ABSTRACT
The objective response rate (ORR) of tyrosine kinase inhibitors (TKIs) therapy in metastatic renal cell cancer (mRCC) patients was not satisfactory. Effective indicator of mRCC patient selection for TKI therapy is urgently needed. The function of tumor infiltrating B lymphocytes (TIBs) in tumor immune elimination is still unclear. We aim to investigate the prognostic and predictive value of TIBs for TKI therapy in mRCC patients in this study. 108 eligible patients treated with TKI were enrolled in this study. TIBs was estimated by immunohistochemical staining of CD19 in the resected tumor, and its relationship with clinicopathological features, clinical outcomes and CD8+ tumor infiltrating T lymphocytes (CD8+ TILs) were evaluated. Associations between the expression level of CD19 and CD8+ TILs associated cytotoxic effectors were also assessed in public databases. Results showed TIBs positive infiltration predicted better therapeutic response to sunitinib (p = 0.006), longer overall survival (OS) (p < 0.001) and progression-free survival (PFS) (p = 0.028) in mRCC patients. Combining TIBs and International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) model showed a better predict value of OS in TKI-treated mRCC patients than IMDC model alone. We also found a positive correlation between TIBs and CD8+ TILs (p < 0.001). Patients with both cells high infiltration showed markedly better OS compared with those infiltrated by CD8+ T cells alone (p = 0.015). To conclude, TIBs density was not only an independent prognostic factor for mRCC patients, but also a predictive marker for TKI therapy response. It may potently enhance the antitumor effect by recruiting and activating CD8+ TILs in mRCC.
KEYWORDS: tumor infiltrating B lymphocyte, renal cell carcinoma, tyrosine kinase inhibitor, biomarkers, survival
Introduction
Renal cell carcinoma (RCC) is a common type of cancer worldwide.1,2 The prognosis of RCC patients significantly deteriorated if the disease turn into metastatic renal cell carcinoma (mRCC)3. Tyrosine kinase inhibitors (TKIs) such as sunitinib and sorafenib have been approved for usage in clinic, which have significantly improved the prognosis of patients with mRCC.4-6 Several new tools have been developed to improve the clinical outcome prediction for mRCC patients. Based on the Memorial Sloan-Kettering Cancer Center (MSKCC) model, Heng et al established the IMDC model for mRCC patients treated with vascular endothelial growth factor (VEGF) targeted agents and only showed limited improvements compared with the former.7,8 This indicated that a ceiling had been reached in predicting prognosis based solely on clinical factors,which suggested tumor-specific biomarkers should be added to achieve better prediction.
In fact, relatively small proportion of patients will respond to TKI therapy.9 However, there is no widely accepted effective indicator of patient selection for TKI therapy. In several studies, the immune-regulating effect of TKIs has been established.10-12 These results all implied the tumor immune microenvironment played a pivotal role in TKI anti-tumor process. Thus, tumor-specific immune signature may affect TKI therapeutic efficacy and therefore be an ideal indicator to improve patient selection for TKI therapy.
Compared with the strongly focused T lymphocytes, B cells are often overlooked in tumor immunology research, which actually are critical for human adaptive immune system. The role of tumor infiltrating B cells (TIBs) in the host immune response upon malignancy is complex and somewhat controversial.13,14 On one hand, TIBs work as antigen-presenting cells (APC), anti-tumor cytokine producer and even direct tumor killer to limit tumor progression. On the other hand, it performs immunosuppressive functions by secreting IL-10, TGF-β and IL-35. Therefore, the ultimate effect of TIBs in mRCC patients need to be clarified.
Several studies have showed that TIBs could influence tumor immunity and further affect tumor treatment.15,16 B cells can present antigens to CD8+ T cells, activate and promote CD8+ T cell proliferation.17-19 In high-grade serous ovarian tumors, TIBs colocalized with CD8+ TIL and was associated with prolonged survival.20 While, in an EMT-6 mammary tumor murine model, extensive B-cell infiltration into the tumor bed decreased CD8+ T cell infiltration and markedly reduced cytotoxic T-cell response.21 Considering CD8+ T cells play a central role in adaptive immunity against tumor, the relation between TIBs and CD8+ TILs in mRCC patients need to be ascertained.
In this study, we identified TIBs in mRCC patients treated with sunitinib or sorafenib and assess the prognostic value of TIBs in these patients. Besides, the relationship between TIBs and CD8+ T cells in mRCC patients would be explored.
Results
Patient characteristics and tibs status
The major characteristics of these 108 patients were listed in Table 1. The cohort included 76 males and 32 females. The median age at first TKI medication was 60 years old (14–78 years old), and the median follow-up was 23.35 months (1.1–92.6 months). 58 (53.7%) patients were diagnosed with TNM stage IV RCC at the initial diagnosis. For pathology, 87 patients (80.6%) were clear cell subtype. According to the IMDC risk stratification model criteria, the number of patients in favorable, intermediate and poor risk group is 23 (21.3%), 58 (53.7%) and 27 (25%), respectively. 73 (67.6%) patients were treated with sunitinib as first-line systemic therapy, while 35 (32.4%) patients took sorafenib.
