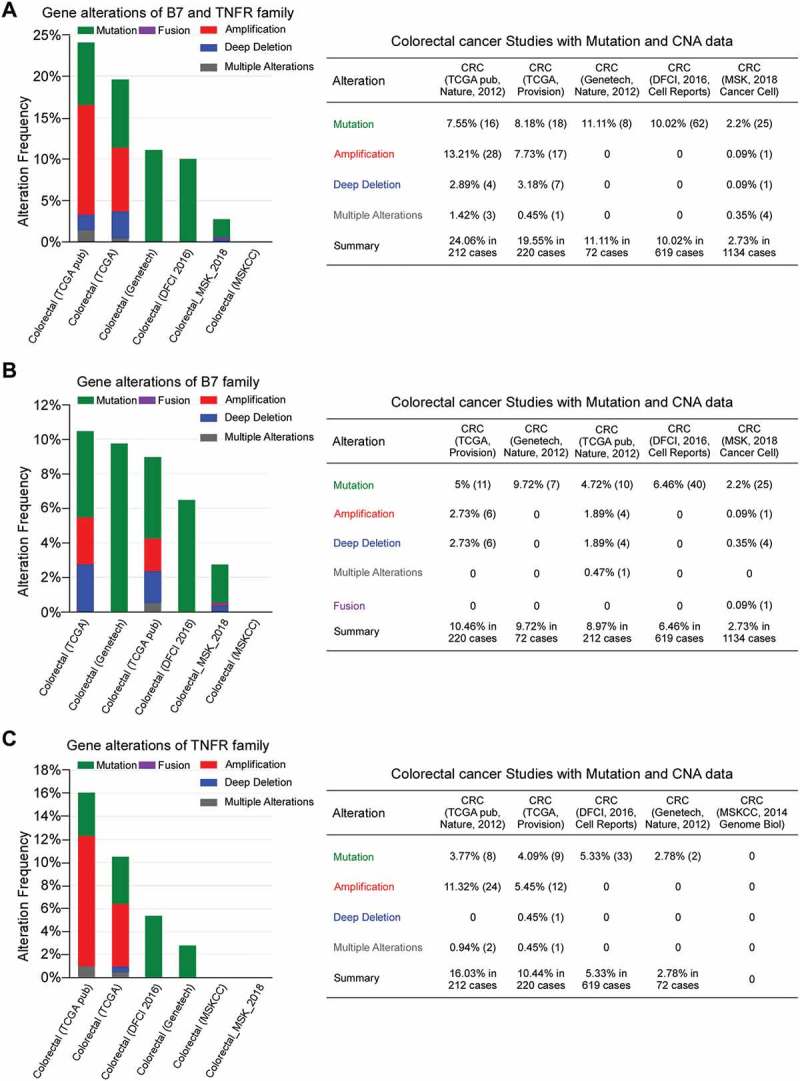
The gene alteration frequencies in the B7 and TNFR families across 6 colorectal cancer studies. (A) Gene alterations of B7 and TNFR family. (B) Gene alterations of B7 family. (C) Gene alterations of TNFR family.
Expression of B7 and TNFR proteins in CRC
Given the high frequency of B7 and TNFR gene copy number variations, their expression were also likely dysregulated. Therefore, we assessed the mRNA alterations of the B7 and TNFR family members across 379 sequenced CRC samples with data from The Cancer Genome Atlas (TCGA), queried with cBioPortal (Figure 2A). For each of the ten B7 and six TNFR genes, mutations were either not observed, or present at less than 2.2% of patients (Figure 2B and C). The frequency of B7-DC and B7- H1 CNA was about 2%, while CNA were more common in the TNFRSF5 gene (5.0%; Figure 2D and E). Interestingly, B7 mRNA levels were upregulated in more than 26% (102/379) of CRC, while TNFR mRNA levels were only upregulated in about 16% of cases (61/379). Except for B7-H2 and B7-H3, all other genes were exclusively upregulated, ranging from 1.1% to 5.8%, which was relatively more common than mutations and CNAs in CRC (Figure 2F and G). Meanwhile, the CNA rates of TNFR genes were less than 0.9%, except for TNFRSF5 (Figure 2E). The mRNA upregulation rates ranged from 1.1% to 4.7% in TNFR family (Figure 2F). For TNFRSF5, the gene amplification and mRNA upregulation rates were similar (Figure 2E and F). Moreover, the levels of TNFRSF5 mRNA were slightly increased in cases with CNAs (shallow deletions, diploid, copy number gains and amplification; Supplementary Figure S1), which suggests that TNFRSF5 mRNA levels could be partially regulated by copy number variation.
