ABSTRACT
Radiotherapy can induce toxicity in healthy tissues such as radiation-induced fibrosis (RIF), and macrophages are proposed as new profibrogenic cells. In this Point-of-View, we summarize the role of the immune response in ionizing radiation injury, and we focus on macrophages as a new therapeutic target in RIF.
Keywords: Radiation injury, macrophages, immune system, radiation toxicity, lung fibrosis
Introduction
While antitumor immune response to radiotherapy (RT) has currently been broadly investigated, the precise impact of the immune component on radiation-induced healthy tissue toxicity remains poorly understood. Recent insights regarding the functional role of inflammatory cells suggest that inflammation could play a role beyond the classical “acute” phase. The initial effect of gamma irradiation (IR) on tissues is the production of reactive oxygen and nitrogen species (ROS and NOS, respectively) that cause damage to DNA, proteins and lipids leading to cell injury or death.1 Upon IR, the secretion of growth factors is stimulated to promote cell growth, and the wound repair process is activated to allow removal/renewal of dead cells.2 Pioneer studies conducted by Rubin and Casarett (1968)3, Travis (1980)4 and Fajardo (1982)5 showed that tissue response to IR is characterized by an acute phase involving the recruitment of inflammatory cells at the site of injury. After a period of latency, a late phase characterized by a chronic inflammation and chronic tissue injury (i.e., fibrosis) follows. During the radiation wound repair process, recruitment of inflammatory cells occurs at the site of injury, which can contribute to late tissue damage.6–8 Here, we discuss in more detail the immune system/macrophage responses to radiotherapy and their involvement in the development of radiation injury.
Immediate immunological consequences of IR
Interestingly, IR is able to induce an immune response that is displaced in time but also in space (i.e., abscopal effect). It has been reported that IR can induce inflammation in nonirradiated sites in vivo.5,9 IR can also induce inflammatory bystander signals in vitro.10,11 Bystander factors are produced by irradiated cells and can activate nonirradiated cells.9 It has been reported that the initial response to RT involves the production of damaged-associated molecular patterns (DAMPs) from irradiated cells.12 Associated to immunogenic cell death,13 DAMPs production is followed by a cascade of proinflammatory cytokine secretion leading to the initiation of inflammation.12 Several studies have described the secretion of inflammatory cytokines after IR in vivo.4,8 Inflammatory cytokines produced in vivo by irradiated cells could act as bystander factors to induce the abscopal effect. Interestingly, macrophages have been proposed as an important source of bystander signals in vivo.9
Macrophage response to radiotherapy
Macrophages as innate immune cells are recruited at sites of injury where they can perform their phagocytic function.14 Accordingly, after IR, macrophages are recruited at the irradiated site.4,8 The direct effect of IR on macrophages remains poorly explored.15 Mukherji et al. proposed that macrophage activation is a secondary effect of IR, which results from cellular damage signals and the clearance of radiation-induced apoptotic cells, rather than a direct effect of IR.9 Accordingly, in our recent study, we showed that lung alveolar macrophages (AM) that are newly recruited to the lungs after the depletion induced by thoracic IR exposure are activated, as they present an increase in both the expression of Arg1, CD206 and Icam1.8 This result suggests that these AMs, which have not been directly exposed to IR, are activated by the existing signals rather than directly by IR. However, several in vitro studies underscore the direct effect of IR in macrophage activation.16–18 After in vitro cumulative ionizing radiation doses (2Gy/fraction/day), human monocyte-derived