ABSTRACT
Immunomodulatory therapies can effectively control haematological malignancies. Previously we reported the effectiveness of combination immunotherapies that centre on 4-1BB-targeted co-stimulation of CD8 + T cells, particularly when simultaneously harnessing the immune adjuvant properties of Natural Killer T (NKT) cells. The objective of this study was to assess the effectiveness of agonistic anti-4-1BB antibody-based combination therapy against two aggressive forms of acute myeloid leukemia (AML).
Anti-4-1BB treatment alone resulted in transient suppression of established AML-ETO9a tumor growth in 50% of mice, however the majority of these mice subsequently succumbed to disease. Combining alpha-galactosylceramide (α-GalCer)-loaded tumor cell vaccination with anti-4-1BB antibody treatment increased the proportion of responding mice to 100%, and protection led to long-term, tumor-free survival, demonstrating complete eradication of AML. This finding was extended to established mixed lymphocytic leukemia (MLL)-AF9 tumors, whereby vaccine plus anti-4-1BB combination similarly resulted in 100% protection. The addition of anti-PD-1 to anti-4-1BB treatment, although improving survival outcomes compared to anti-4-1BB alone, was not as effective as NKT cell vaccination.
The effectiveness of 4-1BB combination therapies was dependent on IFN-γ signaling within host cells, but not tumors. Vaccine plus anti-4-1BB therapy elicited potent generation of functional effector and memory CD8 + T cells in all tumor-associated organs. Therapy induced KLRG1+ effector CD8 T cells were the most effective at controlling disease. We show that combining NKT cell-targeting vaccination with anti-4-1BB provides excellent therapeutic responses against AML and MLL in mice, and these results will guide ongoing efforts in finding immunotherapeutic solutions against acute myeloid leukemias.
Keywords: Acute myeloid leukemia, mixed lineage leukemia, NKT cells, alpha-galactosylceramide, cancer vaccine, immunotherapy, monoclonal antibody, anti-PD-1, anti-4-1BB, CD8 T cells
Introduction
Acute myeloid leukaemia (AML) is a hematological malignancy that is characterized by the expansion and impaired differentiation of immature myeloid cells.1 Despite advances in understanding AML pathogenesis, treatment is largely restricted to chemotherapy (e.g. cytarabine, anthracycline), radiotherapy and allogeneic stem cell transplantation.2 Chemotherapy is often associated with toxicities and increases risk for relapse.2–4 These therapies are also poorly tolerated by elderly patients, who make up the majority of AML cases.2,4 Owing to the foregoing therapeutic challenges, immunotherapy has become an important alternative for cancer treatment, and is associated with greater tumor specificity and lower toxicity.5–14 Current designs in developing immunotherapies against AML include immune or tumor targeting antibodies, adoptive T cell therapy, tumor vaccines and immune checkpoint modulation.6–9,12 One of the ongoing challenges for AML immunotherapy is immune evasion resulting from shedding of stress proteins,15 downregulation of MHC expression,16 immunosuppressive cells4 or inhibitory receptors like cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) on T cells.17
Adoptive CAR T cell therapy has the advantage of circumventing immune exhaustion, however, exploitation of this technology in AML is limited by the difficulty in identifying appropriate target antigens (e.g. CD33, CD123) as their expression strongly overlaps with those in healthy cells.18 Whole tumor vaccines can activate an immune response against multiple tumor antigens, and thus overcoming immune evasion arising from mutations of tumor antigens.6,17,19,20 The glycolipid α-GalCer, which directly targets CD1d-restricted NKT cells, that have downstream regulatory effects on NK and T cell activation, and exhibits strong vaccine adjuvant properties that can be exploited for immunotherapy.11,14,19,21 By administering irradiated α-GalCer-pulsed tumor cells to mouse models of B cell lymphoma, we have shown that induction of effector CD8 + T cells is associated with suppression of tumor growth.10,11,14 These results indicate that cancer treatments can be improved if tumor vaccines are combined with agents that promote the immune adjuvant effects of NKT cells. The importance of NKT cells is further supported by the evidence that AML patients with low NKT cell numbers have poor survival rates.22
PD-1 is an immune inhibitory molecule that interacts with its ligands PD-L1 or PD-L2 on antigen presenting cells to maintain immunologic tolerance.8,23,24 Increased expression of PD-1 on T cells or PD-L1 on tumor cells or myeloid cells represses CD8 + T cell cytolytic activity and enhances tumor growth;23,25–28 suggesting that antagonizing the PD-1/PD-L axis could boost antitumor immunity. Nivolumab (anti-PD-1) was recently approved by FDA for treatment of advanced melanoma and for non-small-cell lung cancer.2,6 Targeting PD-1 is also effective against Hodgkin lymphoma, colorectal cancer and renal cell carcinoma.29–34 There are currently phase 1 and 2 clinical trials for anti-PD-1 monotherapy, or combinations with chemotherapy or stem cell transplant against AML or Myelodysplastic Syndrome.2,6,27,35,36 Given that tumors hijack host immune tolerance mechanisms to avoid detection, effective therapies must aim to enhance T cell immunity while relieving tumor-mediated immune inhibition7,28
The co-stimulatory molecule 4-1BB (CD137) is widely expressed on hematopoietic cells and can contribute to proliferation, survival and enhanced activity of CD8 + T and NK cells.13,37–39 Addition of soluble 4-1BBL to co-cultures of AML-derived DC and T cell, induces differentiation of T cells to effector and central memory CD8 + T cells.40 Adoptive transfer of ex-vivo 4-1BB stimulated T cells or in vivo anti-4-1BB antibody therapy have been shown to eradicate established P815 mastocytoma, Ag104A sarcoma and other forms of cancer.12,13,39,41,42 Using anti-4-1BB and an NKT cell targeting vaccine, we showed 50–70% long-term mouse survival in B-cell lymphoma.5,10 However, such therapeutic effects mediated by 4-1BB co-stimulation can be diminished under conditions of immune exhaustion. This implies that combinational therapies involving inhibitory checkpoint blockade may increase anti-tumor immunity.8,24,34,43 However, designing such therapies should be taken with caution. We have shown in a spontaneous model of B-cell lymphoma, that 4-1BB-induced therapeutic effects is dampened by PD-1 blockade.5
In this study, we report that an NKT cell-targeting vaccine and anti-4-1BB combination therapy resulted in 100% mouse survival against AML or MLL tumor challenge. However, only 40–60% of the mice survived following the combined anti-PD-1 and anti-4-1BB therapy. Our study demonstrates that the vaccine and anti-4-1BB combination induced enhanced CD8 + T cell activation and IFN-γ production, and these responses were associated with AML tumor clearance. Collectively, these results suggest that NKT cell targeting vaccination in combination with 4-1BB co-stimulation may offer attractive alternatives for treatment of acute myeloid leukemia.
