ABSTRACT
Hepatocellular carcinoma (HCC) is the most common liver malignancy with a poor prognosis and an overall 5-year survival rate of approximately 5–6%. This is due because standard of care treatment options are limited and none of them shows a sufficient efficacy.
HCC is an “inflammation-induced cancer” and preliminary preclinical and clinical data suggest that immunotherapeutic approaches may be a good alternative candidate for the treatment of HCC patients improving the dismal prognosis associated with this cancer.
However, recent findings strongly suggest that an optimal immunotherapy in HCC requires the combination of an immune activator with immune modulators, aiming at compensating the strong liver immune suppressive microenvironment. One of the most promising strategy could be represented by the combination of a cancer vaccine with immunomodulatory drugs, such as chemotherapy and checkpoint inhibitors. Very limited examples of such combinatorial strategies have been evaluated in HCC to date, because HCC easily develops resistance to standard chemotherapy, which is also poorly tolerated by patients with liver cirrhosis. The present review describes the most update knowledge in this field.
Keywords: Vaccine, liver cancer, checkpoint inhibitors, metronomic chemotherapy, chemotherapy, combinatorial strategy, models of anticancer vaccination, therapeutic trials, therapeutic vaccination
1. Introduction
Hepatocellular carcinoma (HCC) is one of the ten most frequent solid cancers and the third leading cause of cancer-related death worldwide and its incidence is still rising in many Countries. A wide range of therapies are used in the management of HCC according to the extent and severity of liver disease, however, the overall prognosis for HCC patients is poor.1–3
The major risk factors for HCC are viral hepatitis chronic infections, which are responsible for persistent inflammation in the liver. Consequently, HCC can be classified as an “inflammation-induced cancer” and the immune-based therapies may be valid alternative treatments, as suggested by preliminary preclinical and clinical data.
However, despite developing in a context of chronic inflammation, HCC is characterized by an immunosuppressive tumor microenvironment. Indeed, liver is considered “an immunological organ” characterized by a strong intrinsic immune tolerance, strong innate immunity and poor adaptive immune response.4 In the context of the healthy adult liver, the resident immune cell populations play central roles in regulating inflammation and maintaining organ homeostasis, resulting in a balanced microenvironment between tolerance and inflammation.
The HCC tumor microenvironment (TME) includes different cell types which, as whole, contributes to the favourable tissue context to the tumor growth. Three distinct subsets of phagocytic cells are responsible for the intra-hepatic tolerogenicity: liver sinusoidal endothelial cells (LSECs), Kupffer cells and liver dendritic cells (DCs) (reviewed in5) and even hepatocytes, which have been shown to drive T cells toward an anergic cytotoxic phenotype and a clonal deletion.6,7 The two principal immunosuppressive cell populations are present, namely myeloid-derived suppressor cells (MDSCs)8,9 and CD4+CD25+FoxP3+ regulatory T cells (Tregs).10,11 Both immune regulatory cells mediate their suppressive activity through the production of the immunosuppressive cytokines IL-10 and TGFβ which inhibit the T cell activation and proliferation.
Several studies demonstrated that the accumulation of these immunosuppressive cell populations within the tumor are correlated with disease progression and poor prognosis and can regulate the response of cancer cells to chemotherapy.12 In particular, Tregs play a role in the development of cirrhosis, the transformation of cirrhosis to HCC, and the progression and metastasis of HCC. Higher level of Tregs in the peripheral blood and/or tumor sites is correlated with a poorer prognosis in HBV-related liver conditions. Further, the findings of Kobayashi et al suggest that the prevalence of CD8+ tumor-infiltrating lymphocytes decreases significantly during hepatocarcinogenesis and is inversely correlated with the one of infiltrating Tregs.13
Moreover, the immune suppressive TME of HCC is further enhanced by the expression of immune checkpoint molecules (i.e. programmed death ligand-1 PD-L1) on liver sinusoidal endothelial cells, interacting with programmed death 1 (PD-1) molecules on T cells and leading to the induction of antigen-specific T cell tolerance.6,14
All the components of the HCC intra-tumoral immunosuppressive environment lead to tumor escaping, inefficient immune mediated control and, consequently, the unsatisfactory results observed in cancer immunotherapy clinical trials.15
As a result, there is growing need for new therapeutic strategies in HCC to improve induction of anti-tumor immune response by counteracting the immune suppressive TME. Combinatorial protocols combining specific immunotherapy approaches (i.e. cancer vaccine) with immune modulator strategies (i.e. chemotherapy and anti-checkpoint molecules) would be highly effective (reviewed in16), providing better results than individual treatments (reviewed in17,18 (Figure. 1)
Figure 1.