Figure 2.
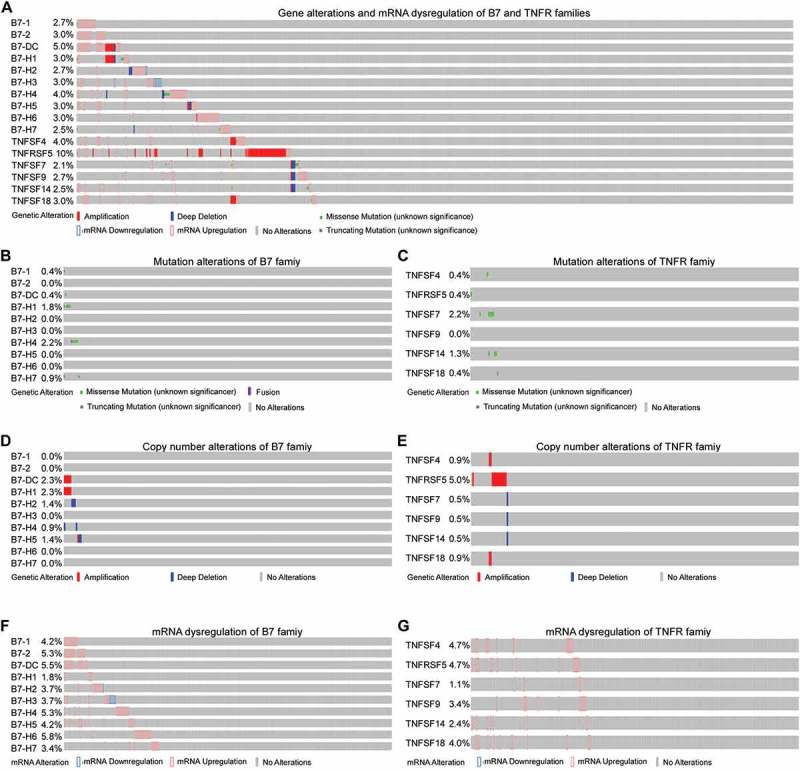
The gene alterations and mRNA dysregulations of B7 and TNFR family genes in colorectal cancer. (A) Gene alterations and mRNA dysregulations of B7 and TNFR family genes. (B) Mutation alterations of B7 family genes. (C) Mutation alterations of TNFR family genes. (D) Copy number alterations (CNA) of B7 family genes. (E) CNAs of TNFR family genes. (F) mRNA dysregulations of B7 family genes. (G) mRNA dysregulations of TNFR family genes.
Although B7 and TNFR proteins are constitutively expressed in CRC, the mechanisms underlying the mRNA dysregulation remains unclear. Therefore, we investigated the correlation between promoter DNA methylation with the mRNA expression level. The mRNA levels of B7-1, B7-H3, B7-H6, B7-H7, TNFSF4, TNFRSF5, TNFSF7, and TNFSF9 were negatively correlated with promoter methylation status (Figure 3). Notably, B7-H3 and TNFRSF5 mRNA levels were relatively strongly correlated with promoter methylation (Spearman: −0.383 and −0.633, respectively). Taken together, these results indicate that the expression of B7 and TNFR family members, especially B7-H3, may be epigenetically regulated in CRC.
Figure 3.