macrophages remain viable despite DNA damages, with an active metabolism. Cumulative doses of 10Gy increased proinflammatory, and decreased anti-inflammatory markers in macrophages.19 In vivo, it is rather difficult to assess the direct effect of IR on macrophage activation. However, recent studies in the tumor stroma showed that high doses of IR (> 8Gy) promote anti-inflammatory activation of macrophages,20 and low doses (< 2Gy) combined with immunotherapy induce proinflammatory activation of macrophages that favor tumor elimination.21 These data suggest that there is a direct effect of IR on macrophage activation. Upon activation, macrophages produce bystander signals9 and play an important role in the development of radiation injury.8 Interestingly, RT can strongly affect the activated phenotypes of macrophages and thus shape the macrophage response and role. Rubin and colleagues have shown that isolated AMs from lungs of mice that were irradiated (17.5 Gy) in vivo produce large amounts of TGFα and TGFβ, suggesting that in response to a high dose of IR, resident and recruited macrophages were stimulated to produce profibrotic growth factors that would induce the differentiation of fibroblasts into secretory myofibroblasts.22 Accordingly, we have shown that high doses of IR (16Gy) increase the level of Arg1+ lung interstistial macrophages (IMs), which are able to induce in vitro activation of fibroblasts.8 Interestingly, RT induces apoptosis in cancer cells,23 and apoptosis is known to drive immunosuppressive macrophages,24 which are characterized by the production of anti-inflammatory cytokines such as TGFβ1, a powerful fibrogenic factor. The excessive production of fibrogenic factors induces an unbalance in wound healing causing tissue toxicity.25
Immune response to radiotherapy and lung radiation injury
Inflammation constitutes an important step in the process of repair of IR-induced wounds.6–8 Tissue response to IR can be dichotomized into two important phases, acute and late. In the lung, as a paradigmatic organ affected by radiation toxicity, during the acute phase, inflammatory cells are mobilized into irradiated sites (Figure 1).8 An in-depth exploration of acute phase is necessary to better understand how IR modulates the immune system within few hours/days. Interestingly, inflammation occurs within parenchyma and then within alveoli. Whether this is a simple spatial displacement of inflammatory response or a differential response between to different compartments is not known. In the first case, inflammatory cells would cross the epithelial barrier, while in the second, an immune response within parenchyma would be totally independent of that within alveoli. It has recently become evident that the immune system also plays a role in the late phases of IR-induced toxicities. We (and other colleagues) have recently shown a key role for macrophages in radiation-induced lung fibrosis.7,8 One of the important questions that was asked and that persists is whether the acute inflammation is at the origin of the late chronic inflammation/tissue injury. Interestingly, the late inflammation/injury arises after a latent period during which nothing occurs at the clinical level suggesting that this period is more infraclinical rather than an inert phase.
Figure 1.
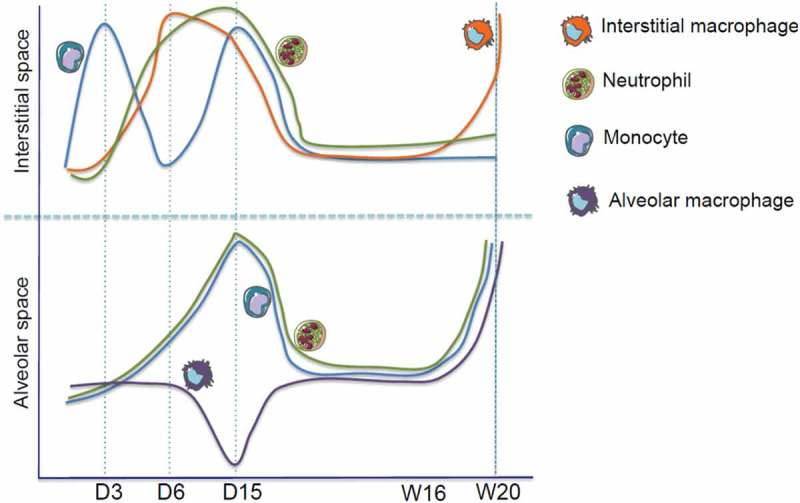
Dynamic changes in myeloid cells in the lung after IR. Upper panel: a wave of monocytes is rapidly (3 days post IR, D3) recruited to lung parenchyma, followed by a second wave at day 15 (D15). Interstitial macrophages peak at 6 days (D6) post IR and, after a latent phase, they increase again starting from 16 weeks (W16) after IR. Neutrophil levels are increased at D6-15 before returning to basal. Lower panel: while levels of alveolar neutrophils and monocytes increase at D15 post IR, at this time point, alveolar macrophages are depleted. The levels of the three populations increase again starting from W16.