Table 1.
Clinical characteristics of patients according to CD19+ TIBs number.
| patients |
TIBs |
||||
|---|---|---|---|---|---|
| Characteristics | n | % | Negative | Positive | p |
| All patients | 108 | 100 | 77 | 31 | |
| Age* | 0.523† | ||||
| ≤ 59 | 54 | 50 | 40 | 14 | |
| >59 | 54 | 50 | 37 | 17 | |
| Gender | 0.931† | ||||
| Female | 32 | 29.6 | 23 | 9 | |
| Male | 76 | 70.4 | 54 | 22 | |
| Tumor size** | 0.911† | ||||
| ≤ 4 cm | 18 | 16.7 | 13 | 5 | |
| > 4 and ≤ 7 cm | 48 | 44.4 | 34 | 14 | |
| > 7 and ≤ 10 cm | 28 | 25.9 | 21 | 7 | |
| > 10 cm | 14 | 13 | 9 | 5 | |
| Histology | 0.581† | ||||
| Clear cell | 87 | 80.6 | 61 | 26 | |
| Non-clear cell | 21 | 19.4 | 16 | 5 | |
| Initial TNM stage | 0.259† | ||||
| I–III | 50 | 46.3 | 33 | 17 | |
| IV | 58 | 53.7 | 44 | 14 | |
| Fuhrman (missing=6) | 0.870‡ | ||||
| 1 | 2 | 1.9 | 1 | 1 | |
| 2 | 52 | 49.1 | 38 | 14 | |
| 3 | 41 | 38.6 | 28 | 13 | |
| 4 | 7 | 6.6 | 5 | 2 | |
| IMDC risk stratification | 0.006† | ||||
| Favorable risk | 23 | 21.3 | 12 | 11 | |
| Intermediate risk | 58 | 53.7 | 40 | 18 | |
| Poor risk | 27 | 25 | 25 | 2 | |
| Number of metastatic organs | 0.206† | ||||
| 1 | 74 | 68.5 | 50 | 24 | |
| ≥2 | 34 | 31.5 | 27 | 7 | |
| Systemic therapy | 0.022† | ||||
| Sunitinib | 73 | 67.6 | 47 | 26 | |
| Sorafenib | 35 | 32.4 | 30 | 5 | |
*Age at initiation of systemic therapy, split at median; **determined after radical or palliative nephrectomy; †Data obtained from χ2 test,‡Data obtained from Fisher’s exact test, P-value<0.05 was regarded as statistically significant.
TIBs infiltration was evaluated by IHC staining on primary RCC specimens by using CD19 antibody. CD19 staining was mainly distributed in the membrane of TIBs (Figure 1A). The density of CD19+ TIBs varied in mRCC samples (Figure 1B). According to the grouping criterion described above, 31 patients (28.7%) were in TIBs positive group and 77 (71.3%) were in negative. TIBs status has no association with tumor size, histological subtype, TNM stage, tumor nucleus grade and metastatic organ number. However, IMDC risk stratification (p = 0.006) and systemic treatment (p = 0.022) were associated with TIBs status. For metastatic sites, CD19+ TIBs were also observed. There was a positive correlation between CD19+ TIBs density at primary tumor sites and paired metastatic sites (Spearman’s correlation = 0.532, p = 0.034, Figure 1C). It suggested the homogeneity of immune microenvironment between primary and metastatic sites of mRCC.
Figure 1.
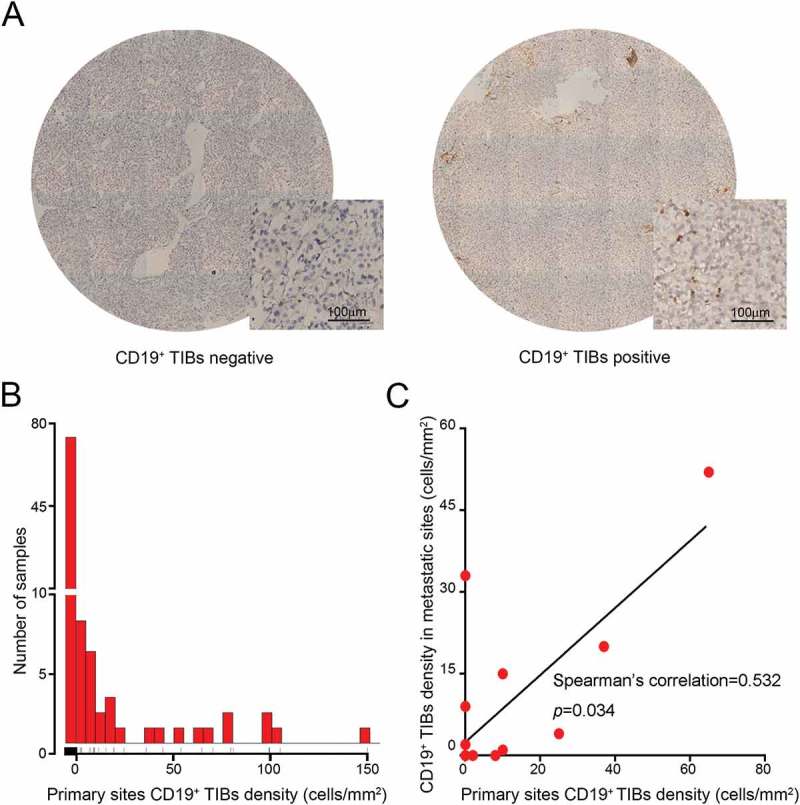
Evaluation of CD19+ TIBs cells by immunohistochemistry in mRCC primary tumor samples. (A) Representative images of CD19 staining in tumor samples. (B) Distribution of CD19+ TIBs density in tumor samples of the study cohort. (C) Spearman’s correlation between CD19+ TIBs density at primary tumor sites and paired metastatic sites from the same patients. Six points overlapped on the origin of the coordinate system.