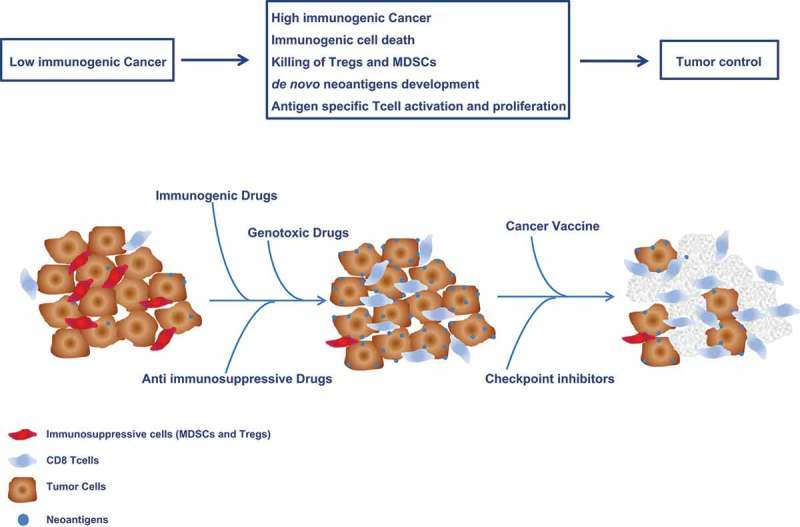
Combinatorial strategies with immunogenic, anti immunosuppressive and genotoxic chemotherapy to enhance efficacy of cancer vaccine and checkpoint inhibitors.
2. Immunomodulatory approaches in HCC
The efficacy of anti-tumoral immune response can be enhanced by contrasting the different components of the immunesuppressive TME using different immunomodulatory treatments, represented by conventional or low-dose chemotherapy and/or anti-immune checkpoint inhibitors. The first type of treatment aims at reducing the suppressor immune cells or at increasing the tumor mutational burden. On the contrary, the second type blocks the inhibitory pathway mediated by immune checkpoint molecules.
2.1. Conventional chemotherapy in HCC treatment
Chemotherapy agents may enhance antitumor immunity by several mechanisms. Indeed, when administered at standard or low-dose metronomic regimens, they have been shown to selectively kill immunosuppressive cell populations. Moreover, they induce an immunogenic cell death in cancer cells with the release of danger signals able to polarize DCs towards a pro-inflammatory phenotype driving an anti-tumor T helper 1 (Th1) response.19,20 Furthermore, they can modulate the expression of tumor antigens and immune checkpoint molecules as well as molecules directly involved in antigen processing and presentation (i.e. MHC class I molecules). Finally, they may counteract the immune tolerance and suppression to promote antigen-specific immunity.21 Overall, these mechanisms would generate a favorable tumor immune environment and may potentiate effects of anticancer vaccines (reviewed in22 and23).
Conventional chemotherapy is based on the pharmacological maximum tolerate dose (MTD), the highest effective single dose usually administered at defined intervals (3 weekly, fortnightly, weekly). MTD has a strong anti-angiogenic effect on the tumor and mainly targets replicating tumor cells, including immune cells, causing a long-term toxicity and massive lymphodepletion.24–27 As reviewed by Shurin et al., the main immunosuppressive effect of MTD chemotherapy is the depletion of T lymphocytes, while restoration of peripheral CD4+ cell levels takes significantly longer than CD8+ cells.28–30
However, in the last years, many studies have reported that standard chemotherapy is not fully immunosuppressive. Indeed, after the initial T cell depletion, it would induce a rebound replenishment of various immune cell subsets, leading to positive effects on anticancer immune response.31 Numerous evidences indicate the benefits of chemotherapy on T-cell-mediated immune responses. Mice vaccinated with doxorubicin- or cisplatin-treated ovarian cancer cells show an enhanced antitumor immunity, and prolonged survival largely dependent on CD4 T-cell-mediated immune responses.32
Moreover, several clinical studies demonstrate that chemotherapy can interact positively with immunotherapy treatments improving their efficacy by several means and, in particular, potentiating the effects of anticancer vaccines (reviewed in22).
More recently it has been demonstrated that cytotoxic agents may increase the anti-tumor immunity by making more immunogenic the tumor, inducing a higher number of tumor-specific mutated neo-antigens. In particular, the Temozolomide drives the inactivation of the DNA mismatch repair (MMR) machinery promoting a dynamic increase in the number of mutations and de novo neoantigens in cancer cells. The induction of high numbers of tumor-specific non-self antigens results in a strong antitumor immune response and improved efficacy of immune-checkpoint inhibitors.33
All these observations indicate that immunomodulatory chemotherapeutic agents may be good candidates also for combination with different immune-based therapeutic approaches such as cancer vaccines.
However, although this can be proposed for many cancer types, MTD chemotherapy is not feasible in HCC. Indeed, systemic chemotherapy has been used for palliative treatment in advanced HCC, but severe toxicities have been reported compared to other cancers, due to the liver cirrhosis and the intrinsic chemoresistence of hepatocytes.24,34 For such a reason, systemic chemotherapy is proposed only for patients with good performance status and preserved hepatic function.35 However, single agents such as doxorubicin, cisplatin and fluorouracil give a 10% response rates which increases to 20% in combination regimens, without any impact on survival.36–38 Therefore, using a full therapeutic dose of cytotoxic agents in HCC will not be considered ethical until their immunological effects will be assessed as more relevant than their cytotoxic effects.