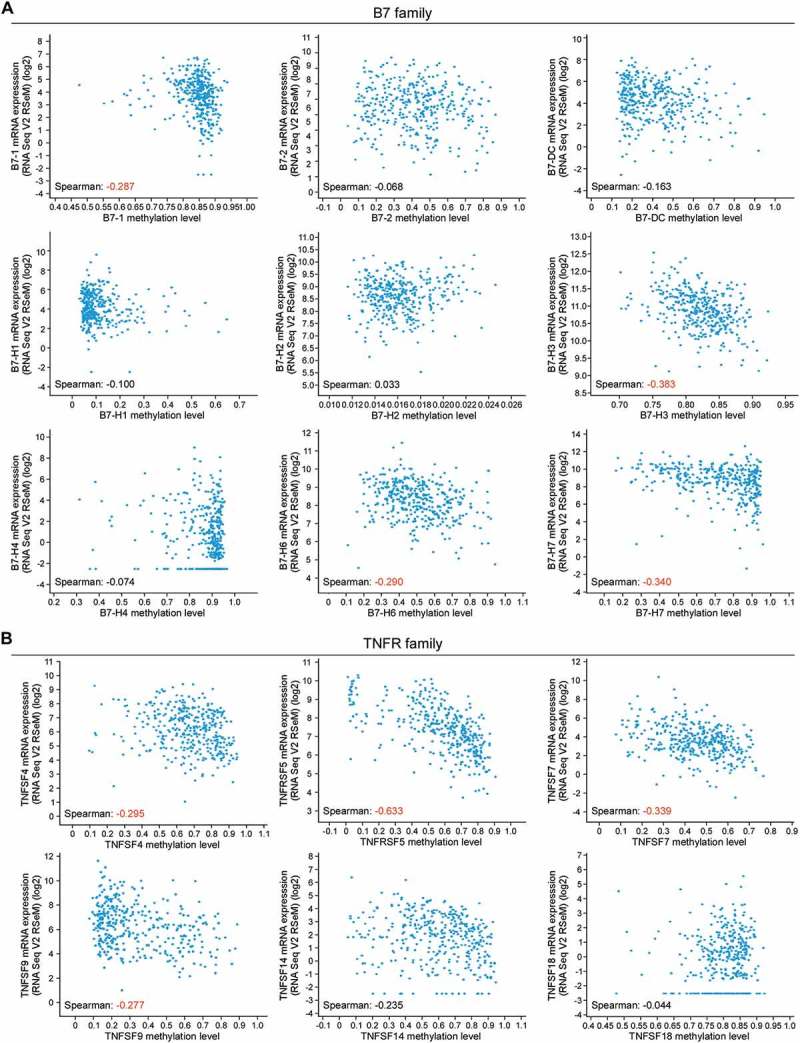
The correlation between the promoter DNA methylation status and mRNA expression level of the B7 and TNFR families in colorectal cancer. (A) The mRNA levels of B7-1, B7-H3, B7-H6 and B7-H7 are negative correlated with their promoter methylated status respectively in 379 colorectal cancer (CRC) samples. (B) The mRNA levels of TNFSF4, TNFRSF5, TNFSF7 and TNFSF9 are negative correlated with their promoter methylated status respectively in 379 colorectal cancer samples. Spearman coefficient in red, relatively strong correlation; Spearman coefficient in black, poor correlation.
B7-H3 and TNFSF7 as a potential prognostic biomarker in CRC
Furthermore, we evaluated the clinical value of the B7 and TNFR members’ mRNA dysregulation by accessing the overall survival (OS) and disease-free survival (DFS). Patients with high B7-H3 expression had significantly worse OS (p = 0.026) and DFS (p = 0.023; Figure 4A). In other B7 family members, no obvious correlations with survival were observed. We also examined whether B7-H3 was genetically dysregulated in four different cBioPortal data sets. The results showed that B7-H3 was amplified in CRC, although at relatively low frequencies (Figure 4B). We also found that the levels of B7-H3 mRNA were increased gradually in cases with gene copy number alterations (shallow deletions, diploid and copy number gains; Figure 4B), which suggests that B7-H3 mRNA levels may also be regulated by copy number variation.
Figure 4.
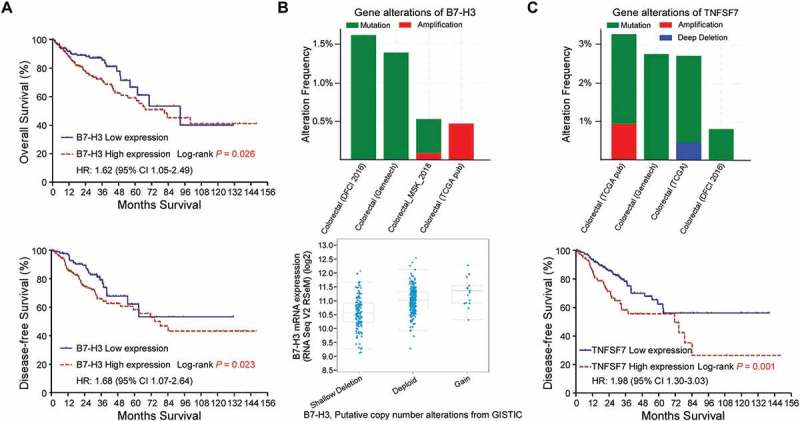
B7-H3 and TNFSF7 are potential biomarkers in colorectal cancer. (A) Overall survival and disease-free survival of patients with upregulated B7-H3 mRNA in CRC. (B) B7-H3 genetic alteration in four studies from cBioPortal and increased B7-H3 mRNA levels with copy number variations in CRC samples. (C) TNFSF7 genetic alteration in four studies from cBioPortal and disease-free survival of patients with upregulated TNFSF7 mRNA in CRC. P values were determined using the log-rank test.