Material and methods
Mice handling
Mice were housed and maintained in pathogen-free conditions at the Translational Research Institute Biological Research Facility (TRI-BRF; Brisbane, Australia) of the University of Queensland. Six to twelve week old C57BL/6J female mice, C57BL/6.Rag1 knockout (KO) mice and congenic C57BL/6J.Ptprca mice were obtained from Animal Resource Centre in Perth, Australia. The IFN-γ and IFN-γ receptor KO (IFN-γ KO, IFN-γR KO) mice on a C57BL/6 background were bred in-house and maintained as previously described.44 Mice were age matched for individual experiments, and all animal procedures were approved by the University of Queensland Health Sciences Animal Ethics Committee (UQDI/TRI/288/15/NHMRC/NIH) and conducted in accordance with animal ethics guidelines provided by the Australian National Health and Medical Research Council.
Reagents and antibodies
The reagents used include phorbol 12-myristate 13-acetate (PMA), ionomycin (Sigma-Aldrich), BD Cytofix/Cytoperm kit for intracellular staining (BD Biosciences) and α-GalCer (Avanti Polar Lipids, Alabaster, Alabama). Fluorochrome-conjugated anti-mouse monoclonal antibodies (mAbs) to KLRG1 (2F1/KLRG1), CD127 (A7R34), NK1.1 (PK136), PD-1 (RMP1-30), PD-L1 (10F.9G2), TCRb (H57–597), CD3e (145–2C11), CD8b (YTS156.7.7), CD44 (IM7), CD62L (MEL-14),IFN-γ (XMG1.2) and associated isotype control antibodies were purchased either from Biolegend (San Diego, CA), eBioscience or BD Biosciences (San Diego, CA). Anti-4–1BB (3H3) and anti-PD-1 (RPM1-14) in vivo antibodies were obtained from Bio-X-cell (West Lebanon, NH).
Cell preparation, staining and flow cytometry
Blood was collected via retro-orbital bleeding into anticoagulant of 1% heparin (in PBS) in a 1:1 ratio. To harvest bone marrow or spleen cells, mice were euthanized and the femurs and spleens were collected in incomplete media (DMEM, 1%Penicillin-Streptomycin-Glutamine (PSG), 1%Sodium pyruvate (NaPyr), Gibco). The femurs were cut on both ends and the bone marrow was flushed out and collected in incomplete media. The harvested bone marrow and spleens were subjected to homogenization through a 70μm cell strainer to generate singe-cell suspensions. Hypotonic Ammonium-Chloride-Potassium (ACK) buffer (prepared in-house) (0.15M NH4Cl, 1mM KHC03, 0.1mM EDTA) was used to lyse red blood cells in whole blood or in cells derived from the bone marrow or spleen, as previously described.5,10,11,14 Cell lysis was quenched by adding fluorescence-activated cell sorting (FACS) buffer (2% newborn calf serum and 2mmol/L EDTA in PBS) in a ratio of 3: 2 (ACK: FACS buffer). Cells were then exposed to two rounds of washes using FACS buffer, centrifuged at 1300 rpm, 4°C, for 5 min. Single cells were then labelled at optimal concentrations of monoclonal antibodies (mAbs) for 30 minutes at 4°C in FACS buffer. This was followed by two washes as above. Flow-count fluorospheres (Beckman Coulter) was added to the samples prior to acquisition for cell number calculation. Intracellular staining of IFN-γ was performed using BD Cytofix/Cytoperm kit (BD Biosciences) according to manufacturer’s instructions. Briefly, cells were stimulated in vitro with a combination of PMA (25 ng/ml) and Ionomycin (1 µg/ml) for 4 hours in RPMI-1640 (Gibco) medium containing 10% fetal calf serum and BD GolgiPlug (BD Biosciences) at 37°C and 5% CO2. Sample acquisition was performed on Gallios (Beckman Coulter) or LSR Fortessa (BD Biosciences), and data analysis was done using Kaluza version 1.2 software (Beckman coulter).
Induction of leukemia models and in vivo imaging
The GFP- and luciferase co-expressing AML-ETO9a or MLL-AF9 tumor cells were created as previously described,14,45 and they were passaged through Rag1-KO mice to expand tumor cell numbers. Freshly isolated tumor cells from spleens were frozen down for future transplantation experiments. For leukemia inoculation, frozen tumor cells were thawed, washed, enumerated using a haemocytometer (Marienfeld GmbH, Lauda-Königshofen, Germany) and resuspended in incomplete media (above) and inoculated intravenously (i.v) into mice. Using MDS Nordion Gammacell 40 Exactor (Kanata, Ontario, Canada), mice were sub-lethally irradiated (3 Gy) followed by inoculation of either 1 × 105 AML-ETO9a, or 5 × 104 MLL-AF9 tumor cells. Mice were subsequently monitored for tumor establishment in the blood by detecting GFP+ cells using flow cytometry or IVIS Spectrum whole body bioluminescent imaging of luciferase+ cells. The IVIS imaging technology is non-invasive and conveniently enables longitudinal monitoring of tumor progression in live mice. Briefly, luciferin (1.5 mg/ml) substrate in 100 µL was injected intraperitoneally (i.p) to shaved mice and mice were anaesthetized with 2.5% isoflurane followed by whole body imaging. The acquired bioluminescent images were analysed using Living Image software.14 In all the experiments, at least three independent experiments were performed in each of the test conditions in AML, but only one experiment was conducted in MLL.
Therapeutic vaccination and immunotherapy
The NKT-cell-targeting tumor cell vaccine was prepared as previously described. Briefly, autologous tumor cells at 5 × 106 cells/ml were loaded in an overnight culture with α-GalCer (500ng/ml), and subjected to 5000 cGy to arrest further cell proliferation and enhance antigen presentation.5,10,14 Tumor cells loaded with α-GalCer was inoculated i.v 4 days after tumor inoculation, at a time where established disease was confirmed by bioluminescent imaging (Supplementary Fig 1). When the vaccine was given with anti-4-1BB (100μg) in combination therapy, mAb were delivered 2 days following vaccination, and repeated weekly for a total of 3 doses in any given experiment. In separate experiments, anti-4-1BB, anti-PD-1 (200μg) or their combination were administered i.p in a single injection once a week, for a total of 3 doses. mAb administration was given 6 days post-tumor inoculation.