A valid alternative should be the low dose metronomic chemotherapy which has been shown to have anti-immunosuppressive effects without toxicity and potentiate the efficacy of cancer vaccines.
2.2. Metronomic chemotherapy in HCC
Metronomic chemotherapy refers to a regular administration of a chemotherapeutic drug for a long time with no extended drug-free breaks.39 Multiple repeated low doses of chemotherapy drugs administered at short intervals were originally reported to inhibit tumor neo-angiogenesis and significantly suppress tumor growth. More recently, it has been shown that metronomic chemotherapy exerts the anti-tumor effect via additional mechanisms (reviewed in40). In particular, as for the full dose, metronomic chemotherapy induces an immunogenic cell death (ICD), generating a favorable tumor immune environment (reviewed in19). Indeed, low-dose cyclophosphamide is toxic to immunosuppressive Treg cells improving the anti-tumor T cell response.41–43 Similarly, gemcitabine selectively kills myeloid-derived suppressor cells (MDSCs) in vitro and in vivo.44 Finally, docetaxel has been reported to modulate different cell subsets, enhancing CD8+ function and deleting Tregs.45 Overall, these effects on the tumor microenvironment can significantly improve immunotherapy approaches, including cancer vaccines.
In this framework, we have previously reported in preclinical studies a novel combinatorial strategy, based on peptide vaccine and a metronomic chemotherapy including taxanes and alkylating agents. This combinatorial strategy showed to significantly delay tumor growth and prolong animal survival. Such anti-tumor biological effects were shown to be directly correlated with induction of immunological cell death (ICD), enhanced T cell response and reduction of the immune suppressive Tregs cell population.20,46
To date, more than 50 clinical trials of metronomic chemotherapy have been reported in patients affected by different cancers showing an enhancement of anti-tumor immunity.23 However, only four clinical trials evaluating metronomic chemotherapy in HCC have been registered to date. Treiber et al. reported no effects in terms of time to progression and overall survival.47 Hsu et al. reported that metronomic tegafur/uracil (UFT) could be safely combined with Sorafenib, enhancing its antitumor efficacy without additional severe side effects.48,49 Woo et al. reported that combination of metronomic epirubicin with cisplatin and 5-FU is a potentially useful treatment for HCC patients with portal vein thrombosis.35 In addition, Shao et al. reported a modest activity of a metronomic UFT plus thalidomide, sorafenib, or bevacizumab in patients with advanced HCC.50 Very recently a phase II clinical trial evaluating metronomic capecitabine in advanced hepatocellular carcinoma patients showed efficacy in treatment-naive patients as well as in those previously treated with Sorafeni.51 Moreover, two case reports provided encouraging results in individual HCC patients treated with metronomic capecitabine.52,53
Overall, results of the metronomic chemotherapy in HCC patients are encouraging and motivate the design of large randomized phase II/III trials to confirm its efficacy.
2.3. Checkpoint inhibitors in HCC
Immune checkpoints are a wide range of membrane molecules expressed in cancer cells as well as in different cell types involved in the immune response, including B and T cells, natural killer (NK) cells, DC, tumor associated macrophages (TAM), monocytes, and myeloid-derived suppressor cells (MDSC). These molecules inhibit T cell activation and proliferation during the immune response against non-self antigens.54–56 In particular, high expression of programmed cell death protein 1(PD-1) and PD-L1 in liver cancer tissue is correlated to poor prognosis in HCC patients as well as to more aggressive tumor characteristics.57,58 There are other inhibitory membrane molecules which are expressed on HCC cells, including cytotoxic T-lymphocyte protein 4 (CTLA-4), the lymphocyte activation gene 3 protein (LAG-3), T-cell immunoglobulin and mucin-domain containing (TIM-3) and B and T lymphocyte attenuator (BTLA).59–61 However, only CTLA-4 and PD-1/PD-L1 pathways have been pursued for HCC in clinic evaluation, and the anti-CTLA-4 inhibitor Tremelimumab, was the first molecule to be clinically evaluated in HCC62,63 (Table 1).
Table 1.