In TNFR family, patients with high TNFSF7 expression had significantly more unfavorable DFS (p = 0.001; Figure 4C), while other TNFR family members did not showed obvious correlations with survival. The genetic alteration analysis showed that TNFSF7 was amplified and deleted in different CRC cBioPortal datasets (Figure 4C). However, TNFSF7 mRNA levels showed no correlations with gene copy number variations. As Figure 3B showed that TNFSF7 mRNA expression was inversely correlated with promoter methylation, it indicates that DNA dysmethylation may play important role in regulating TNFSF7 expression. Collectively, B7-H3 and TNFSF7 could be potential prognostic markers in evaluating CRC survival.
B7-H1 and PD-1 as potential prognostic biomarkers in CRC with msi-high status
Microsatellite instability (MSI) is an important molecular mechanism for CRC initiation and progression1. Patients with MSI-high (MSI-H) exhibits high levels of tumour neoantigens, tumour-infiltrating lymphocytes, and checkpoint regulators, which are associated with the response to B7-H1 (PD-1) blockade.12,13 Here, we examined the expression levels of B7 and TNSF family genes in cases with different MSI status. The results revealed that MSI status was associated with both PD-L1 (B7-H1, Spearman: −0.497, p < 0.001) and PD-1 (Spearman: −0.158, p < 0.001) mRNA levels (Figure 5A). The significant correlations were also observed between MSI status and TNFSF7, B7-1, B7-2, B7-2H, TNFSF4, TNFRSF5, TNFSF9, TNFSF14 and TNFSF18, respectively (Figure 5A, p < 0.005).
Figure 5.
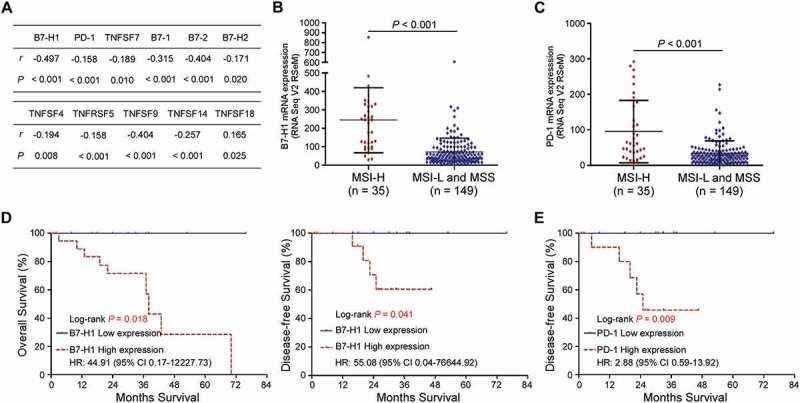
B7-H1 and PD-1 are potential prognostic biomarkers in colorectal cancer with MSI-High status. (A) Spearman’s correlation between MSI status and B7 or TNSF mRNA expression. (B and C) B7-H1 (B) and PD-1 (C) mRNA expression levels in CRC with high-microsatellite instable (MSI-high, n = 35) and low- microsatellite instable/microsatellite stable (MSI-L/MSS, n = 149) status, respectively. Student’s t-test. (D) Overall survival and disease-free survival of patients with upregulated B7-H1 mRNA in CRC. (E) Disease-free survival of patients with upregulated PD-1 mRNA in CRC. P values were determined using the log-rank test.
Furthermore, we evaluated the relation between survival and these gene mRNA expression level in CRC patients with different MSI status. The results showed that only PD-L1 and PD-1 mRNA expressions were correlated with survival. Moreover, patients with MSI-H (n = 35) had significantly higher PD-L1 (p < 0.001) or PD-1 (p < 0.001) mRNA expression than the patients (n = 149) with MSI-low (MSI-L) and microsatellite stability (MSS; Figure 5B and C). In MSI-H group, patients with PD-L1 upregulation experienced significantly shorter OS (3-year OS; 71.4% vs. 100%, P = 0.018) and DFS rates (3-year DFS; 55.6% vs. 100%, P = 0.041) than patients with PD-L1 downregulation. Meanwhile, patients with high PD-1 expression levels also showed statistically worse DFS (3-year DFS; 45.7% vs. 100%, P = 0.009) than patients with low PD-1 expression levels in MSI-H group. However, no association with survival was found in cases with different PD-1 or PD-L1 expression levels in MSI-L/MSS group. These findings suggest that PD-1 or PD-L1 mRNA upregulation is clinically associated with unfavorable outcomes in MSI-H CRC patients and the combination of PD-1/PD-L1 pathway and MSI status may help to classify subgroup candidates for checkpoint immunotherapy.
