Abstract
Vascular cognitive impairment (VCI) describes all forms of cognitive impairment caused by any type of cerebrovascular disease. Early identification of VCI is quite difficult due to the lack of both sensitive and specific biomarkers. Extensive damage to the white matter tracts, which connect the cortical and subcortical regions, has been shown in subcortical VCI (SVCI), the most common subtype of VCI that is caused by small vessel disease. Two specific MRI sequences, including diffusion tensor imaging (DTI) and functional MRI (fMRI), have emerged as useful tools for identifying subtle white matter changes and the intrinsic connectivity between distinct cortical regions. This review describes the advantages of these two modalities in SVCI research and the current DTI and fMRI findings on SVCI. Using DTI technique, a variety of studies found that white matter microstructural damages in the anterior and superior areas are more specific to SVCI. Similarly, functional brain abnormalities detected by fMRI have also been mainly shown in anterior brain areas in SVCI. The characteristic distribution of brain abnormalities in SVCI interrupts the prefrontal-subcortical loop that results in cognitive impairments in particular domains, which further confirms the ‘disconnection syndrome’ hypothesis. In addition, another MRI technique, arterial spin labelling (ASL), has been used to describe the disconnection patterns in a variety of conditions by measuring cerebral blood flow. The role of the ASL technique in SVCI research is also assessed. Finally, the review proposes the application of multimodality fusion in the investigation of SVCI pathogenesis.
Keywords: diffusion tensor imaging, disconnection syndrome, functional magnetic resonance imaging, subcortical vascular cognitive impairment
Introduction
Vascular cognitive impairment (VCI) encompasses all forms of cognitive impairment that affect at least one cognitive domain and are caused by any type of vascular abnormality. These cognitive deficits range from the mildest form to fully developed dementia, including vascular dementia (VaD).1 Traditionally, VaD is the second most common type of dementia after Alzheimer’s disease (AD). VaD accounts for 15%–20% cases of dementia in Europe and America,2 3 and it is even more common than AD in Asia due to the high prevalence of stroke.4 5 Almost 15%–30% subjects develop dementia within 3 months after a stroke.6 The prevalence of VCI may even be much higher. However, the prevalence of VCI has been underestimated due to the lack of a consistent criterion for the diagnosis. The concept of VCI has gained increasing attention worldwide because most of the vascular risk factors, such as ischaemic heart disease, hypertension, obesity, smoking, diabetes, hyperlipidaemia and hyperhomocysteinaemia, are modifiable. Early identification of VCI is of great importance, since managing these risk factors in a timely manner may prevent disease development and reduce disease progression.7 However, the diagnosis of VCI still commonly depends on the clinical signs and symptoms and the exclusion of other aetiologies, which are usually insufficient to differentiate various dementias. Biomarkers that precede and predict the onset of VCI are in the early developmental stages in clinical research.