Correlation between baseline characteristics including tibs and mrcc patients prognosis
In this cohort, 89.8% (97 of 108) of patients with mRCC deceased during the followup period. Patients with positive TIBs had better response to TKIs treatment (p = 0.001, Figure 2A) and this benefit only exist in sunitinib treated group (p = 0.006; Figure 2A). Analysis showed positive TIBs were linked with longer OS by Kaplan-Meier analysis (p < 0.001, Figure 2B). Univariate Cox regression model revealed that initial TNM stage (p = 0.021), systemic therapy (p = 0.019), IMDC risk stratification (p<0.001), metastatic organ number (p = 0.038) and TIBs (p < 0.001) were significant prognostic factors for OS. Histological type had been well proved to be a parameter that affect the prognosis of mRCC patients.22 We then involved these parameters in multivariate Cox analysis. Results confirmed positive TIBs was still an independent predictor for better prognosis of mRCC patients (p = 0.002, Figure 2D).
Figure 2.
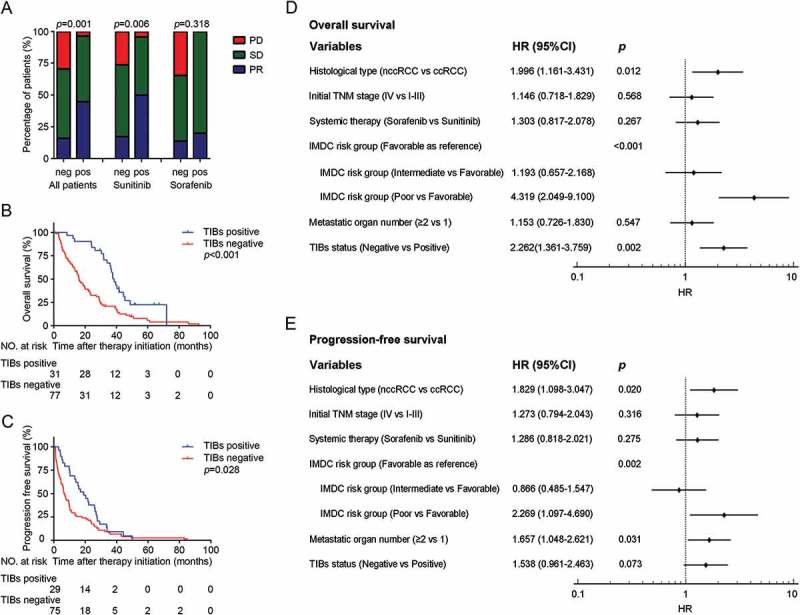
CD19+ TIBs status predicts sunitinib therapeutic response and survival in mRCC patients. (A) TKI treatment response in different CD19+ TIBs groups. PR, partial response; SD, stable disease; PD, progressive disease. (B,C) Kaplan–Meier survival analysis of OS (B) and PFS (C) according to CD19+ TIBs grouping. (D,E) Multivariate analysis identified independent prognostic factors of OS (D) and PFS (E). HR, hazard ratio; CI, confidence interval.
As of the last followup, almost all (103 of 104, four patients were excluded from PFS analysis because of incomplete imaging data during followup) patients experienced disease progression. Patients with positive TIBs demonstrated longer PFS by Kaplan-Meier analysis (p = 0.028, Figure 2C). Initial TNM stage (p = 0.016), metastatic organ number (p = 0.003), IMDC risk stratification (p < 0.001) and TIBs status (p = 0.029) had strong relevance to the PFS in Univariate Cox regression model. From Multivariate Cox analysis, although there was no statistical significance, TIBs status was still a moderate predictor of PFS in mRCC patients treated with TKIs (p = 0.073, Figure 2E).