Adoptive transfer experiments
Single cell suspensions were prepared from spleens as described above. CD8 + T cells from pooled spleen samples were isolated using fluorescence-activated cell sorting. All samples went through an “enrichment” sort to exclude the bulk of tumor cells present, before a secondary sort to isolate CD8 + T cells with >90% purity. For some experiments, CD8 T cells were further divided into effector sub-populations by sorting based on expression of CD44, CD62L and KLRG1. Sorted CD8 + T cells were then assessed for viability and enumerated via trypan blue exclusion and delivered into mice i.v at equal numbers 24 hours prior to tumor inoculation.
Statistical analysis
The results were averaged by group and treatment, and presented as means ± SE. The results were compared by student t-test, one-way or two-way ANOVA followed by Tukey’s multiple comparisons post hoc analysis to determine significance (p < 0.05) using Graphpad Prism 7. Survival curves were analysed by Mantel-Cox log-rank multiple comparison test. Flow cytometry plots were analysed by Kaluza analysis software version 1.3.
Results
Agonistic anti-4–1bb antibody treatment suppresses growth of aml-eto9a tumors and can be enhanced with PD-1 blockade
Previously in a model of Eµ-myc lymphoma, we showed that stimulation of 4–1BB using an agonistic monoclonal antibody (clone 3H3) led to an increased T cell response that was associated with reduced tumor load and enhanced mouse survival.10 In this study, using a murine model of AML, we show that administration of weekly anti-41BB, which commenced following tumor establishment (Supplementary Fig 1) six days after tumour challenge, promotes complete clearance of tumor in 40% of treated mice. However, AML progression subsequently occurred in 30% of responding mice (Fig 1A). We discovered that anti-4-1BB administration led to a rapid and sustained increase in PD-1+ CD8 + T cells (Fig 1B). This treatment did not affect PDL-1 expression on tumor cells (Fig 1C), but significantly increased PDL-1 expression on Ly6Clo monocytes (Fig 1D). Anti-PD-1 blockade alone had no effect on tumor growth (Fig 1E/F/G), but in combination with anti-4-1BB prevented tumor dissemination in the blood in 70% of the mice, of which 40% remained tumor-free (Fig 1 E/F/G). As shown previously in a B cell lymphoma model, anti-4-1BB treatment leads to systemic depletion of NK cells and increases in CD8 + T cell numbers.46 Whilst we observed similar trends in AML, there were no significant differences in proportions of these key effector cells were observed when comparing responding and non-responding mice over time (Fig 1 H/I).
Figure 1.
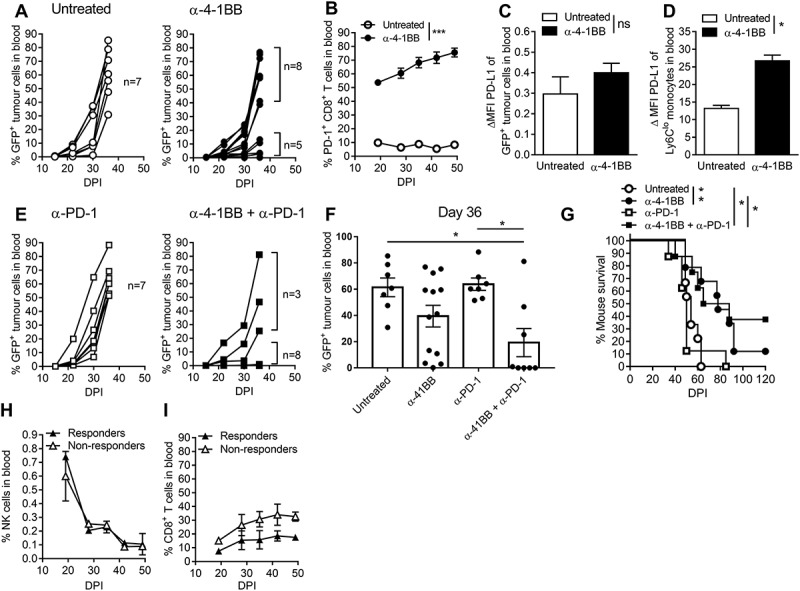
The anti-4-1BB and anti-PD-1 combination therapy suppresses AML tumor growth and enhances mouse survival.. AML-ETO9a tumor-bearing mice were treated with anti-4-1BB, anti-PD-1 or their combination. Individual tumor growth curves from untreated or anti-4-1BB treated mice (A), the percentage of PD-1 expressing CD8 + T cells (B), PD-L1 expression on tumor cells (C), and monocytes (D) is shown. Tumor growth curves from mice treated with anti-PD-1 or in combination with anti-4-1BB (E) is plotted, in addition to summary data at day 36 (F), survival curves (G) and the fraction of NK cells (H) and CD8 + T cells (I) grouped into responders (tumor free) and non-responders. The data is presented as individual mice or mean ± SEM (n = 3 to 7) and was analyzed by student t-test, or Two-way ANOVA followed by Tukey’s post-hoc test to determine the significance (p < 0.05). Survival curves were analysed by the Mantel-Cox log-rank multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Delaying the administration of anti-PD-1 (Supplementary Fig 2A) in an attempt to 1) allow initial CD8 + T cell activation through PD-1 signalling (activation of PD-1 during early phases of T cell activation is important)25,47 and 2) to allow anti-4-1BB-induced increases in PD-1 expression, resulted in complete abrogation of the therapeutic effect (Supplementary Fig 2B/C). This also prevented the capacity for combination treatment to enhance CD8 + T cell IFN-γ production, measured directly ex vivo (Supplementary Fig 2D), or after PMA/Ionomycin re-stimulation (Supplementary Fig 2E). These results indicate that AML tumors cannot be suppressed by delayed administration of anti-PD-1, and possibly, addition of anti-PD-1 at later stages may negate the therapeutic effect of anti-4-1BB treatment.
NKT cell-targeting vaccination and anti-4-1bb combination therapy completely eradicates aml-eto9a tumors
Anti-PD-1 and anti-4-1BB antibodies only effectively protected a proportion of AML tumor-challenged mice. Considering that an NKT cell-targeting vaccine with anti-4-1BB therapy yielded effective therapeutic outcomes in Eµ-myc lymphoma,5,10,11,14 we tested the ability of this combination to inhibit AML growth. We demonstrate that the vaccine alone was sufficient to delay AML outgrowth in the majority of mice (78%). Vaccine and anti-4-1BB combination cured 100% of the mice with AML, resulting in long term (>120 days) tumor-free survival (Fig 2A/B). Vaccination did not overtly affect NK cell levels in the blood by day 20, but prevented early loss of NK cells induced by anti-4-1BB treatment. (Fig 2C). The availability of NK cells in the blood is likely regulated by the vaccine-induced, NKT cell-mediated NK cell expansion, followed by IFN-γ-induced NK cell depletion.46 Addition of vaccine did not further increase the proportion of CD8 + T cells in the blood induced by anti-4-1BB (Fig 2D), however combination treatment led to significant increases in the numbers of CD8 + T cells with effector (TEM) and memory (TCM) phenotype (Fig 2E/F). Collectively, these data indicate that the vaccine and anti-4-1BB combination immunotherapy effectively clears AML likely through induction of NKT/NK cell innate immunity and increased CD8 + T cell activation and differentiation.