Published and ongoing HCC cancer vaccines based on peptides alone or in combination with chemotherapy, evaluated in human clinical trials.
| Antigen |
Vaccine strategy |
Combination |
Ref |
| Telomerase | Single Peptide | No | 73 |
| AFP | Single Peptide | No | 75 |
| GPC3 | Single Peptide | No | 76 |
| Telomerase | Single Peptide | Low-dose cyclophosphamide | 93 |
| HEPAVAC | Multiple Peptides | Low-dose cyclophosphamide | NCT03203005 |
The study by Sangro et al. involved HCC patients not eligible for surgery or locoregional therapies. The authors reported a good safety profile, with a 17.6% partial response rate and 76.4% disease control rate. Time to progression was 6.48 months (95% CI 3.95–9.14). Antitumor activity induced by Tremelimumab appeared promising in all measured outcomes, including objective tumor responses, durable stabilizations and long time to progression (TTP).62
In a very recent clinical trial by Duffy et al. was described that the efficacy of Tremelimumab can be improved when combined with tumor ablation, enhancing a specific tumor immune response and leading to the accumulation of intratumoral CD8+ T cells.63
As regards the PD-L1/PD-1 pathway, results of a very recent clinical trial has been reported on the use of the anti PD-1 inhibitor Nivolumab in HCC patients.64
The trial was conducted in HCC patients after treatment with Sorafenib. Treatment was well tolerated without any significant side effects. Convincing signs of efficacy were reported and, in particular, tumor responses were observed in about 20% of patients. The responses were meaningful and durable for a median of 17 months. An additional 45% of patients had stable disease lasting more than 6 months in most cases. Response rates were similar across different etiologies, and both in Sorafenib-naïve and Sorafenib-exposed patients. These results support Nivolumab as a viable second-line therapy following Sorafenib in HCC patients.
3. Active immunotherapy approaches for HCC
Several passive immunotherapies, based on immune cells transfer, have been tested in early phases clinical trials in HCC with limited clinical benefit for patients and none of them has been moved in phase III efficacy clinical trials. Active immunotherapy approaches may provide more promising outcomes.5,65,66
3.1. Preventive vaccine for HCC
Hepatitis B virus (HBV) infection is one of the main etiologic factors of hepatocellular carcinoma (HCC).67 The universal preventive hepatitis B vaccination for infants has been launched in the late ‘80s – early ‘90s in most Countries all over the World with high incidence of HBV infection. As direct consequence of prevention from HBV infection, the HBV vaccine is a successful preventive vaccine for HCC occurrence. Indeed, reduced incidence of liver cancer in children and adolescents have been reported in studies performed in different Countries.68–72
3.2. Cancer vaccines in HCC
Over the past 15 years, a number of tumor-associated antigens (TAAs) have been identified in HCC, some of which elicit tumor specific immune responses.73 Unfortunately, only a limited number of such TAAs have been used because most of them are not specific to HCC, such as telomerase reverse transcriptase – TERT;74,75 Wilms’ tumor 1 -WT-1;76,77 alpha fetoprotein – AFP,78 glypican 3 – GPC3;79,80 MAGE-A, SSX-2, NY-ESO-1.81
Among such TAAs, only a limited number of vaccines based on HLA class-I restricted epitopes derived from AFP, TERT and GPC3 have been tested so far in human clinical trials with limited results.82–84 A vaccine based on an AFP-derived peptide was evaluated in the first clinical trial conducted in the early 2000’s, showing the induction of an AFP specific T cell response in six patients.84 More recently, GPC3 peptides were proven to induce specific CD8+ CTLs in the tumor microenvironment (TME).85 A phase II clinical trial testing the GPC3-based vaccine as adjuvant therapy for patients after surgery or RFA is currently ongoing (UMIN-CTR: 000002614). An open label phase II clinical trial based on telomerase peptide did not lead to any complete or partial responses in advanced HCC patients.82 A very innovative strategy is currently pursued for identification of shared “off-the-shelf” HCC-specific antigens within the HEPAVAC project (www.hepavac.eu).65 In particular, novel HCC-associated antigens have been identified and a multi-epitope, multi-HLA peptide vaccine has been produced. A phase I/II clinical trial is currently ongoing to assess safety and immunogenicity in early-intermediate stage HCC patients undergoing surgical and/or loco-regional treatments (NCT03203005). The vaccination protocol will include also an actively personalized vaccine (APVAC) in a subset of vaccinees, based on patient-specific HCC-specific neo-antigens.
3.3. Enhancing cancer vaccine efficacy in HCC by combinatorial strategies
As discussed above, immunomodulatory treatments such as standard as well as metronomic chemotherapy and checkpoint inhibitors, may significantly improve the efficacy of anticancer immune responses.86–89 Consequently, cancer vaccines may result more effective when combined with such treatments, due to the sum of different biological effects such as potentiating the effector functions of activated T cells and NK, control of the suppressive activity of Tregs, enhancement of antibody-dependent cellular cytotoxicity.17
Indeed, clinical trials have shown that the combination of chemotherapy and cancer vaccines induces in cancer patients a better clinical outcome than individual treatments.90,91 Chemotherapy can improve anti-tumor effects of cancer vaccines not only by overcoming the immune-suppression, but also by enhancing cross-presentation of tumor antigens as well as increasing the number of effector cells in the tumor microenvironment.20,46,92–94
Combination of low-dose metronomic chemotherapy and cancer vaccines could represent a potentially attractive option for HCC patients, given the strong immunosuppressive microenvironment in the liver for the presence of several immunosuppressive cells such as CD4+CD25+ regulatory T cells,95 which are known to suppress the function of antigen-specific T cell responses and are increased in patients with HCC.96
Greten TF et al. have demonstrated that low-dose cyclophosphamide systemic treatment, but not the full dose, decreases the frequency and suppressor function of circulating CD4+CD25+Foxp3+ regulatory T cells in peripheral blood and unmasks α-fetoprotein-specific CD4+ T-cell responses in patients with advanced HCC. Moreover, in such patients affected by high tissue damage due to liver cirrhosis, Authors demonstrated that low-dose metronomic chemotherapy is safe without any significant hematologic side effects.97 Afterwards, the same Authors investigated the effect of a cancer vaccine based on hTERT peptide (GV1001) in combination with a low-dose cyclophosphamide treatment in a single arm phase II trial. Unfortunately, the combination did not show antitumor efficacy in respect to tumor response and time-to-progression.82 No additional attempts have been done subsequently in such a tumor setting.