VCI is mainly caused by three forms of cerebrovascular disease, including large vessel strokes, small vessel disease and cerebral amyloid angiopathy. The most common cause of VCI, small vessel disease, is characterised by microangiopathy and arteriolosclerosis and yields lacunes and diffuse injuries in both subcortical grey matter nuclei and white matter.8 VCI associated with small vessel disease, which is also termed subcortical VCI (SVCI), can progress in the absence of new cerebral infarcts but in the presence of diffuse white matter lesions, which are known as leukoaraiosis.9 SVCI has been considered a ‘disconnection syndrome’ due to the extensive damage to the white matter tracts or U-fibres, which connect cortical and subcortical regions. The concept of ‘disconnection syndrome’ refers to that a neurological deficit may result from a disturbance of effective connectivity between two cerebral processes than from primary injuries to those cerebral processes themselves.10 11
The pathology of SVCI
SVCI is a homogeneous form of VCI caused by small vessel disease. It is characterised by extensive white matter hyperintensities and multiple lacunar infarctions on MRI.12 The structural and functional injuries of small vessels in the brain play an important role in the pathology of SVCI. Vascular risk factors (eg, hypertension, diabetes mellitus, atrial fibrillation and hypercholesterolaemia) may induce neurovascular dysfunction through pathways mediated by vascular oxidative stress and inflammation.7 Oxidative stress promotes the release of prostanoids and vascular endothelial growth factor by inducing endothelial dysfunction. Then, prostanoids and vascular endothelial growth factor accelerate protein extravasation, vascular leakage and cytokine production. Inflammation, in turn, downregulates antioxidant defenses and upregulates the expression of reactive oxygen species-producing enzymes.7 The vicious circle disrupts the brain microenvironment and increases the brain’s susceptibility to ischaemic-hypoxic damages.13 Vascular risk factors are associated with diverse vascular pathologies, including atherosclerotic plaques in small arteries, segmental arterial disorganisation, hyaline deposition in small vessel wall and fibrinoid degeneration.14 15 These vascular pathologies reduce cerebral blood flow through deep penetrating arteries that supply subcortical nuclei, cortical projection fibres and association fibres. As a result, communication within and between cortical and subcortical regions is disrupted.16
Disrupted cortical–subcortical or cortical–cortical connections may result in ensuing cognitive impairments. Cognitive impairments in SVCI vary depending on the involvement of various brain areas. Generally, cognitive impairments in executive function, information processing and attention are relatively more common in SVCI.17 Deficits in these domains are associated with vascular lesions within frontosubcortical white matter and basal ganglia regions, which reflect impairments of the prefrontal-subcortical loop.8 18 Neuroimaging techniques are highly valuable for identifying diffuse white matter injuries and evaluating patients with SVCI. In general, MRI detects the white matter injuries more sensitively than cranial CT. MRI may detect impaired neurovascular function in the white matter that appears normal in CT.19 Two specific MRI sequences, diffusion tensor imaging (DTI) and functional MRI (fMRI) have been increasingly shown to detect early structural and functional brain alterations related to SVCI, especially in the last 2 years (figure 1). DTI and fMRI have emerged as useful tools for identifying subtle white matter changes and intrinsic connectivity between distinct cortical regions, respectively.20–22 A close link between DTI measurements and fMRI measurements has been confirmed by a series of studies.23–25 Combining DTI and fMRI findings may be highly valuable for the investigation of early and specific brain alterations in SVCI. Here, we review studies investigating structural and functional changes in SVCI using DTI or fMRI techniques (table 1). Furthermore, another MRI technique, arterial spin labelling (ASL), has been applied to measure cerebral blood flow (CBF) alterations in SVCI and will promisingly become an useful tool to detect the disconnection patterns in SVCI. We also discuss the role of the ASL technique in SVCI research and propose the application of multimodality fusion in the investigation of SVCI pathogenesis.
Figure 1.
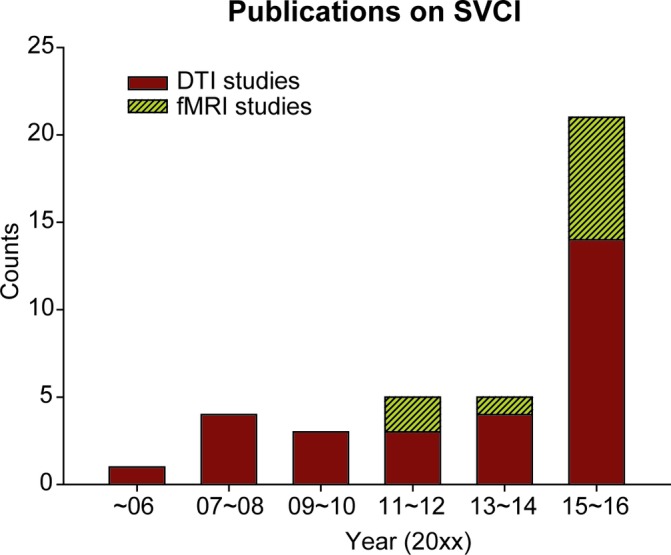
The number of publications on subcortical vascular cognitive impairment (SVCI) using diffusion tensor imaging (DTI) or functional MRI (fMRI) techniques. DTI and fMRI technique have been increasingly used to detect structural and functional brain alterations related to SVCI, especially in the last 2 years.