Figure 2.
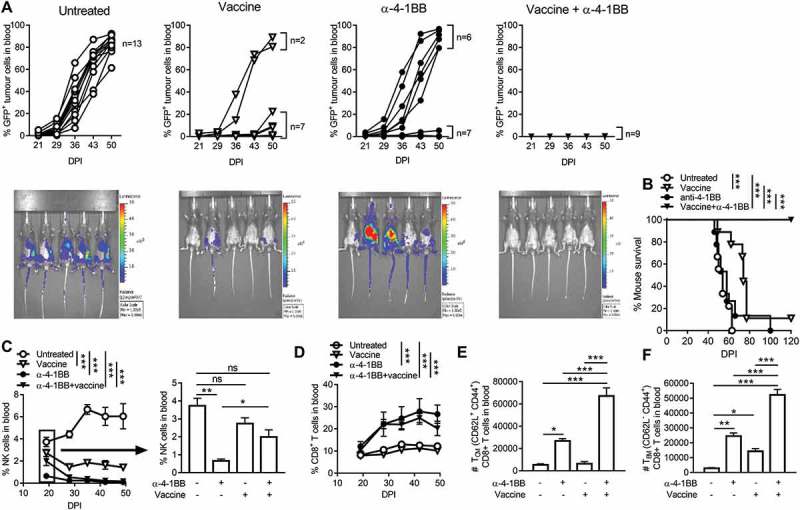
The α-GalCer-loaded tumor cell vaccine combined with anti-4-1BB antibody treatment eradicates blood tumor, activates effector CD8 + T cells and depletes NK cells.. Mice that were challenged with GFP and luciferase-expressing AML-ETO9a tumor cells were subjected to α-GalCer-loaded tumor cell vaccine, anti-4-1BB or their combination. For in vivo imaging, mice were injected with luciferin (1.5 mg/ml) on day 38, followed by acquisition of bioluminescence data of each mouse using IVIS Spectrum In Vivo Imaging System (A). The survival curves (B) and proportion of NK cells (C), CD8 + T cells (D), or numbers of central memory CD8 + T cells (TCM) (E) and effector memory CD8 + T cells (TEM) (F) were analysed following immunotherapy. The results are displayed as individual mice and mean ± SEM (n = 4 to 9) and was analyzed by One or Two-way ANOVA followed by Tukey’s post-hoc test to determine the significance (p < 0.05). Statistical analysis for (C) and (D) indicates overall differences between groups inclusive of all time-points. Survival curves were analysed by the Mantel-Cox log-rank multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Only vaccine and anti-4-1bb combination treatment leads to tumor clearance in bone marrow and spleen and enhancement of CD8 + t cell differentiation and function
AML tumor cells primarily establish in the bone marrow and disseminate and colonize other organs like the spleen. This means that undetectable tumor cells in the blood may not reflect tumor loads at those sites. We therefore next examined whether the vaccine and anti-4-1BB combination eradicates AML tumor cells in the bone marrow and the spleen. For these experiments vaccine was delivered on day 4 after tumor inoculation, followed by three weekly injections of anti-4-1BB and/or and-PD-1 commencing on day 6. Organs were harvested on day 29 for analysis. Vaccine and anti-4-1BB combination therapy effectively inhibited growth of AML tumor in both bone marrow (Figs 3A) and spleen (Fig 3B), consistent with long term tumor-free survival observed with this combination (Fig 2). In contrast, anti-4-1BB treatment alone had no effect on tumor growth in these organs, suggesting that decreased tumor load observed in blood with anti-4-1BB (Fig 1, 2) was due to decreased or delayed tumor dissemination. Addition of anti-PD-1 to anti-4-1BB treatment led to moderate suppression of tumor growth in bone marrow and spleen (Fig 3A/B). Anti-4-1BB led to increased numbers of total CD8 + T cells in these organs (Fig 3C/D) the majority constituting a TEM phenotype. The addition of anti-PD-1 did not alter these numbers. Vaccine plus anti-4-1BB combination significantly increased the numbers of effector T cells in both bone marrow and spleen, in particular TCM cells (Fig 3C/D). Furthermore, the addition of anti-PD-1 or vaccine to anti-4-1BB treatment was required to stimulate IFN-γ production by both TEM and TCM cells (Fig 3E/F/G). Such responses in TEM cells were significantly higher in vaccine combination treated mice. These results indicate that the vaccine and anti-4-1BB combination induces enhanced CD8 + T cell responses in the tumor-bearing bone marrow and spleen, which evoked superior anti-tumor protection.
Figure 3.
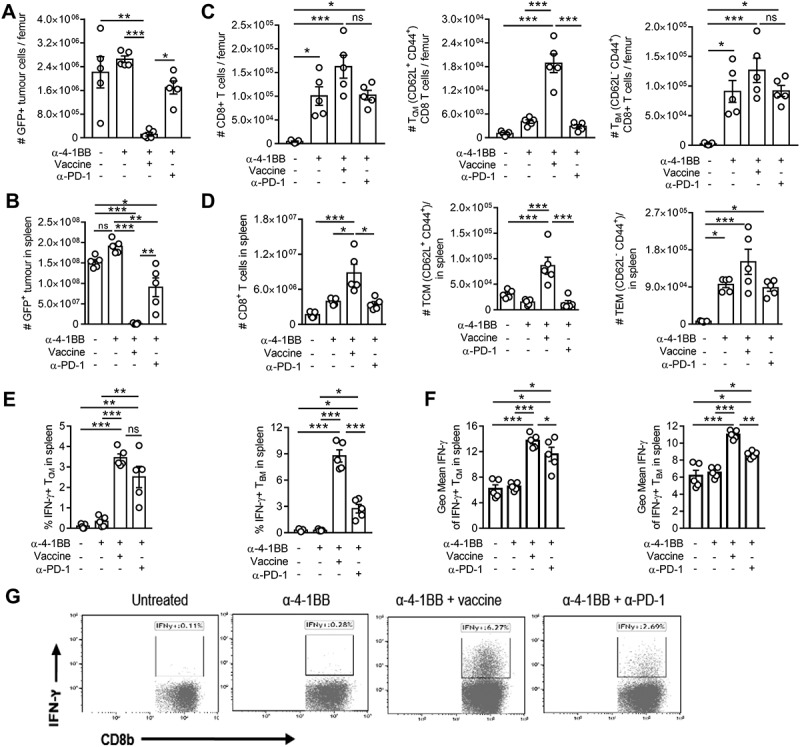
Clearance of AML-ETO9a tumor cells from the bone marrow or spleen by vaccine and anti-4-1BB co-stimulation is associated with increases in CD8+ TCM and TEM responses.. Mice were lethally challenged with AML-ETO9a tumor cells, therapeutically administered with the vaccine, anti-4-1BB or their combination, or anti-4-1BB + anti-PD-1 followed by analysis of bone (A + C) or spleen (B + D) for tumor cell numbers (A + B), CD8 + T cells, TCM and TEM numbers (C + D) on day 29. The frequency of IFN-γ releasing TCM or TEM cells (E) or the expression level of IFN-γ within TCM and TEM cells (F) along with representative plots of IFN-γ production from spleen after different treatments (G) is displayed. The data is presented as mean ± SEM (n = 4) or dot plots of representative data, and was analyzed by One-way ANOVA followed by Tukey’s post-hoc test to determine the significance (p < 0.05) or by Kaluza analysis program. * p < 0.05, ** p < 0.01, *** p < 0.001.