Alternatively, combination of checkpoint inhibitors and cancer vaccines could represent an additional attractive option for HCC patients.98 Several pre-clinical studies have investigated combination strategies including cancer vaccines and checkpoint inhibitors, all of them showing significant enhancement of cancer vaccine effects associated with increased infiltration of effector CD8+ T cell.99–101 All such pre-clinical data have provided the rationale for designing and conducting several clinical trials for testing the efficacy in different cancer settings of such combinations. All such clinical trials are currently recruiting patients and results are expected to be available in the coming months (review in.102) None of such combinatorial strategies have been evaluated in HCC yet, but positive results reported in clinical trials evaluating checkpoint inhibitors in liver cancer103 strongly suggest that cancer vaccine and checkpoint combinations may show better efficacy also in HCC. Instead, the combination of checkpoint inhibitors with co-stimulatory molecules such as CD137 (4-1BB), CD134 (OX40), glucocorticoid-induced tumor necrosis factor receptor (GITR) or CD40 has been evaluated in a preclinical study using an aggressive transgenic hepatocellular carcinoma mouse model. This study demonstrated that combinatorial strategy is effective to potentiate the effector functions of activated T cells and to decrease the density of immunosuppressive cells in tumor microenvironment.104
4. Conclusions
HCC is a tumor with very high medical need. The current lack of an effective therapy, especially in more advanced stages, makes urgent the need for developing alternative treatments such as immunotherapies.
However, the immunosuppressive liver tumor microenvironment must be counterbalanced by combinatorial strategies, in order to improve the efficacy of such immunotherapies. Full dose chemotherapy is not applicable, due to drug resistance of HCC and toxicity. Therefore, the only treatments possibly available for combination with cancer vaccines are low-dose chemotherapy and checkpoint inhibitors.
The first option has been evaluated in several clinical trials with unsatisfactory results. The second one has not been yet evaluated and represents a priority goal. The recent favorable results from the CheckMate 040 clinical trial, showing safety and efficacy of anti-PD-1 in HCC patients, opens a new horizon in this setting.
Furthermore, cancer vaccines for HCC needs to be improved, identifying more specific target antigens. To this aim, results from the currently ongoing Hepavac-101 clinical trial (NCT03203005) will hopefully provide promising results. In addition, personalized HCC cancer vaccines based on patient-specific mutated neo-antigens represent the ultimate frontier and is yet an unexplored field.
All such considerations suggest that the field of HCC immunotherapy will see a blossom of pre-clinical and clinical evaluations of combinatorial strategies in the coming years.
Funding Statement
This work was supported by the European Commission Directorate-General for Research and Innovation FP7-HEALTH, Contract nr. 602893;
References
- 1.European Association for the Study of the Liver EOfRaToC EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56: 908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Fong ZV, Kk T.. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014; 120: 2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D. Bray F: cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136: E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Racanelli V. Rehermann B: the liver as an immunological organ. Hepatology. 2006; 43: S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 5.Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Fm B. Challenges in cancer vaccine development for hepatocellular carcinoma. JHepatol. 2013; 59: 897–903. doi: 10.1016/j.jhep.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AW, Pa K. Antigen-presenting cell function in the tolerogenic liver environment. NatRevImmunol. 2010; 10: 753–766. [DOI] [PubMed] [Google Scholar]
- 7.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004; 114: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res. 2015; 128: 95–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achyut BR, As A. Myeloid cell signatures in tumor microenvironment predicts therapeutic response in cancer. Onco Targets Ther. 2016; 9: 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006; 6: 295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le CA, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer ImmunolImmunother. 2007; 56:: 641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tredan O, Galmarini CM, Patel K, If T. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007; 99: 1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007; 13: 902–911. [DOI] [PubMed] [Google Scholar]
- 14.Keir ME, Butte MJ, Freeman GJ, Ah S. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26: 677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero P, Banchereau J, Bhardwaj N, Cockett M, Disis ML, Dranoff G, Gilboa E, Hammond SA, Hershberg R, Korman AJ, et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Sci Transl Med. 2016; 8: 334–339. doi: 10.1126/scitranslmed.aaf0685. [DOI] [PubMed] [Google Scholar]