Table 1.
The examples of DTI and fMRI studies in SVCI
| Study | Subjects | N | Neuroimaging technique | Main findings |
| Xu et al 32 | SVCI and normal controls | 42 | DTI and conventional MRI | DTI detected FA and MD alterations in normal-appearing white matter in SVCI subjects. DTI changes correlated with cognition better than did conventional MRI. |
| Kim et al 37 | SVCI | 61 | DTI and conventional MRI | DTI abnormalities in supratentorial regions correlated with cognitive deficits better than did the ischaemic burden detected by conventional structural MRI. |
| Lin et al 38 | SVCI and cognitively normal subjects with subcortical ischaemic vascular disease | 50 | DTI | SVCI subjects displayed decreased FA and increased MD in all supratentorial regions, which correlated with cognitive dysfunction. |
| Kim et al 39 | Subcortical VaD, AD and normal controls | 128 | DTI | Patients with subcortical VaD showed decreased FA and increased MD in all white matter regions. |
| Zhou et al 40 | SVCI and normal controls | 36 | DTI | SVCI subjects showed lower FA values throughout the brain. |
| Jung et al 41 | SVCI and normal controls | 169 | DTI | SVCI subjects displayed decreased FA in multiple white matter tracts neighbouring and providing connections between grey matter regions. |
| Shim et al 42 | SVCI, MCI and normal controls | 57 | DTI | A greater decrease in FA in the centrum semiovale and parietal regions in SVCI subjects, and the lowest FA in the hippocampus in MCI subjects. |
| Chen et al 43 | Subcortical VaD, MCI, AD, FTD and normal controls | 85 | DTI | White matter abnormalities mainly in the frontal cortical regions, the genu of the corpus callosum and periventricular regions in subcortical VaD subjects. |
| Zarei et al 44 | VaD, AD and normal controls | 51 | DTI | The decreased FA in the transcallosal prefrontal tracts was the most significant biomarker for VaD. |
| Sun et al 55 | SVCI and cognitively normal elderly with subcortical ischaemic vascular disease | 34 | Resting-state fMRI | Decreased DMN FC with frontal, anterior cingulate and temporal regions and increased FC with temporal and parietal regions in SVCI subjects. |
| Kim et al 57 | Subcortical VaD, AD, mixed dementia and normal controls | 152 | Resting-state fMRI | Lower FC in frontal and anterior insular regions in subcortical VaD subjects. |
| Zhou et al 56 | SVCI and normal controls | 55 | Structural and resting-state fMRI | Decreased FC between medial prefrontal cortex and anterior cingulate cortex and supplementary motor area in SVCI subjects. |
| Yi et al 58 | SVCI and normal controls | 54 | Structural and resting-state fMRI | Decreased low-frequency oscillations amplitudes in the anterior part of the DMN and increased amplitudes in the posterior part of the DMN in SVCI subjects. |
| Yi et al 59 | SVCI and normal controls | 47 | Resting-state fMRI | Decreased intramodular connectivity in the prefrontal cortex, parietal cortex, anterior insula and middle cingulate cortex and increased intermodular connectivity in the parietal cortex in SVCI subjects. |
| Li et al 62 | Subcortical VaD, AD and normal controls | 20 | Task-fMRI | The activation in the frontal, parietal and anterior cingulate cortex was reduced in subcortical VaD subjects during performing a Stroop test. |
| Li et al 63 | SVCI, subcortical VaD and normal controls | 35 | Task-fMRI | Mild SVCI subjects displayed significantly increased activation in frontal regions, whereas VaD subjects showed decreased activation during performing a Stroop test. |
AD, Alzheimer’s disease; DMN, default mode network; DTI, diffusion tensor imaging; FA, fractional anisotropy; FC, functional connectivity; fMRI, functional MRI; FTD, frontotemporal dementia; MCI, mild cognitive impairment; MD, mean diffusivity; SVCI, subcortical vascular cognitive impairment; VaD, vascular dementia; VCI, vascular cognitive impairment.