The univariate and multivariate analyses of TIBs and other clinical characteristics with OS and PFS were listed in Supplement table 1.
Tibs and prognosis in different subgroups of mrcc patients
Results demonstrated high TIBs infiltration tended to prolong OS (p = 0.004, Supplement Figure 1A), but not PFS (p = 0.193, Supplement Figure 1B), in ccRCC patients. Meanwhile, in nccRCC patients, positive TIBs status was a predictor for better OS (p = 0.016, Supplement Figure 1C) and PFS as well (p = 0.008, Supplement Figure 1D).
When stratified by initial TNM stage, Kaplan-Meier analyses also showed the prognostic value of TIBs in patients’ OS in both stage I-III (p = 0.013, Supplement Figure 2A) and stage IV (p = 0.008, Supplement Figure 2C). For PFS, TIBs have a forecasting effect only in stage IV (p = 0.027, Supplement Figure 2D), but not stage I-III (p = 0.331, Supplement Figure 2B).
The prognostic value of tibs status combined with IMDC risk stratification
IMDC risk stratification model was used for predicting the survival of mRCC patients treated with VEGF targeted agents. In order to better estimating the OS of TKI-treated mRCC patients, we combined TIBs status with IMDC risk stratification to generate a new model. Receiver operating characteristic (ROC) analysis was performed at 12, 24 and 36-month followup, and the combined model demonstrated a better prognostic value than IMDC risk stratification model alone (Figure 3, p = 0.063, 0.003 and 0.023 respectively). C-index also represented that incorporating TIBs with IMDC model could improve the predictive accuracy of OS, compared with IMDC model or TIBs alone. The c-index of our integrated system, IMDC model and TIBs was 0.707, 0.674 and 0.618, respectively.
Figure 3.
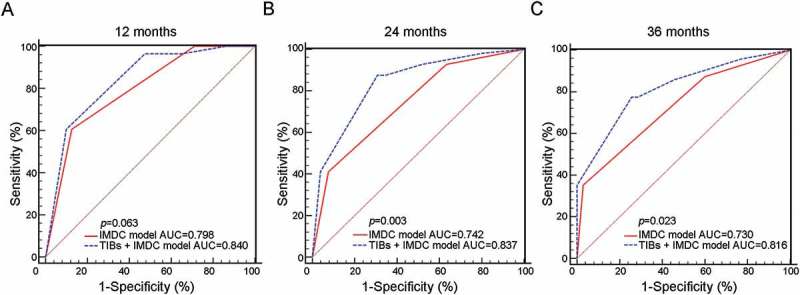
Comparison of combined model with IMDC model alone in predicting OS of mRCC patients. ROC analysis for predictive accuracy of IMDC model alone and combined with CD19+ TIBs status for OS in mRCC patients at 12 months (A), 24 months (B) and 36 months (C).
Correlation between tibs and CD8+ tumor infiltrating lymphocytes
To investigate the potential mechanism of TIBs anti-tumor effects, we made gene profile investigation in the TCGA KIRC cohort (n = 530). The top 1000 co-expression genes of CD19 were incorporated into the gene enrichment analysis. Of note, CD19 was related to multiple T cell-related process and signaling pathways including T cell receptor signaling pathway,T cell costimulation, positive regulation of T cell proliferation and CTL mediated immune response against target cells (Supplement table 2). Through TCGA KIRC cohort (Figure 4A) and the Sato cohort (Supplement Figure 3), we found the expression of CD8 and CD8+ TILs-associated cytotoxic effectors were up-regulated along with elevating CD19 expression to varying degrees. Further study by Spearman correlation analysis proved the same conclusion, as there existed a significant positive correlation between CD19 and CD8+ TILs-associated molecules (Figure 4B).
Figure 4.

Correlation between TIBs and CD8+ tumor infiltrating lymphocytes (TILs). (A) Changing of CD8 and CD8+ TILs-associated cytotoxic effectors expression levels as CD19 expression elevating in the KIRC cohort (TCGA). (B) Heatmap shows Spearman’s correlation coefficients of CD19 with CD8 and CD8+ TILs-associated cytotoxic effectors in the KIRC cohort (TCGA) and the Sato cohort. All p ≤ 0.001. (C) Representative images of co-rich infiltration of CD19+ TIBs and CD8+ TILs in one sample (up) and co-rare infiltration in another (down). Scale bar is 150 μm. (D) Comparison of CD8+ TILs density between CD19+ TIBs positive and negative groups. (E) Spearman’s correlation between CD19+ TIBs and CD8+ TILs density in mRCC patients.
Thus, we investigated the underlying interaction between CD19+ TIBs and CD8+ T in mRCC patients. Similar to previous results, TIBs positive cancer tissue specimens were more likely to be high CD8+ T cell infiltration (Figure 4C). The number of infiltrated CD8+ T was remarkably higher in TIBs positive group in compared with negative one (p = 0.004, Figure 4D). Significant positive correlation was also detected between the presence of CD8+ TILs and CD19+ TIBs (Spearman correlation coefficient r = 0.374, p < 0.001, Figure 4E).