- 16.Tagliamonte M, Petrizzo A, Tornesello ML, Ciliberto G, Buonaguro FM, Buonaguro L. Combinatorial immunotherapy strategies for hepatocellular carcinoma. Curr Opin Immunol. 2016; 39: 103–113. doi: 10.1016/j.coi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Sr G. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. SeminOncol. 2012; 39: 323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012; 12: 237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J ClinInvest. 2008; 118: 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Rea D, Barbieri A, Arra C, Maiolino P, Tornesello M, Ciliberto G, et al. A novel multi-drug metronomic chemotherapy significantly delays tumor growth in mice. J Transl Med. 2016; 14: 58. doi: 10.1186/s12967-016-0867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emens LA, Machiels JP, Reilly RT, Em J. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001; 3: 77–84. [PubMed] [Google Scholar]
- 22.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015; 28: 690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Gnoni A, Santini D, Scartozzi M, Russo A, Licchetta A, Palmieri V, Lupo L, Faloppi L, Palasciano G, Memeo V, et al. Hepatocellular carcinoma treatment over sorafenib: epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin Ther Targets. 2015; 19: 1623–1635. doi: 10.1517/14728222.2015.1071354. [DOI] [PubMed] [Google Scholar]
- 24.Boucher E, Corbinais S, Brissot P, Boudjema K, Jl R. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen). Cancer Chemother Pharmacol. 2002; 50: 305–308. doi: 10.1007/s00280-002-0503-x. [DOI] [PubMed] [Google Scholar]
- 25.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS. Folkman J: Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000; 60: 1878–1886. [PubMed] [Google Scholar]
- 26.Bergers G. Benjamin LE: Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003; 3: 401–410. [DOI] [PubMed] [Google Scholar]
- 27.McWhinney SR, Goldberg RM. McLeod HL: Platinum neurotoxicity pharmacogenetics. MolCancer Ther. 2009; 8: 10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shurin MR. Dual role of immunomodulation by anticancer chemotherapy. Nat Med. 2013; 19: 20–22. doi: 10.1038/nm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Magrath IT, Wexler LH, Dimitrov DS. Gress RE: Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997; 89: 3700–3707. [PubMed] [Google Scholar]
- 30.Azuma E, Nagai M, Qi J, Umemoto M, Hirayama M, Kumamoto T, Hiratake S, Komada Y. Sakurai M: CD4+ T-lymphocytopenia in long-term survivors following intensive chemotherapy in childhood cancers. Med PediatrOncol. 1998; 30: 40–45. [DOI] [PubMed] [Google Scholar]
- 31.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell DeathDiffer. 2014; 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JE, Jang MJ, Lee JI, Chung YH, Jeong JH, Hung CF, Kim D. Cancer cells containing nanoscale chemotherapeutic drugs generate antiovarian cancer-specific CD4+ T cells in peritoneal space. JImmunother. 2012; 35: 1–13. doi: 10.1097/CJI.0b013e3182328569. [DOI] [PubMed] [Google Scholar]
- 33.Germano G, Lamba S, Rospo G, Barault L, Magrì A, Maione F, Russo M, Crisafulli G, Bartolini A, Lerda G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017; 552: 116–120. doi: 10.1038/nature24673. [DOI] [PubMed] [Google Scholar]
- 34.Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005; 11: 7391–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo HY, Youn JM, Bae SH, Jang JW, Cha JH, Kim HL, Chun HJ, Choi BG, Choi JY, Sk Y. Efficacy and safety of metronomic chemotherapy for patients with advanced primary hepatocellular carcinoma with major portal vein tumor thrombosis. Korean J Hepatol. 2012; 18: 32–40. doi: 10.3350/kjhep.2012.18.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005; 97:: 1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 37.Tetef M, Doroshow J, Akman S, Coluzzi P, Leong L, Margolin K, Morgan RJ Jr., Raschko J, Shibata S, Somlo G. 5-Fluorouracil and high-dose calcium leucovorin for hepatocellular carcinoma: a phase II trial. Cancer Invest. 1995; 13: 460–463. [DOI] [PubMed] [Google Scholar]
- 38.Parikh PM, Fuloria J, Babu G, Doval DC, Awasthy BS, Pai VR, Prabhakaran PS, Ab B. A phase II study of gemcitabine and cisplatin in patients with advanced hepatocellular carcinoma. Trop Gastroenterol. 2005; 26: 115–118. [PubMed] [Google Scholar]
- 39.Hanahan D, Wagner EF, Rd P. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 2007; 21: 2258–2270. doi: 10.1101/gad.1583307. [DOI] [PubMed] [Google Scholar]
- 40.Maiti R. Metronomic chemotherapy. J Pharma Col Pharmacother. 2014; 5: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L. Chauffert B: Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007; 56: 641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer ImmunolImmunother. 2012; 61: 353–362. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermans IF, Chong TW, Palmowski MJ, Harris AL. Cerundolo V: synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003; 63: 8408–8413. [PubMed] [Google Scholar]
- 44.Suzuki E, Kapoor V, Jassar AS, Kaiser LR. Albelda SM: gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005; 11: 6713–6721. [DOI] [PubMed] [Google Scholar]