DTI and white matter integrity
White matter tracts consist of association fibres interconnecting cortical regions within each hemisphere (eg, superior/inferior longitudinal fasciculus, cingulum and uncinate fasciculus), projection fibres interconnecting cortical regions with subcortical regions, brain stem or spinal cord (eg, geniculocalcarine tracts, frontopontine tracts and thalamocortical tracts) and commissural fibres interconnecting brain regions between hemispheres (eg, anterior commissure and corpus callosum).26 These white matter tracts are vital for maintaining normal cortical–cortical and cortical–subcortical connections. DTI can potentially detect the microstructural integrity of white matter by measuring the directional water diffusion within tissues. Axon and myelin sheath membranes restrict the movement of water molecules in a single direction; water molecules can diffuse along the different directions of the axon but is obstructed orthogonally by the myelin sheath. Two common DTI-derived measurements, fractional anisotropy (FA) and mean diffusivity (MD), characterise the patterns of water diffusion within tissues. FA describes the anisotropy of diffusion, and MD reflects the average magnitude of diffusion in all directions.27 Thus, tract injuries are represented by a decreased FA and an increased MD.
Several studies found that DTI measurements correlated with conventional structural measurements, including brain or lesions volume measured by T1 sequence and the intensity and volume of white matter hyperintensities measured by fluid attenuation inversion recovery sequence.28–30 Currently, DTI may be the most sensitive structural technique for assessing white matter microstructural damages due to cerebrovascular disease.31 DTI quantitatively measures microstructural changes in brain tissue that appears normal on other MRI sequences. For example, a longitudinal study investigated the alterations of DTI measurements and conventional structural MRI measurements in subjects with leukoaraiosis and lacunar stroke.20 The study demonstrated that DTI detected aberrant FA and MD at a 1-year follow-up, whereas no detectable changes were observed in other structural MRI measurements.20 Another study also found alterations in FA and MD in normal-appearing white matter in SVCI subjects. In addition, these alterations in FA and MD were associated with memory and attention-executive scores in the patients.32 Another advantage of DTI is that it displays a higher correlation with cognitive function than conventional structural MRI. Conventional structural MRI does not show a correlation between white matter lesions and cognitive impairments,33 and these white matter lesions are even common in the cognitively normal elderly population.34 35 In contrast, multiple associations between specific tract alterations and certain cognitive impairments have been demonstrated by DTI. A study explored the behavioural significance of DTI measurements in subjects with small vessel disease and found that DTI measurements (FA and MD) in the cingulum bundle correlated with verbal memory scores, while the MD value in the frontal lobe correlated with psychomotor speed performance.36 In addition, DTI measurements in the corpus callosum correlated with global cognitive function, executive function, psychomotor speed and concept shifting.36 This study suggested that DTI could serve as a useful tool to predict the cognitive consequences of small vessel disease. Another study of patients with SVCI also demonstrated that DTI abnormalities in supratentorial regions correlated with cognitive deficits better than the ischaemic burden detected by conventional structural MRI.37 These findings suggest that the white matter integrity measured by DTI correlates with cognitive deficits better than does the burden of the ischaemic lesions. The disconnection of specific white matter tracts connecting grey matter regions may contribute to the cognitive deficits in SVCI, which supports the ‘disconnection’ hypothesis. Therefore, DTI may serve as a sensitive tool to explore subtle white matter abnormalities and predict ensuing cognitive deficits in SVCI.