Macrophages involvement in radiation-induced injury
The Travis group in the 1980s described macrophage infiltration in air space with mononuclear inflammatory cells in alveolar walls as the criterion of acute injury phase. Furthermore, alveolitis was described as one hallmark of radiation-induced fibrosis (RIF) in the lung.4 Accordingly, recent studies have confirmed earlier data reporting macrophage infiltration within the site of the radiation-induced toxicity in the heart26 and in the lung.7,8 Scientists have long wondered whether the recruitment of macrophages is the cause or the consequence of the development of the RIF. The Huber group showed that CTGF inhibitor protects against radiation-induced fibrogenesis via normalization of the expression of genes that is associated with anti-inflammatory macrophage influx.7 We recently showed that radiation-induced fibrosis is associated with a large increase in Arg1 mRNA (an anti-inflammatory marker) in IMs with an increase in membrane expression of CD206 suggesting that IMs adopt an anti-inflammatory profile during radiation-induced lung toxicity.8 It seems that anti-inflammatory macrophages are more radio-resistant than proinflammatory macrophages.27 Our study clearly showed that interstitial and alveolar macrophages were modulated differently during the acute phase of IR response (Figure 1); interestingly, 15 days post-IR AMs were depleted but not IMs, suggesting that IMs are more radio-resistant than AMs.8 Furthermore, IMs are profibrosant but not AMs. The radio-resistance of IMs and the ability of IR to induce an anti-inflammatory profile in IMs favor fibrogenesis. Indeed, a macrophage has been reported as the one of the most radio-resistant cell in humans due to its substantial production of antioxidant molecules such as manganese superoxide dismutase (MnSOD), a scavenger of superoxide ions (O−2).28 This scenario could explain the major role of macrophages in the development of IR-induced tissue injury. Therefore, targeting macrophages to prevent or treat IR-induced tissue toxicity becomes a new therapeutic strategy. Our group has shown that the specific depletion of profibrogenic anti-inflammatory IMs but not AMs using a monoclonal antibody against colony stimulating factor 1 receptor (CSF1R) protect against IR-induced lung fibrosis. Targeting the CCL2/CCR2 axis to inhibit the migration of monocytes into the irradiated site could also constitute a potential strategy to protect against IR-induced tissue toxicity, including lung fibrosis.29 However, it is important to get a clear understanding of the involvement of resident and recruited macrophages in the development of tissue toxicity to optimize such potential therapeutic intervention. Another therapeutic strategy is represented by macrophage reprogramming and immune system reeducation. In tumor models, macrophage reprograming using a low dose of IR resulted in an antitumor activity.21 Accordingly, in IR-induced tissue toxicity, the use of a proinflammatory agent could be a potential strategy to reprogram macrophage polarization and switch the profibrotic profile of macrophages to a more proinflammatory, antifibrotic one.
Conclusions
Macrophage as an immune cell plays an important role in pathophysiological tissue response to radiotherapy, and it represents a novel profibrogenic actor that plays an important role during radiation-induced normal tissue toxicity. Radiation-induced normal tissue toxicity is a complex response, which depends on the microenvironment, on the dose and fractionation of IR, on the target site and volume irradiated, on concomitant or sequential treatments (chemo and/or immunotherapy). Each of these parameters and their interplay is very complex and still needs to be completely understood. Within the microenvironment, macrophages respond differently to radiotherapy in normal tissue depending on both time and IR/fractionation dose, and a complete understanding of their response to IR has yet to be achieved. Nevertheless, macrophages appear as a promising therapeutic target for the prevention or the treatment of radiation-induced toxicities. Immunomodulation can thus represent not only a groundbreaking approach to increase the antitumor efficacy of radiotherapy, but also to limit its side effects. We anticipate that the radiotherapy community will put substantial research efforts in this field and that significant advances in the management of radiation toxicities will be achieved in the next years.
Funding Statement
This work was supported by the Eli Lilly and Company;INCA [2014-1-PL BIO-03];INSERM;SIRIC SOCRATE;
Acknowledgments
The Molecular Radiotherapy Unit of Gustave Roussy is supported by INSERM and SIRIC SOCRATE. LM is supported by an Inca 2014-1-PL BIO-03 fellowship, MM and ED are grant recipients from Eli Lilly.
Disclosure statement
MM and ED are recipients of a grant from Eli Lilly concerning the use of anti-CSF1R to limit radiation-induced fibrosis.