- 45.Garnett CT, Schlom J. Hodge JW: combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008; 14: 3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Arra C, Maiolino P, Izzo F, Tornesello ML, Aurisicchio L, Ciliberto G, et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer ImmunolImmunother. 2015; 64:: 1305–1314. doi: 10.1007/s00262-015-1698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treiber G, Wex T, Malfertheiner P. Impact of different anticancer regimens on biomarkers of angiogenesis in patients with advanced hepatocellular cancer. J Cancer ResClin Oncol. 2009; 135: 271–281. doi: 10.1007/s00432-008-0443-x. [DOI] [PubMed] [Google Scholar]
- 48.Ch H, Yc S, Zz L, Pj C, Yy S, Yh D, Hsu C, Al C. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010; 53: 126–131. doi: 10.1016/j.jhep.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 49.Hsu CY, Shen YC, Yu CW, Hsu C, Hu FC, Hsu CH, Chen BB, Wei SY, Cheng AL, Tt S. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011; 55: 858–865. doi: 10.1016/j.jhep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Shao YY, Lin ZZ, Hsu C, Lee KD, Hsiao CH, Lu YS, Huang CC, Shen YC, Hsu CH, Al C. Efficacy, safety, and potential biomarkers of thalidomide plus metronomic chemotherapy for advanced hepatocellular carcinoma. Oncology. 2012; 82: 59–66. doi: 10.1159/000336126. [DOI] [PubMed] [Google Scholar]
- 51.Brandi G, De RF, Agostini V, Di GS, Andreone P, Bolondi L, Serra C, Sama C, Golfieri R, Gramenzi A, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013; 18: 1256–1257. doi: 10.1634/theoncologist.2013-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballardini P, Marri I, Margutti G, Aliberti C, Benea G. Manfredini R: long-lasting response with metronomic capecitabine in advanced hepatocellular carcinoma. Tumori. 2010; 96: 768–770. [DOI] [PubMed] [Google Scholar]
- 53.Marinelli S, Granito A, Piscaglia F, Renzulli M, Stagni A, Bolondi L: metronomic capecitabine in patients with hepatocellular carcinoma unresponsive to or ineligible for sorafenib treatment: report of two cases. HepatMon 2013, 13:e11721 10.5812/hepatmon.11721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J. Lim M: Immune checkpoint modulators: An emerging antiglioma armamentarium. J Immunol Res. 2016; 2016: 4683607. doi: 10.1155/2016/4683607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:: 252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nirschl CJ. Drake CG: Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013; 19: 4917–4924. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P. Wang FS: PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011; 128: 887–896. [DOI] [PubMed] [Google Scholar]
- 58.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009; 15: 971–979. [DOI] [PubMed] [Google Scholar]
- 59.Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014; 59: 567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 60.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014; 2: 393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. NatImmunol. 2003; 4: 670–679. [DOI] [PubMed] [Google Scholar]
- 62.Sangro B, Gomez-Martin C, De La Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. JHepatol. 2013; 59: 81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Duffy AG, Ma C, Ulahannan SV, Rahma OE, Makarova-Rusher O, Cao L, Yu Y, Kleiner DE, Trepel J, Lee MJ, et al. Phase I and preliminary phase ii study of TRC105 in combination with sorafenib in hepatocellular Carcinoma. Clin Cancer Res. 2017; 23: 4633–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ab E-K, Sangro B, Yau T, Ts C, Kudo M, Hsu C, Ty K, Sp C, Trojan J, Thr W, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389: 2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buonaguro L. Developments in cancer vaccines for hepatocellular carcinoma. Cancer ImmunolImmunother. 2016; 65: 93–99. doi: 10.1007/s00262-015-1728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S, Loecher M, Iyer R. Immunomodulation in hepatocellular cancer. J Gastrointest Oncol. 2018; 9: 208–219. doi: 10.21037/jgo.2017.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988; 61: 1942–1956. [DOI] [PubMed] [Google Scholar]
- 68.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Ds C. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997; 336: 1855–1859. doi: 210.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 69.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009; 101: 1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 70.McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Aj P. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011; 54: 801–807. [DOI] [PubMed] [Google Scholar]
- 71.Wichajarn K, Kosalaraksa P, Wiangnon S. Incidence of hepatocellular carcinoma in children in Khon Kaen before and after national hepatitis B vaccine program. Asian Pac J Cancer Prev. 2008; 9: 507–509. [PubMed] [Google Scholar]
- 72.Qu C, Chen T, Fan C, Zhan Q, Wang Y, Lu J, Lu LL, Ni Z, Huang F, Yao H, et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: a cluster randomized controlled trial. PLoS Med. 2014; 11: e1001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Honda M. Kaneko S: comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011; 53: 1206–1216. [DOI] [PubMed] [Google Scholar]