In addition to its high sensitivity, DTI also detects specific distributions of DTI alterations in SVCI relative to other types of dementia. Compared with cognitively normal subjects, subjects with various stages of SVCI, including mild SVCI and subcortical VaD, displayed DTI abnormalities in almost all white matter regions.38–41 However, the DTI abnormalities in the anterior and superior subcortical regions are more specific to SVCI. A study investigated the microstructural alterations between SVCI and non-vascular mild cognitive impairment (MCI), an intermediate state between normal ageing and early AD. Compared with the non-vascular MCI subjects, SVCI subjects showed a greater decrease in FA in the centrum semiovale and parietal regions. In contrast, the non-vascular MCI subjects had the lowest FA in the hippocampus.42 A study compared the DTI changes between subcortical VaD and other types of dementia and found that subcortical VaD subjects had white matter abnormalities mainly in the frontal cortical regions, the genu of the corpus callosum and periventricular regions.43 Tractography analysis also suggested that the decreased FA in the transcallosal prefrontal tracts was the most significant biomarker for VaD.44 On the other hand, white matter damages in the temporal lobe and hippocampus, which are associated with episodic memory deficits, may be more specific for AD.45 Additionally, DTI changes in the frontal and temporal lobes seem to be more pronounced in frontotemporal dementia.43 The characteristic distribution of tract damages in SVCI interrupts the prefrontal-subcortical loop that results in cognitive impairments in executive function, information processing and attention,17 which further confirms the ‘disconnection syndrome’ hypothesis.
fMRI and functional networks
Another MRI technique, fMRI, provides new ways to explore the ‘disconnection’ in SVCI. fMRI detects regional brain activity by measuring alterations in local blood flow and oxygenation, namely, blood-oxygen-level-dependent (BOLD) contrast. According to the task status (ie, task and no task) of subjects during fMRI scanning, fMRI is divided into task-fMRI and resting-state fMRI. Particularly, task-fMRI has been applied to map and localise functionally specialised brain areas related to specific task performance, and resting-state fMRI has been used to assess the synchronous activity of distant brain areas. A series of resting-state brain functional networks have been identified on the basis of the temporal correlations between the intrinsic fluctuations of BOLD signals across functionally related areas, which is also known as functional connectivity (FC).22 Notably, resting-state functional networks resemble the patterns of networks activated during the performance of specific tasks.46 The physiological basis of the fMRI signals has been revealed by the significant correlation between fMRI signals and local field potentials measured by intracortical recordings.47 Furthermore, a genetic basis underlying the synchronous activity in functional networks has also been shown by a recent study. Functional networks are supported by the orchestrated activity of dozens of genes regulating synaptic function and ion channel activity.48
Similar to DTI measurements, functional brain abnormalities detected by fMRI have also been detected prior to conventional structural changes (eg, atrophy) and clinical symptoms.49 These functional abnormalities can even be detected in healthy subjects at risk of cognitive impairment.50 51 Furthermore, the FC patterns of resting-state functional networks exhibit a high correlation with cognition in specific domains. This correlation has been often confirmed in three commonly described networks, including the default mode network (DMN), the executive control network and the dorsal attention network. The DMN is associated with episodic memory, executive function and processing speed.52 The executive control network is associated with executive control and working memory. And the dorsal attention network is associated with cortical attention and voluntary orienting.53