References
- 1.Zhao W, Robbins ME.. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–143. PubMed PMID: 19149566. [DOI] [PubMed] [Google Scholar]
- 2.Trott KR, Herrmann T, Kasper M. Target cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys. 2004;58(2):463–469. PubMed PMID: 14751516. [DOI] [PubMed] [Google Scholar]
- 3.Rubin P, Casarett GW. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 1968;22(4):767–778. PubMed PMID: 5212301. [DOI] [PubMed] [Google Scholar]
- 4.Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 1980;6(3):345–347. PubMed PMID: 7390907. [DOI] [PubMed] [Google Scholar]
- 5.Fajardo LF. Pathology of radiation injury. Masson Pub: USA; 1982. [Google Scholar]
- 6.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature Reviews Cancer. 2006;6(9):702–713. PubMed PMID: 16929324. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 7.Bickelhaupt S, Erbel C, Timke TC, Wirkner U, Dadrich M, Flechsig P, Tietz A, Pfohler J, Gross W, Peschke P, et al. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J Natl Cancer Inst. 2017;109(8). PubMed PMID: 28376190. doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 8.Meziani L, Mondini M, Petit B, Boissonnas A, Thomas de Montpreville V, Mercier O, Vozenin MC, Deutsch E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur Respir J. 2018;51(3). PubMed PMID: 29496785. doi: 10.1183/13993003.02120-2017. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee D, Coates PJ, Lorimore SA, Wright EG. Responses to ionizing radiation mediated by inflammatory mechanisms. J Pathol. 2014;232(3):289–299. PubMed PMID: 24254983. doi: 10.1002/path.4299. [DOI] [PubMed] [Google Scholar]
- 10.Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res. 2011;176(2):139–157. PubMed PMID: 21631286. [DOI] [PubMed] [Google Scholar]
- 11.Wright EG. Manifestations and mechanisms of non-targeted effects of ionizing radiation. Mutat Res. 2010;687(1–2):28–33. PubMed PMID: 20080112. doi: 10.1016/j.mrfmmm.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: anticancer radioimmunotherapy. Oncoimmunology. 2013;2(9):e25595 PubMed PMID: 24319634; PubMed Central PMCID: PMC3850274. doi: 10.4161/onci.25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. PubMed PMID: 17979839. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. PubMed PMID: 22772564; PubMed Central PMCID: PMC3405917. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi X, Shiao SL. The role of macrophage phenotype in regulating the response to radiation therapy. Translational Research: the Journal of Laboratory and Clinical Medicine. 2018;191:64–80. PubMed PMID: 29175267. doi: 10.1016/j.trsl.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi G, Sodhi A. In vitro activation of murine peritoneal macrophages by ultraviolet B radiation: upregulation of CD18, production of NO, proinflammatory cytokines and a signal transduction pathway. Mol Immunol. 2004;40(18):1315–1323. PubMed PMID: 15072850. doi: 10.1016/j.molimm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Sodhi A, Sethi G. Involvement of MAP kinase signal transduction pathway in UVB-induced activation of macrophages in vitro. Immunol Lett. 2003;90(2–3):123–130. PubMed PMID: 14687713. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Allouch A, Paoletti A, Leteur C, Mirjolet C, Martins I, Voisin L, Law F, Dakhli H, Mintet E, et al. NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy. Cell Death Differ. 2017;24(9):1632–1644. PubMed PMID: 28574504; PubMed Central PMCID: PMC5563995. doi: 10.1038/cdd.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teresa Pinto A, Laranjeiro Pinto M, Patricia Cardoso A, Monteiro C, Teixeira Pinto M, Filipe MA, Castro P, Figueira R, Monteiro A, Marques M, et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep. 2016;6:18765 PubMed PMID: 26735768; PubMed Central PMCID: PMC4702523. doi: 10.1038/srep18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, Weichselbaum RR. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70(4):1534–1543. PubMed PMID: 20145121. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. PubMed PMID: 24209604. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophage and the septal fibroblast. Int J Radiat Oncol Biol Phys. 1992;24(1):93–101. PubMed PMID: 1512168. [DOI] [PubMed] [Google Scholar]
- 23.Muschel RJ, Soto DE, McKenna WG, Bernhard EJ. Radiosensitization and apoptosis. Oncogene. 1998;17(25):3359–3363. PubMed PMID: 9916998. doi: 10.1038/sj.onc.1202580. [DOI] [PubMed] [Google Scholar]
- 24.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26(4):653–656. PubMed PMID: 10047800. [DOI] [PubMed] [Google Scholar]
- 25.Rieder F, Brenmoehl J, Leeb S, Scholmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56(1):130–139. PubMed PMID: 17172588; PubMed Central PMCID: PMC1856649. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monceau V, Meziani L, Strup-Perrot C, Morel E, Schmidt M, Haagen J, Escoubet B, Dorr W, Vozenin MC. Enhanced sensitivity to low dose irradiation of ApoE-/- mice mediated by early pro-inflammatory profile and delayed activation of the TGFbeta1 cascade involved in fibrogenesis. PloS One. 2013;8(2):e57052 PubMed PMID: 23451141; PubMed Central PMCID: PMC3579799. doi: 10.1371/journal.pone.0057052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leblond MM, Peres EA, Helaine C, Gerault AN, Moulin D, Anfray C, Divoux D, Petit E, Bernaudin M, Valable S. M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget. 2017;8(42):72597–72612. PubMed PMID: 29069812; PubMed Central PMCID: PMC5641155. doi: 10.18632/oncotarget.19994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828 PubMed PMID: 28769933; PubMed Central PMCID: PMC5509958. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groves AM, Johnston CJ, Williams JP, Finkelstein JN. Role of infiltrating monocytes in the development of radiation-induced pulmonary fibrosis. Radiat Res. 2018;189(3):300–311. PubMed PMID: 29332538; PubMed Central PMCID: PMC5862390. doi: 10.1667/RR14874.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