Adoptive transfer of TEM, but not TCM, CD8 + t cells were effective at controlling AML tumor growth
Having demonstrated that the vaccine and anti-4-1BB combination increased in TEM and TCM CD8 + T cells in the bone marrow and the spleen that were associated with tumor clearance, we next investigated whether specific subsets of CD8 + T cells were responsible for therapy-induced protection. Tumor-bearing recipient mice received CD8 + T cell populations derived from donor mice that were treated with vaccine and anti-4-1BB combination or left untreated. Those receiving adoptive transfer of unfractionated CD8 + T cells isolated from healthy mice (Fig 4B) or untreated tumor-bearing mice (Fig 4C) displayed tumor outgrowth similar to mice that did not receive CD8 + T cell transfers (Fig 4A). Similarly, CD8+ TCM cells isolated from mice originally exposed to the vaccine and anti-4-1BB combination therapy did not control AML growth (Fig 4D). However, adoptive transfer of CD8+ TEM from mice treated with the vaccine and anti-4-1BB combination, resulted in tumor growth suppression in majority of cases (75%) (Fig 4E/F). Within the TEM population, specific transfer of KLRG1+ cells was most effective at clearing tumor (100%) (Supplementary Fig 3), suggesting that KLRG1+ CD8+ TEM cells play a significant role in anti-AML immunity elicited by vaccine plus anti-4-1BB therapy.
Figure 4.
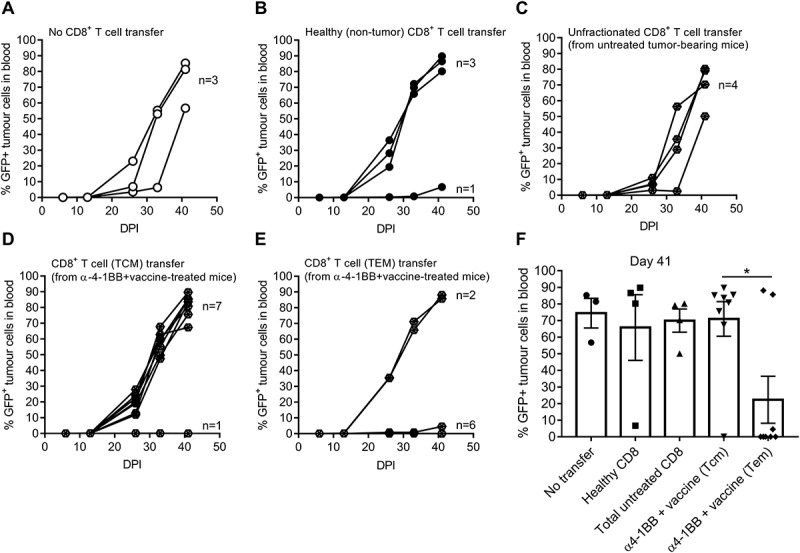
Adoptive transfer of effector CD8 + T cells from vaccine and anti-4-1BB-treated mice induced protection against AML-ETO9a tumor challenge.. Mice either received no CD8 + T cell transfer (A), naïve CD8 + T cells (B), unfractionated CD8 + T cells from untreated tumor-bearing mice (C) or from TCM (D) and TEM (E) from mice previously treated with vaccine plus anti-4-1BB followed by determination of tumor growth displayed as individual curves. Summary data of tumor burden at day 41 is shown (F) and presented as mean ± SEM, analyzed by One-way ANOVA to determine the significance; * p < 0.05.
Host ifn-γr signaling is critical for therapeutic efficacy of combination immunotherapy
Increased IFN-γ production is important for the anti-tumor effect elicited by NKT cell-targeting vaccination5,10,11,14 or anti-4-1BB therapy.10,13 We therefore hypothesized that IFN-γ/IFN-γR signalling was critical for inhibition of AML growth in our combination treatments. In wildtype (WT) mice, vaccine and anti-4-1BB combination again completely cleared AML tumors in 100% of mice, which was more effective than the combination of anti-4-1BB and anti-PD-1 (Fig 5A). The efficacy of both combination treatments was completely lost in IFN-γRKO mice, despite the presence of IFN-γ and IFN-γR+ tumors in these recipients, suggesting a critical role for host (and not tumor) IFN-γ signalling in the efficacy of these treatments (Fig 5B). Treatment efficacy was also significantly suppressed in IFN-γKO hosts, however, the average survival of untreated mice was increased from day 40 DPI in WT mice to 60 DPI in IFN-γKO mice (Fig 5C), suggesting likely existence of alternative pathways that feed into the downstream signalling mechanisms mediated through the IFN-γR receptor.48,49
Figure 5.