Discussion
This current study portrayed the systemic perspectives and clinical relevance of immune co-stimulator B7 and TNSF family genes in CRC. Mutations and CNAs were observed in both family genes. We found that their mRNA dysregulation was more common and associated with promoter DNA methylation. Moreover, the B7-H3 and TNFSF7 mRNA overexpression were correlated with unfavorable survival. In MSI-H CRC group, patients exhibited higher levels of PD-L1/PD-1 expression. We further provided evidence that MSI-H patients with PD-1/PD-L1 pathway upregulation experienced poor clinical outcomes, which representing a promising strategy for the CRC immune checkpoint therapy.
Growing evidence has highlighted the importance of the immunotherapy for cancer4. Antibodies to PD-1/PD-L1 (B7-H1) or B7-2/CTLA-4 pathway molecules have shown promise for inducing tumor responses, even in late-stage patients who have failed multiple lines of therapy.24,25 However, only a small number of patients respond.7,8 Therefore, it has been urgent to identify the best candidates for this therapy and explore novel targets to develop new treatment strategy. In this study, we found that the B7 and TNFR family genes were mutated, amplified or deleted in CRCs, which is consistent with previous studies in other cancer types, including breast cancer, head and neck cancer.26,27 Although the mutation and CNA rates were relatively low in CRC, a total of about 20% of patients had B7 and TNFR gene alterations, which may help to stimulate or suppress immune response in CRC. Moreover, we found that the upregulated B7-H3 and TNFSF7 mRNA expressions were associated with poor OS or DFS. Previous studies have demonstrated that B7-H3 participates in both immune stimulatory and inhibitory signals,20 while TNFSF7 involves in stimulatory signals.19 Therefore, our findings may provide significant prognostic factors and help to develop new target therapy.
Although anti-PD-1/PD-L1 antibodies have been demonstrated to induce significant durable tumor regressions in patients with renal cancer, melanoma and non-small cell lung cancer,24,28,29 CRC cases exhibit very low response rates, except those with MSI-H status.6,7 MSI is a consequence of impaired DNA mismatch repair, resulting in mutation accumulations to create a rich source of tumor-specific neoantigens.13 Some of which will be presented on the major histocompatibility complex (MHC), recognized as foreign by T cells, and thereby might stimulate lymphocytic reaction.12 It has been supported by the findings that MSI CRCs have higher level of TILs and a better prognosis than MSS.14 However, MSI tumors highly up-regulate expression of multiple immune checkpoints.11,30 Consistently, our study demonstrated that MSI-H CRCs displayed PD-1/PD-L1 overexpression, which might help CRC cells to survive from naturally immune eradication. Moreover, we found that MSI-H patients with PD-1/PD-L1 upregulation developed poorer survival than those with PD-1/PD-L1 downregulation, which may potentially define subsets of CRC that might be more sensitive to checkpoint blockade.
Our study had several limitations. First, the expression of B7 and TNFR genes was measured at mRNA level, the precise protein expression has not been validated by immunohistochemistry in CRC sample sections. Second, the value of combining MSI status and PD-1/PD-L1 expression remains unknown in forecasting benefit from immunological therapy, and further investigations into their roles in pre-clinical and clinical checkpoint therapy might provide novel treatment strategies. Moreover, our findings require further validation in prospective studies and multicenter clinical trials.
In conclusion, our study provided an overview of B7 and TNFSR family gene alterations in CRC. We also highlighted the prognostic value of PD-1/PD-L1 overexpression in MSI-H CRC patients, thereby facilitate the development of novel predictive strategies and immune checkpoint therapy against CRC.