CD19+ TIBs and CD8+ TILs double positive/high infiltration favorable survival of TKIs treated mRCC patients
Finally, we assessed the impact of different CD19+ TIBs and CD8+ TILs infiltration situation on patient survival. By Kaplan–Meier analysis, CD19+ TIBs and CD8+ TILs double positive/high cases demonstrated markedly better OS compared with those double negative/low cases (p<0.001, Figure 5D), followed by those CD8+ TILs high meanwhile CD19+ TIBs negative cases (p = 0.015, Figure 5D).
Figure 5.

Kaplan–Meier survival analysis of overall survival according to CD19+ TIBs and CD8+ TILs grouping.
Discussion
B lymphocytes are a kind of cells derived from hematopoietic stem cells. Unlike the concentratedly studied CD8+ T lymphocytes, which directly killing tumor cells and are strongly associated with patient survival in many cancers,23 the function of tumor infiltrating B cells in tumor immunity is complex and still not explicitly stated.
In this study, we found the homogeneity of immune microenvironment between primary and metastatic sites of mRCC (Figure 1C). Therefore, the immune environment of the primary tumor can be used to predict the immune milieu of metastatic sites in mRCC patients. We explored CD19+ TIBs high infiltration in primary tumor as an independent predictor of better TKI therapeutic response of sunitinib (Figure 2A), better OS (Figures 2B) and PFS (Figure 2C) in mRCC patients. Combining TIBs and current IMDC risk stratification showed a better prognostic value than IMDC risk stratification model alone (Figure 3). Through gene enrichment analysis, we found CD19 was related to multiple T cell-related process and signaling pathways (Supplement table 2). Then, we disclosed a significant positive correlation between CD19 and CD8+ TILs-associated molecules by public databases analysis (Figure 4A, B; Supplement Figure 3). It implied that CD19+ TIBs might attract CD8+ TIL or potentially enhance the antitumor effect of CD8+ TIL in mRCC. The subsequent immunohistochemistry confirmed the positive correlation between CD19+ TIBs and CD8+ TILs (Figure 4E). CD19+ TIBs and CD8+ TILs double positive/high cases showed markedly longer OS compared with double negative/low cases and those CD8+ TILs high meanwhile CD19+ TIBs negative cases (Figure 5).
For a long time, B cells were believed to play a negative role in tumor immunity.24 Depleting B lymphocytes lead to slower tumor growth or/and higher rejection rates in mice model.25,26 However, this phenomenon did not appear in clinical trials. As an adjunct to IL-2 therapy, rituximab induced normal B cells exhaustion didn’t increase the response rate in RCC and melanoma patients.27 This may because tumor-reactive B cells play a more important role in long-term T cell responses against cancer rather than short-term.28 So TIBs anti-tumor function cannot be fully played in relatively short-term tumor-bearing mice experiments. It is noteworthy that B cells are not homogenous with respect to cytokine production. B cells activated by Th1 cells secrete cytokines like IFN-γ and IL-12,which are connected with type 1 immune responses.29 Combination of IL-21 and B-cell receptor stimulation enables B cells to secrete granzyme B and to mediate an early antigen-specifc, cytotoxic immune responses. In gastric cancer, B cells mostly infiltrate as tertiary lymphoid structures and are associated with better prognosis.30 Overall, B cells can directly kill tumor cells, enhance T cell responses by producing Abs, stimulatory cytokines, and chemokines, serve as local APCs, and organize the formation of tertiary lymphoid structures that sustain long-term immunity.28,31 Our results also showed that high TIBs were associated with better prognosis in mRCC patients treated with TKIs and therefore an independent predictor of better TKI therapeutic response.
Immune checkpoint inhibitors have emerged as promising new therapies in advanced renal cancer. A recent randomized, phase 3 trial involving previously untreated patients with advanced renal cell carcinoma showed that the overall survival and objective response rates were significantly higher with nivolumab plus ipilimumab than with sunitinib.32 Even so, the response rates to anti-PD-1 antibody were only 27%, which is far from satisfied, in patients with renal cancer.33 Predictive biomarkers exist to guide selection for immune checkpoint inhibitors are still not routinely used in the clinic yet. In fact, B cells constitutively express PD-L1, which significantly increased in TIBs compared with splenic B cells.34 These PD-L1hi TIBs can directly mediate the exhaustion of CD8+ TILs or induce the formation of regulatory T cells (Tregs) in tumor.21 On the other hand, current researches of PD-1 mostly focused on T cells. It is worth mention that B cells can also express PD-1 when they were activated. PD-1hi B cells can operate T-cell dysfunction via interleukin-10-dependent pathways and thereby create an immunosuppressive microenvironment to permit tumor evasion.35 Thus, due to the presence of PD-1hi TIBs, anti–PD-1/anti–PD-L1 antibody can also control some tumors with PD-1- TILs.36 Therefore, TIBs may be a potential predictor to guide selection for anti-PD-1/anti-PD-L1 antibody therapy and further study is required to verify this hypothesis.