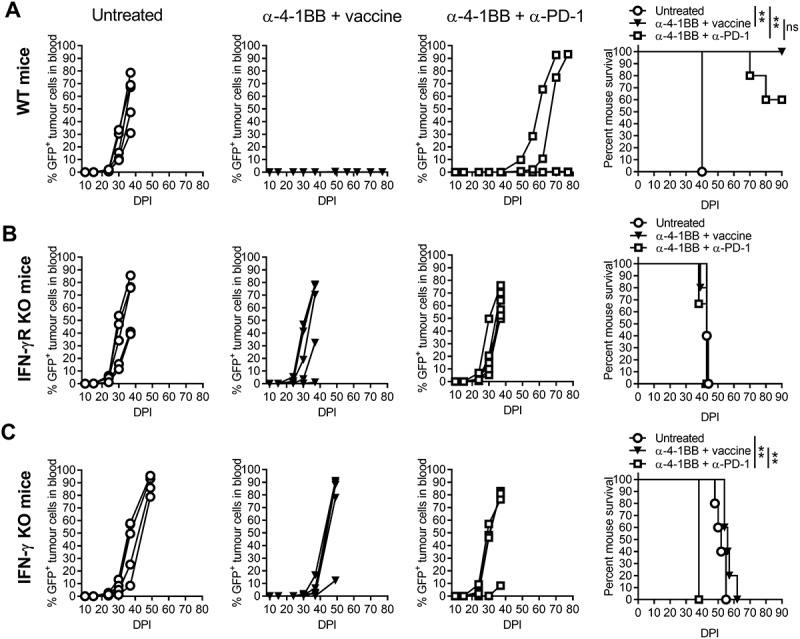
The effects of combination immunotherapy were lost in IFN-γ or IFN-γR deficient mice following AML-ETO9a tumor challenge.. Wildtype (WT) (A), IFN-γRKO (B) or IFN-γKO (C) mice were injected with AML-ETO9a tumor cells followed by administration of vaccine and anti-4-1BB or anti-PD-1 and anti-4-1BB combination and determination of GFP+ AML-ETO9a tumor growth, and survival curves shown. The tumor growth curves are presented as individual mice (n = 5), and survival curves were analysed by the Mantel-Cox log-rank multiple comparison test to determine significance (p < 0.05). ** p < 0.01.
Vaccine and anti-4-1bb combination immunotherapy is also highly effective against MLL
To determine whether vaccine and anti-4-1BB immunotherapy is effective against other AML tumors, we treated mice harbouring aggressive MLL-AF9 tumors.50 Similar to that observed for AML-ETO9a tumors, mice receiving vaccine and anti-4-1BB therapy completely prevented MLL-AF9 tumor growth and dissemination, as depicted by absence of detectable tumor cells in the blood (Fig 6A-C), resulting in long term tumor-free survival (Fig 6D). These results indicate the broader effectiveness of the vaccine plus anti-4-1BB therapy against multiple AML leukaemia sub-types
Figure 6.
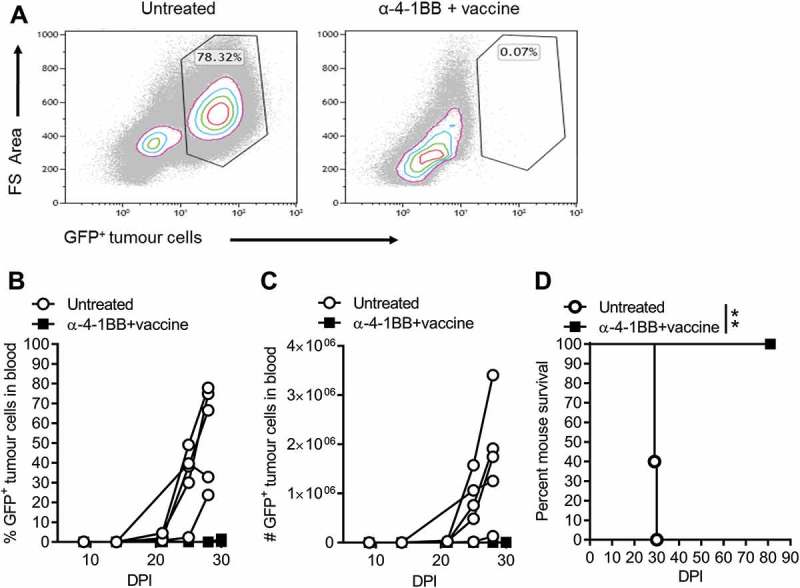
The vaccine and anti-4-1BB combination therapy eradicates established MLL-AF9 tumors.. Mice were administered with MLL-AF9 tumor cells followed by vaccine and anti-4-1BB therapy. Indicated are dot plots of representative data displaying the levels and percent blood tumor (day 28) from control and experimental mice (A), GFP+ MLL-AF9 tumor cells in the blood (B, C) and mouse survival curves (D). Tumor data is displayed as individual curves (n = 5), and survival curves were analysed by the Mantel-Cox log-rank multiple comparison test to determine significance (p < 0.05). Dot plots were analysed by Kaluza analysis program. **p < 0.01.
Discussion
In this study, we have demonstrated that an NKT cell targeting vaccine plus agonistic, anti-4-1BB antibody therapy eradicates aggressive mouse AML-ETO9a and MLL-AF9 tumors. Tumor clearance was associated with increased CD8 + T cell numbers and IFN-γ secreting TEM and TCM cells. These findings are consistent with previous reports indicating that 4-1BB stimulation potentiates various vaccine regimens such as HPV E7 DNA vaccines for TC-1 tumors,51 Trp2 peptide vaccines in combination with CpG-oligodeoxynucleotides (TLR9 agonist) for B16 melanoma,52 DC vaccines for metastatic CT26 colon cancer,53 and tumor cell vaccines for preclinical models of lymphoma.5,10 In these studies, combination therapies showed that CD8 + T cell expansion, IFN-γ production and CTL activity were important for tumor regression. Similarly, we show here that adoptive transfer of TEM to AML-ETO9a tumor-bearing mice, leads to growth inhibition and eradication of established tumors in 75% of mice. We found that effective TEM resided mostly within the KLRG1+ TEM pool.
We have demonstrated that NK cells progressively decline following anti-4-1BB therapy in AML, and previously in B-cell lymphoma.10 Similar observations have been made by other researchers in CT26 colon cancer, renal cancer (RENCA), EL4 thymoma and MC38 adenocarcinoma.10,46 According to Choi et al., 2010, the decline of NK cells appears to be linked to 4-1BB signalling on peripheral NK cells and CD8 + T cells resulting in IFN-γ production that suppresses NK cell development in the bone marrow.46 This decline in NK cells and an increase in CD8 + T cells following therapy, indicates that CD8 + T cells may play a dominant role in long-term protection against AML. Several lines of evidence support the protective role of CD8 + T cells against haematological and solid tumors.5,10,11,14
While vaccine or anti-4-1BB alone were partly protective, anti-PD-1 treatment alone was completely ineffective against AML. Interestingly vaccine, anti-4-1BB alone or in combination with anti-PD-1, promoted AML tumor clearance (responders) in some mice, but tumor grew progressively in others (non-responders). While the exact effector mechanism leading to responders and non-responders following this immunotherapy remains to be investigated, the lack of tumor regression in a proportion of mice may be attributed to poorly immunogenic or immune resistant leukemic cell clones that escape immune attack. Similar observations of responding and non-responding mice to anti-4-1BB therapy have been reported in murine models of hepatocellular carcinoma.54 In the current study, we observed increased PD-1 expressing CD8 + T cells, and PDL-1 expression on monocytes in response to anti-4-1BB. These markers are associated with immune exhaustion.27 This implies that, although anti-4-1BB alone increased CD8 + T cells and effector memory CD8 + T cells in the bone marrow and spleen, the effectiveness of such an immune response could be hindered by subsequent immune exhaustion. The role of PD-1/PDL-1 axis in impeding immune responses in AML has been extensively reviewed.28 Despite this evidence, the addition of anti-PD-1 to anti-4-1BB treatment did not greatly enhance the suppression of established AML. In fact, our evidence suggests that anti-PD-1 may negate the beneficial effects of anti-4-1BB if anti-PD-1 administration is delayed. The anti-4-1BB/anti-PD-1 combination was more effective if treatment was commenced with minimal tumor load, suggesting that this therapy may be more important in consolidation therapy, following debulking by chemotherapy.