- 74.Saini N, Srinivasan R, Chawla Y, Sharma S, Chakraborti A, Rajwanshi A. Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status. Liver Int. 2009; 29: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 75.Kojima H, Yokosuka O, Imazeki F, Saisho H, Omata M. Telomerase activity and telomere length in hepatocellular carcinoma and chronic liver disease. Gastroenterology. 1997; 112: 493–500. [DOI] [PubMed] [Google Scholar]
- 76.Sera T, Hiasa Y, Mashiba T, Tokumoto Y, Hirooka M, Konishi I, Matsuura B, Michitaka K, Udaka K. Onji M: wilms’ tumour 1 gene expression is increased in hepatocellular carcinoma and associated with poor prognosis. EurJCancer. 2008; 44: 600–608. [DOI] [PubMed] [Google Scholar]
- 77.Berasain C, Herrero JI, Garcia-Trevijano ER, Avila MA, Esteban JI, Mato JM. Prieto J: expression of Wilms’ tumor suppressor in the liver with cirrhosis: relation to hepatocyte nuclear factor 4 and hepatocellular function. Hepatology. 2003; 38: 148–157. [DOI] [PubMed] [Google Scholar]
- 78.Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T. Kaneko S: identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer. 2006; 118: 1194–1204. [DOI] [PubMed] [Google Scholar]
- 79.Ho M. Kim H: Glypican-3: a new target for cancer immunotherapy. EurJCancer. 2011; 47: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Y, Li H, Gao RL, Adeyemo O, Itkin M. Kaplan DE: Expansion of interferon-gamma-producing multifunctional CD4+ T-cells and dysfunctional CD8+ T-cells by glypican-3 peptide library in hepatocellular carcinoma patients. ClinImmunol. 2011; 139: 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caballero OL. Chen YT: Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009; 100: 2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMCCancer. 2010; 10: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bertino G, Demma S, Ardiri A, Proiti M, Mangia A, Gruttadauria S, Toro A, Di CI, Malaguarnera G, Bertino N, et al. The immune system in hepatocellular carcinoma and potential new immunotherapeutic strategies. BiomedResInt. 2015; 2015: 731469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH. Economou JS: T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003; 9: 5902–5908. [PubMed] [Google Scholar]
- 85.Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012; 18: 3686–3696. [DOI] [PubMed] [Google Scholar]
- 86.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. NatMed. 2007; 13: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 87.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. NatMed. 2009; 15: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 88.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010; 29:: 482–491. [DOI] [PubMed] [Google Scholar]
- 89.Lake RA. Robinson BW: Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer. 2005; 5:: 397–405. [DOI] [PubMed] [Google Scholar]
- 90.Schlom J, Arlen PM. Gulley JL: Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007; 13:: 3776–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wheeler CJ, Das A, Liu G, Yu JS. Black KL: clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004; 10: 5316–5326. [DOI] [PubMed] [Google Scholar]
- 92.Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, Fanton A, Bergoin E, Maynadie M, Arnould L, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin ExpImmunol. 2007; 150: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008; 14: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrizzo A, Mauriello A, Luciano A, Rea D, Barbieri A, Arra C, Maiolino P, Tornesello M, Gigantino V, Botti G, et al. Inhibition of tumor growth by cancer vaccine combined with metronomic chemotherapy and anti-PD-1 in a pre-clinical setting. Oncotarget. 2018; 9: 3576–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Terabe M. Berzofsky JA: immunoregulatory T cells in tumor immunity. Curr OpinImmunol. 2004; 16: 157–162. [DOI] [PubMed] [Google Scholar]
- 96.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF. Korangy F: increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005; 65: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 97.Tf G, La O, Fikuart A, Hochst B, Henschen S, Horning M, Mp M, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. JImmunother. 2010; 33: 211–218. [DOI] [PubMed] [Google Scholar]
- 98.Sharma P, Jp A. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015; 161: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015; 38: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ali OA, Lewin SA, Dranoff G, Dj M. Vaccines Combined with Immune Checkpoint Antibodies Promote Cytotoxic T-cell Activity and Tumor Eradication. Cancer Immunol Res. 2016; 4: 95–100. doi: 10.1158/2326-6066.CIR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD. Knutson KL: accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014; 74: 2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye Z, Qian Q, Jin H. Cancer vaccine: learning lessons from immune checkpoint inhibitors. J Cancer. 2018; 9: 263–268. doi: 10.7150/jca.20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017. 68(1): 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morales-Kastresana A, Sanmamed MF, Rodriguez I, Palazon A, Martinez-Forero I, Labiano S, Hervas-Stubbs S, Sangro B, Ochoa C, Rouzaut A, et al. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013; 19: 6151–6162. [DOI] [PubMed] [Google Scholar]