The major limitation of this study is the relatively small sample size. However, despite the insufficient number of enrolled patients, CD19+ TIBs still performed as a robust predictive factor for sunitinib therapeutic response and mRCC patients’ survival. Moreover, TIBs in different locations display characteristic phenotypes and there are some subtypes of TIBs indeed suppress tumor immunity.20,37,38 Further studies are required to investigate explicit function of different TIBs subgroups and the role of them in different tumor sub-regions. The underlying mechanism of how TIBs interact with CD8+ TIL still left to clarify. The retrospective design may also confound the results. Sorafenib, a second-line targeted therapy in patients with mRCC today, had been used as first-line treatment in many patients in the cohort during those years. The predictive value of CD19+ TIBs for other novel TKI agents and immune checkpoint inhibitors needs to be investigated in the future. Finally, prospective external validations are still needed before clinical application.
In conclusion, CD19+ TIBs could be used as a predictor of better therapeutic response of sunitinib and an independent prognostic factor in mRCC patients. High TIBs identified a subgroup of mRCC patients who appeared to benefit from sunitinib therapy. Incorporation of TIBs into IMDC system could further improve the predicting accuracy of OS in mRCC patients. Our results showed a positive correlation between TIBs and CD8+ TILs, which suggested an immune activation function of TIBs in the microenvironment of mRCC.
Patients and methods
Patient and clinical database
A total of 138 mRCC patients treated with sunitinib or sorafenib, between March 2005 and June 2014, were retrospectively screened at the Department of Urology, Zhongshan Hospital, Fudan University. Of which, 108 patients (age range 14 to 78 years, median 59) met the inclusion criteria. The other 30 patients were excluded from analysis due to loss of followup or unavailable tissue samples. All the 108 patients received standard surgical treatment of either radical or cytoreductive nephrectomy. 16 suitable oligometastatic patients among the 108 patients accepted metastasectomy (10 lung metastasis, 3 bone metastasis, 1 liver metastasis and 2 other sites metastasis). Ethical ratification was authorized by the clinical research ethics committee of Zhongshan Hospital (approval number B2015-030) and written informed consent on the use of clinical specimens was obtained from each patient. Patient inclusion criteria involves: a diagnosis of mRCC, no other malignancy history, treatment with sunitinib or sorafenib as first line systemic therapy and available fixed tumor tissue. Exclusion criteria includes: lack of tissue sample, tumor necrosis area greater than 80%, received prior systemic therapy or loss of followup.
Baseline demographic, clinical and laboratory data were collected retrospectively from medical records and electronic databases. Presence of nodal and distant metastasis was diagnosed according to intraoperative, pathologic, and radiographic findings. Initial tumor node metastasis stages were reassigned based on the 2010 AJCC TNM classification.39 The retrospective data on treatment, response and survival were obtained from medical records and followup. Disease progression was defined following the RECIST 1.1 criteria.40 Histological subtypes were divided into clear cell renal cell carcinoma (ccRCC) and non-clear cell renal cell carcinoma (nccRCC) in accordance with the 2004 WHO criteria.41 The nucleus grade was reported in terms of the Fuhrman grading system.42 Kidney Renal Clear Cell Carcinoma (KIRC) cohort from The Cancer Genome Atlas (TCGA) was downloaded from TCGA database (https://cancergenome.nih.gov/). The Sato et al. 43Agilent microarray gene expression dataset was downloaded from ArrayExpress (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-1980/) and all samples (n = 101) were included in the analysis as previously described.44
Immunohistochemical staining and evaluation
Tissue microarrays were established and IHC staining were performed as previously described.45 Primary monoclonal anti-CD19 antibody (ab31947, Abcam, 1:200) and anti-CD8 antibody (clone C8/144B, DAKO,ready to use) were applied in the procedure. For every primary tumor from nephrectomy in 108 patient and metastatic simple from metastasectomy in 16 patients, two independent shots away from tissue margin, obvious inflammation or necrotic domains were obtained and all data were recorded under the same circumstances. Immunohistochemistry sections were scanned by a fully automated microscopy system (Leica DM6000 B; Leica Microsystems GmbH, Mannheim, Germany). Images were captured by Leica CVM2CL camera and analysed by Aperio ImageScope 12.2 (Leica Microsystems GmbH, Mannheim, Germany). Two genitourinary pathologists, masked to the followup data, counts the number of CD19 positive staining cells at 200× magnification and the average number was used as the final number of TIBs. Because of the lower density of CD19 positive staining cells, we defined infiltrating B cells in both tumor parenchyma and stroma as TIBs.