In demonstrating the importance of IFN-γ in tumor control, we show that WT mice were completely protected from AML with vaccine and anti-4-1BB combination. Interestingly, the therapeutic efficacy of both combination treatments was abrogated in IFN-γKO or IFN-γRKO mice, suggesting that IFN-γ/IFN-γR signalling is important in therapy-induced tumor suppression. These results indicate that host IFN-γR signalling is required for protection, and IFN-γ signalling in tumor cells does not overtly affect their growth. However, some enhanced survival was observed in IFN-γKO mice that was not seen in IFN-γRKO mice, suggesting that multiple signalling mechanisms mediated via the IFN-γR pathway are responsible for tumor suppression. While some of these pathways are mediated via the IFN-γ directly, additional mechanisms feed into the downstream IFN-γR signalling cascade. Crosstalk between the IFN-γ and IL-6-mediated mechanisms, have been widely studied.55 IFN-γR signaling activates transcription factor STAT1 and JAK, inducing target gene expression. When IL-6 engages the glycoprotein 30 (gp30) receptor, JAK/STAT3 and mitogen activated protein kinase (MAPK) cascades are stimulated.49,55 This leads to the formation of STAT1/STAT3 heterodimer complexes from the two pathways, that translocate to the nucleus causing target gene transcription,48 and stimulation of pathways that may be protective against AML. These warrant further investigation.
Moreover, when the vaccine and anti-4-1BB combination were tested on a more aggressive type of leukemia, MLL-AF9, 100% of the mice were protected. MLL is notoriously difficult to treat in humans in part because of its aggressiveness that makes the initial management very challenging, but also because of the patient’s vulnerability to complications, toxicities and relapses post therapy.56 Our therapeutic strategy offers a new promising effective therapy for this subtype of leukemia.
In summary, this study unveils a combination immunotherapeutic strategy that confers full protection against two aggressive preclinical models of leukemia, AML-ETO9a and MLL-AF9. Harnessing the immune adjuvant properties of NKT cells through vaccination is an effective approach to elicit anti-AML immunity. Potentiation of therapeutic vaccination by anti-4-1BB resulted in enhancement of CD8 + T cell expansion, differentiation and IFN-γ production. This immune response was associated with total tumor clearance in all affected organs (blood, bone marrow and spleen) of tumor-challenged mice.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding Statement
This work was supported by Project Grant (APP1044355) from the National Health and Medical Research Council (NHMRC) of Australia. TK and M.D.N were supported by an Australian Government Research Training Program (RTP) Scholarship. PL was supported by a University of Queensland International Scholarship. S.R.M. was supported by an NHMRC Career Development Fellowship (APP1061429).
Acknowledgments
The authors acknowledge the Translational Research Institute (TRI) for providing an excellent research environment and core facilities that enabled this research to be conducted. We particularly thank Brian Tse and Kamil Sokolowski (Preclinical Imaging Facility), Alistair Zealey, Karen Knox and Rodrick Rupan (Biological Resources Facility) for mouse husbandry and technical assistance, David Sester, Dalia Khalil and Yitian Ding (Flow Cytometry Facility) for technical assistance, and Ricky Johnstone (Peter MacCallum Cancer Centre) for originally providing the AML-ET09a and MLL-AF9 tumor cells used for this study.
Author contributions
DK designed and conducted the experiments, data analysis and writing of the paper. MSFS and BLD designed and performed experiments. TK, MDN and PL assisted with experiments and writing of the paper. GRL provided intellectual input into the study and helped with manuscript preparation. SRM conceived and designed the study, supervised and directed the experiments, assisted with data analysis and wrote the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Zhou H, Xu R.. Leukemia stem cells: the root of chronic myeloid leukemia. Protein Cell 2015; 6:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H. Acute myeloid leukemia--major progress over four decades and glimpses into the future. Am J Hematol 2016; 91:131–45. [DOI] [PubMed] [Google Scholar]
- 3.Hanekamp D, Denys B, Kaspers GJL, Te Marvelde JG, Schuurhuis GJ, De Haas V, et al. Leukaemic stem cell load at diagnosis predicts the development of relapse in young acute myeloid leukaemia patients. Br J Haematol 2017. [DOI] [PubMed] [Google Scholar]
- 4.Cooper SL, Brown PA.. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am 2015; 62:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee SJ, Doff BL, Soon MS, Mattarollo SR.. Therapeutic Efficacy of 4-1BB Costimulation Is Abrogated by PD-1 Blockade in a Model of Spontaneous B-cell Lymphoma. Cancer Immunol Res 2017; 5:191–7. [DOI] [PubMed] [Google Scholar]
- 6.Assi R, Kantarjian H, Ravandi F, Daver N.. Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors. Curr Opin Hematol 2017. [DOI] [PubMed] [Google Scholar]
- 7.Vick E, Mahadevan D.. Programming the immune checkpoint to treat hematologic malignancies. Expert Opin Investig Drugs 2016; 25:755–70. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Drake CG, Pardoll DM.. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X.. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 2015; 21:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi T, Doff BL, Rearden RC, Leggatt GR, Mattarollo SR.. NKT cell-targeted vaccination plus anti-4-1BB antibody generates persistent CD8 T cell immunity against B cell lymphoma. Oncoimmunology 2015; 4:e990793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattarollo SR, Steegh K, Li M, Duret H, Foong Ngiow S, Smyth MJ.. Transient Foxp3(+) regulatory T-cell depletion enhances therapeutic anticancer vaccination targeting the immune-stimulatory properties of NKT cells. Immunol Cell Biol 2013; 91:105–14. [DOI] [PubMed] [Google Scholar]
- 12.Li SY, Liu Y.. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin Pharmacol 2013; 5:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinay DS, Kwon BS.. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther 2012; 11:1062–70. [DOI] [PubMed] [Google Scholar]
- 14.Mattarollo SR, West AC, Steegh K, Duret H, Paget C, Martin B, et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood 2012; 120:3019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan CJ, Smyth MJ, Martinet L.. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ 2014; 21:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia 2015; 29:1143–52. [DOI] [PubMed] [Google Scholar]