Materials and methods
Determination of B7 and TNFR family member alterations in colorectal cancer
The frequency of genetic alterations (including mutations, amplifications, deletions and fusions) of the B7 and TNFR families was assessed across 6 studies of colorectal cancers (CRC) using the cBioPortal (http://www.cbioportal.org/index.do) for Cancer Genomics database and TCGA31 with more than 1,000 patients’ details. Mutations included missense mutations and truncating mutations. Missense mutations are point mutations changing a single nucleotide that results in the substitution of a different amino acid and a nonfunctional protein. Truncating mutations are point mutations to generate one stop codon, which leads to protein translation interruption. Further, we assessed the genomic alterations, including mRNA dysregulation and promoter methylation of B7 and TNFR families, across 379 sequenced CRC samples with complete TCGA data from cBioPortal. All mRNA data were assayed by mRNA-seq and gene expression values were represented as RNA-Seq by Expectation Maximization (RSEM) data normalized within each sample to the upper quartile of total reads.32
Prognostic significance of B7 and TNFR families in colorectal cancer
We obtained the clinicopathological information, including the microsatellite instability (MSI) status and survival data of all CRC samples from TCGA. Then, we assessed the prognostic effects of B7 and TNFR family members’ mRNA dysregulation in patients with colorectal cancer. The prognostic effects of B7 and TNFR gene expressions in patients with different MSI status were also evaluated. Cut-off value of mRNA was determined by receiver operating characteristic (ROC) curve analysis.
Statistical analyses
Statistical analyses were performed using SPSS 22.0 software (IBM, Armonk, NY, USA) and STATA version 12.0 (Stata Corporation, College Station, TX). Two-tailed Student’s t-tests were used to compare variables between groups. Comparisons among categorical variables were conducted by Pearson chi-square test and Fisher’s exact test. The relationship between mRNA expression and promoter methylation or MSI status was performed using Spearman correlation test. The Kaplan–Meier survival curves and log-rank tests were used to estimate the actuarial rates and comparisons. The hazard ratio was calculated by unadjusted Cox proportional hazards model. The mRNA expression data are presented as the mean ± s.d. P values < 0.05 were considered statistically significant.
Funding Statement
This work was supported by the No.
Acknowledgments
We would like to thank the staff members of the Cancer Genome Atlas for their involvement in the cBioPortal for Cancer Genomics Program. We also thank the anonymous editors and reviewers for their insightful comments and great efforts to improve this manuscript.
Competing interests
The authors have declared that no competing interest exists.
Supplemental Material
Supplemental data for this article can be accessed here.
References
- 1.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okugawa Y, Grady WM, Goel A.. Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology 2015;149:1204–1225. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014;110:1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masugi Y, Nishihara R, Yang J, Mima K, Da SA, Shi Y, Inamura K, Cao Y, Song M, Nowak JA, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut 2017;66:1463–1473. doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov 2015;5:16–18. doi: 10.1158/2159-8290.CD-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 2016;22:813–820. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 15.Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, Wendl MC, Kim J, Reardon B, et al. Comprehensive characterization of cancer driver genes and mutations. Cell 2018;173:371–385. doi: 10.1016/j.cell.2018.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 2016;44:955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 2016;44:1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer 2014;134:2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 21.Clouthier DL, Watts TH. TNFRs and control of chronic LCMV infection: implications for therapy. Trends Immunol 2015;36:697–708. doi: 10.1016/j.it.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Jung K, Choi I. Emerging co-signaling networks in T cell immune regulation. Immune Netw 2013;13:184–193. doi: 10.4110/in.2013.13.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assal A, Kaner J, Pendurti G, Zang X. Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy-Uk 2015;7:1169–1186. doi: 10.2217/imt.15.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shen J, Wang MH, Yi T, Yu Y, Zhu Y, Chen B, Chen J, Li L, Li M, et al. Comprehensive molecular profiling of the B7 family of immune-regulatory ligands in breast cancer. Oncoimmunology 2016;5:e1207841. doi: 10.1080/2162402X.2016.1207841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YP, Zhang J, Wang YQ, Liu N, He QM, Yang XJ, Sun Y, Ma J. The immune molecular landscape of the B7 and TNFR immunoregulatory ligand-receptor families in head and neck cancer: A comprehensive overview and the immunotherapeutic implications. Oncoimmunology 2017;6:e1288329. doi: 10.1080/2162402X.2017.1288329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korehisa S, Oki E, Iimori M, Nakaji Y, Shimokawa M, Saeki H, Okano S, Oda Y, Maehara Y. Clinical significance of programmed cell death-ligand 1 expression and the immune microenvironment at the invasive front of colorectal cancers with high microsatellite instability. Int J Cancer 2018;142:822–832. doi: 10.1002/ijc.31107. [DOI] [PubMed] [Google Scholar]
- 31.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer C, Diehn M, Alizadeh AA, Brown PO. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics 2006;7:115. doi: 10.1186/1471-2164-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