Statistical analyses
Risk stratification of patients was performed according to the IMDC model.7 OS was defined as the duration from the time of systemic therapy to death as a result of any cause or censoring at the date of last followup. PFS was defined as the date from initiation of systemic therapy to the date of disease progression or censoring at last followup. The cut point of TIBs density was determined at 0/mm2 using X-tile software version 3.4.7 (Robert L. Camp, 2005) through minimum p value method based on the patients’ OS information.46 Thus, patients were divided into positive (TIBs >0/mm,2 n = 31) and negative (TIBs = 0/mm,2 n = 77) TIBs groups. Distribution of TIBs density in primary tumor samples of the study cohort was shown in Figure 1B. Similar, the cut point of CD8+ T infiltration was determined at 40/mm,2 and patients were divided into high (CD8+ T ≥ 40/mm2) and low (CD8+ T <40/mm2) CD8+ T groups. GraphPad Prism 6 (GraphPad Software Inc.), SPSS 21.0 (SPSS Inc.) and R software packages (version 3.1.2; The R Foundation for Statistical Computing, http://www.r-project.org/) were used for the statistical analyses in this study. The correlation between TIBs infiltration and baseline characteristics were assessed by the chi-square test or Fisher’s exact method. The Kaplan-Meier method and log rank test was performed to estimate the impact of TIBs/CD8+ T status on OS and PFS. Univariate and multivariate Cox proportional hazard models were applied to evaluate the hazard ratio (HR) and 95% confidence interval (CI). Spearman’s correlation test was used to evaluate the relationship between CD19+ TIBs density at primary lesion and paired metastatic sites, as well as the correlations of CD19 and CD8+TIL associated molecules expression levels. Time dependent ROC analysis was accomplished by adding the weighted value of the TIBs to the IMDC risk stratification model. 2-tailed p < 0.05 was considered as statistically significant.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China (NSFC) [81472227, 81472376, 31770851, 81702496, 81702497, 81702805 and 81772696];Zhongshan Hospital Talented Youth Program [2017ZSYQ26];Zhongshan Hospital Science Foundation [2016ZSQN30 and 2017ZSQN18];Shanghai Health and Family Planning Commission [20174Y0042];
Acknowledgments
This study was funded by grants from National Natural Science Foundation of China (81472227, 81472376, 31770851, 81702496, 81702497, 81702805, 81772696), Shanghai Health and Family Planning Commission (20174Y0042), Zhongshan Hospital Science Foundation (2016ZSQN30, 2017ZSQN18) and Zhongshan Hospital Talented Youth Program (2017ZSYQ26). All these study sponsors have no roles in the study design, in the collection, analysis, and in the interpretation of data.
Conflict of interest statement
The authors declare no potential conflict of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172 [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine. 2007;356:115–124. doi: 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. The Lancet Oncology. 2013;14:552–62. doi: 10.1016/S1470-2045(13)70093-7 [DOI] [PubMed] [Google Scholar]
- 7.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809 [DOI] [PubMed] [Google Scholar]
- 8.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, Rha SY, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. The Lancet Oncology. 2013;14:141–8. doi: 10.1016/S1470-2045(12)70559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10:992–1000. doi: 10.1016/S1470-2045(09)70240-2 [DOI] [PubMed] [Google Scholar]
- 10.Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol. 2012;2012:1–8. doi: 10.1155/2012/492920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. Journal of immunotherapy. 2010;33:991–8. doi: 10.1097/CJI.0b013e3181f4c208 [DOI] [PubMed] [Google Scholar]
- 12.Js Ko, Ah Zea, Bi Rini, Jl Ireland, Elson P, Cohen P, A Golshayan, PA Rayman, L Wood, J Garcia, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhu Y, Wang Z, Zhang T, Wu P, Huang J. Yin-yang effect of tumor infiltrating B cells in breast cancer: from mechanism to immunotherapy. Cancer Lett. 2017;393:1–7. doi: 10.1016/j.canlet.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 14.Shen M, Sun Q, Wang J, Pan W, Ren X. Positive and negative functions of B lymphocytes in tumors. Oncotarget. 2016;7:55828–55839. doi: 10.18632/oncotarget.v7i34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zirakzadeh AA, Kinn J, Krantz D, Rosenblatt R, Winerdal ME, Hu J, Hartana CA, Lundgren C, Bergman EA, Johansson M, et al. Doxorubicin enhances the capacity of B cells to activate T cells in urothelial urinary bladder cancer. Clinical immunology. 2017;176:63–70. doi: 10.1016/j.clim.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 2014;20:5995–6005. doi: 10.1158/1078-0432.CCR-14-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara H, Melenhorst JJ, El Ouriaghli F, Kajigaya S, Grube M, Sconocchia G, Rezvani K, Price DA, Hensel NF, et al. In vitro induction of myeloid leukemia-specific CD4 and CD8 T cells by CD40 ligand-activated B cells gene modified to express primary granule proteins. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2005;11:4495–4503. doi: 10.1158/1078-0432.CCR-04-2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, Xia Z, Zeng WY, Wucherpfennig KW, Nadler LM, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 2002;99:3319–3325. doi: 10.1182/blood.V99.9.3319 [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Lederman MM, Harding CV, Sieg SF. Presentation of soluble antigens to CD8+ T cells by CpG oligodeoxynucleotide-primed human naive B cells. J Immunol. 2011;186:2080–2086. doi: 10.4049/jimmunol.1001869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, Shin S-U, Cho H-M, Bayati AA, Pimentel A, et al. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. International immunology. 2016;28:423–433. doi: 10.1093/intimm/dxw007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Liu L, Qu Y, Xi W, Xia Y, Bai Q, Xiong Y, Long Q, Xu J, Guo J Prognostic Value of SETD2 Expression in Patients with Metastatic Renal Cell Carcinoma Treated with Tyrosine Kinase Inhibitors. The Journal of urology. 2016;196:1363–1370. doi: 10.1016/j.juro.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 23.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416 [DOI] [PubMed] [Google Scholar]
- 24.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627 [DOI] [PubMed] [Google Scholar]
- 25.Monach PA, Schreiber H, Rowley DA. CD4+ and B lymphocytes in transplantation immunity. II Augmented Rejection Tumor Allografts by Mice Lacking B Cells Transplantation. 1993;55:1356–1361. [PubMed] [Google Scholar]
- 26.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I Immunity Chemically Induced Tumor Journal Immunology. 1978;121:359–362. [PubMed] [Google Scholar]
- 27.Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Annals of oncology: official journal of the European Society for Medical Oncology. 2004;15:1109–14. doi: 10.1093/annonc/mdh280 [DOI] [PubMed] [Google Scholar]
- 28.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunology. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323 [DOI] [PubMed] [Google Scholar]
- 29.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakimura C, Tanaka H, Okuno T, Hiramatsu S, Muguruma K, Hirakawa K, Wanibuchi H, Ohira M. B cells in tertiary lymphoid structures are associated with favorable prognosis in gastric cancer. The Journal of surgical research. 2017;215:74–82. doi: 10.1016/j.jss.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 31.Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y, Lundy SK, Ito F, Pan Q, Zhang X, et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. European journal of immunology. 2015;45:999–1009. doi: 10.1002/eji.201444625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan H, Lan Y, Wan Y, Wang Q, Wang C, Xu L, Chen Y, Liu W, Zhang X, Li Y, et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19(+) B lymphocytes and T cells in Invasive breast cancer. Oncoimmunology 2016;5:e1075112. doi: 10.1080/2162402X.2015.1075112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. European journal of immunology. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao X, Xm Lao, Mm Chen, Rx Liu, Wei Y, FZ Ouyang, Chen D-P, Zhao X-Y, Zhao Q, Li X-F, et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer discovery. 2016;6:546–559. doi: 10.1158/2159-8290.CD-15-1408 [DOI] [PubMed] [Google Scholar]
- 36.Ren Z, Peng H, Fu YX. PD-1 Shapes B Cells as Evildoers in the Tumor Microenvironment. Cancer Discov. 2016;6:477–478. doi: 10.1158/2159-8290.CD-16-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, Shi YH, Shi GM, Ding ZB, Ke AW, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 2013;19:5994–6005. doi: 10.1158/1078-0432.CCR-12-3497 [DOI] [PubMed] [Google Scholar]
- 38.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunology. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329 [DOI] [PubMed] [Google Scholar]
- 39.Kim SP, Alt AL, Weight CJ, Costello BA, Cheville JC, Lohse C, Allmer C, Leibovich BC. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: results from a large, single institution cohort. The Journal of urology. 2011;185:2035–2039. doi: 10.1016/j.juro.2011.02.059 [DOI] [PubMed] [Google Scholar]
- 40.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version1.1). European journal of cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 41.Delahunt B, Srigley JR, Montironi R, Egevad L. Advances in renal neoplasia: recommendations from the 2012 International Society of Urological Pathology Consensus Conference. Urology. 2014;83:969–974. doi: 10.1016/j.urology.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 42.Erdogan F, Demirel A, Polat O. Prognostic significance of morphologic parameters in renal cell carcinoma. Int J Clin Pract. 2004;58:333–336. doi: 10.1111/j.1368-5031.2004.00008.x [DOI] [PubMed] [Google Scholar]
- 43.Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nature genetics. 2013;45:860–867. doi: 10.1038/ng.2699 [DOI] [PubMed] [Google Scholar]
- 44.Senbabaoglu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, De Velasco G, Miao D, Ostrovnaya I, Drill E, Luna A, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome biology. 2016;17:231. doi: 10.1186/s13059-016-1092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521 [DOI] [PubMed] [Google Scholar]
- 46.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