- 17.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017; 17:286–301. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenegger FS, Krupka C, Haubner S, Kohnke T, Subklewe M.. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol 2017; 10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbins JD, Ancelet LR, Weinkove R, Compton BJ, Painter GF, Petersen TR, et al. An autologous leukemia cell vaccine prevents murine acute leukemia relapse after cytarabine treatment. Blood 2014; 124:2953–63. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S.. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med 2007; 204:2641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunn MK, Farrand KJ, Broadley KW, Weinkove R, Ferguson P, Miller RJ, et al. Vaccination with irradiated tumor cells pulsed with an adjuvant that stimulates NKT cells is an effective treatment for glioma. Clin Cancer Res 2012; 18:6446–59. [DOI] [PubMed] [Google Scholar]
- 22.Najera Chuc AE, Cervantes LA, Retiguin FP, Ojeda JV, Maldonado ER.. Low number of invariant NKT cells is associated with poor survival in acute myeloid leukemia. J Cancer Res Clin Oncol 2012; 138:1427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Taube JM, Anders RA, Pardoll DM.. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009; 229:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, et al. A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1. Cancer Res 2015; 75:3800–11. [DOI] [PubMed] [Google Scholar]
- 27.Skalniak L, Zak KM, Guzik K, Magiera K, Musielak B, Pachota M, et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 2017; 8:72167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehgal A, Whiteside TL, Boyiadzis M.. Programmed death-1 checkpoint blockade in acute myeloid leukemia. Expert Opin Biol Ther 2015; 15:1191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer 2014; 2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget 2013; 4:2067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960–4. [DOI] [PubMed] [Google Scholar]
- 32.Daskivich TJ, Belldegrun A.. Words of wisdom. Re: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. Eur Urol 2015; 67:816–7. [DOI] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taneja SS. Re: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. J Urol 2012; 188:2149. [PubMed] [Google Scholar]
- 35.Haroun F, Solola SA, Nassereddine S, Tabbara I.. PD-1 signaling and inhibition in AML and MDS. Ann Hematol 2017; 96:1441–8. [DOI] [PubMed] [Google Scholar]
- 36.Choi DC, Tremblay D, Iancu-Rubin C, Mascarenhas J.. Programmed cell death-1 pathway inhibition in myeloid malignancies: implications for myeloproliferative neoplasms. Ann Hematol 2017; 96:919–27. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Flies DB.. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013; 13:227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Lin GH, McPherson AJ, Watts TH.. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009; 229:192–215. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE.. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res 2003; 63:2546–52. [PubMed] [Google Scholar]
- 40.Houtenbos I, Westers TM, Dijkhuis A, de Gruijl TD, Ossenkoppele GJ, van de Loosdrecht AA.. Leukemia-specific T-cell reactivity induced by leukemic dendritic cells is augmented by 4-1BB targeting. Clin Cancer Res 2007; 13:307–15. [DOI] [PubMed] [Google Scholar]
- 41.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 1997; 3:682–5. [DOI] [PubMed] [Google Scholar]
- 42.Strome SE, Martin B, Flies D, Tamada K, Chapoval AI, Sargent DJ, et al. Enhanced therapeutic potential of adoptive immunotherapy by in vitro CD28/4-1BB costimulation of tumor-reactive T cells against a poorly immunogenic, major histocompatibility complex class I-negative A9P melanoma. J Immunother 2000; 23:430–7. [DOI] [PubMed] [Google Scholar]
- 43.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rearden R, Sah A, Doff B, Kobayashi T, McKee SJ, Leggatt GR, et al. Control of B-cell lymphoma by therapeutic vaccination and acquisition of immune resistance is independent of direct tumour IFN-gamma signalling. Immunol Cell Biol 2016; 94:554–62. [DOI] [PubMed] [Google Scholar]
- 45.Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, et al. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol 2011; 29:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi BK, Kim YH, Kim CH, Kim MS, Kim KH, Oh HS, et al. Peripheral 4-1BB signaling negatively regulates NK cell development through IFN-gamma. Journal of immunology (Baltimore, Md : 1950) 2010; 185:1404–11. [DOI] [PubMed] [Google Scholar]
- 47.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017; 114:4993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi YF, Huang YX, Wang HY, Zhang Y, Bao YL, Sun LG, et al. Elucidating the crosstalk mechanism between IFN-gamma and IL-6 via mathematical modelling. BMC Bioinformatics 2013; 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eulenfeld R, Dittrich A, Khouri C, Muller PJ, Mutze B, Wolf A, et al. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol 2012; 91:486–95. [DOI] [PubMed] [Google Scholar]
- 50.Marschalek R. Systematic Classification of Mixed-Lineage Leukemia Fusion Partners Predicts Additional Cancer Pathways. Ann Lab Med 2016; 36:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H, Kwon B, Sin JI.. Combined stimulation of IL-2 and 4-1BB receptors augments the antitumor activity of E7 DNA vaccines by increasing Ag-specific CTL responses. PLoS One 2013; 8:e83765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sin JI, Kim H, Ahn E, Jeon YH, Park WS, Lee SY, et al. Combined stimulation of TLR9 and 4.1BB augments Trp2 peptide vaccine-mediated melanoma rejection by increasing Ag-specific CTL activity and infiltration into tumor sites. Cancer Lett 2013; 330:190–9. [DOI] [PubMed] [Google Scholar]
- 53.Lee H, Park HJ, Sohn HJ, Kim JM, Kim SJ.. Combinatorial therapy for liver metastatic colon cancer: dendritic cell vaccine and low-dose agonistic anti-4-1BB antibody co-stimulatory signal. J Surg Res 2011; 169:e43–50. [DOI] [PubMed] [Google Scholar]
- 54.Gauttier V, Judor JP, Le Guen V, Cany J, Ferry N, Conchon S.. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer 2014; 135:2857–67. [DOI] [PubMed] [Google Scholar]
- 55.Regis G, Pensa S, Boselli D, Novelli F, Poli V.. Ups and downs: the STAT1: STAT3seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol 2008; 19:351–9. [DOI] [PubMed] [Google Scholar]
- 56.Brown P. Treatment of infant leukemias: challenge and promise. Hematology Am Soc Hematol Educ Program 2013; 2013:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