Resting-state fMRI studies of SVCI have revealed specific distributions of FC alterations, mostly in the DMN that consists of bilateral posterior cingulate cortex, anterior cingulate cortex, inferior parietal lobule, medial prefrontal cortex, lateral temporal cortex and hippocampus formation.54 For example, the DMN FC with anterior cingulate cortex, frontal cortex and temporal cortex was decreased in patients with SVCI.55 The FC between the medial prefrontal cortex and the anterior cingulate cortex and between the thalamus and the orbitofrontal lobe were also decreased in patients with SVCI.56 A study comparing the DMN FC patterns between subcortical VaD and AD showed that the FC in the medial frontal and superior frontal gyri was prominently decreased in patients with subcortical VaD.57 A study of SVCI explored the low-frequency oscillation (LFO) amplitudes in the DMN and showed that the LFO amplitudes in the anterior portion of the DMN (eg, the medial prefrontal cortex) were decreased, while those in the posterior cingulate cortex were increased.58 Furthermore, the topological properties of FC were also investigated in patients with SVCI. Both disrupted intramodular connectivity in the prefrontal cortex, parietal cortex, anterior insula and middle cingulate cortex and increased intermodular connectivity in the parietal cortex were observed in patients with SVCI, and the latter finding was associated with impaired memory function in these patients.59 These resting-state fMRI studies support the major role of functional abnormalities in the anterior brain areas in SVCI. On the other hand, a handful of task fMRI studies have also shown similar distributions of regional activity alterations in SVCI subjects. Multiple brain regions are activated on task fMRI signals during performing a task.60 These regions form a particular network displaying similar task-related activities, and the disrupted network pattern suggests the disconnection of these regions in SVCI subjects. A Stroop test is commonly applied in task-fMRI studies to assess cortical attention and executive function and is usually associated with activations of the middle and inferior frontal gyrus, anterior cingulate, inferior parietal lobule and insular in healthy population.61 A task-fMRI study demonstrated that the activation in the frontal regions, parietal regions and anterior cingulate cortex, was markedly reduced in patients with VaD during a Stroop test performance compared with healthy controls. In addition, the reduced activation was related to the impaired performance in patients with VaD, suggesting that the disconnection of these regions may contribute to the cognitive deficits in these patients.62 Another task-fMRI study that used both patients with mild SVCI and patients with VaD found different activation patterns in the frontal cortex between the two groups during a Stroop task. The patients with mild SVCI displayed significantly increased activation, whereas the patients with VaD showed decreased activation, which the authors interpreted as a frontal compensation in the mild SVCI stage and a dysfunction in the VaD stage.63 The disrupted functional synchrony and regional activity shown in SVCI suggest that fMRI can detect functional changes caused by the ‘disconnection’, and these functional changes may reflect cognitive deficits in SVCI.
Considerations for multimodality fusion in SVCI
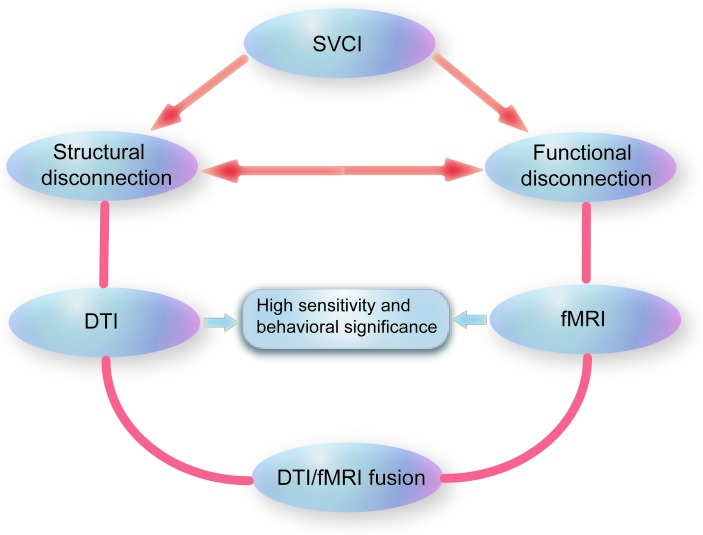
Both DTI and fMRI can describe brain alterations related to the ‘disconnection’ in SVCI; DTI measures the structural changes in tracts connecting distinct brain regions, whereas fMRI measures synchronous activities of functionally related regions (figure 2). Notably, the structures with aberrant DTI changes largely coincided with the brain areas associated with abnormal FC changes in patients with SVCI. All these findings support the major role of the anterior part of the brain in the pathogenesis of SVCI. However, to the best of our knowledge, no study has directly compared or combined DTI changes and fMRI changes in SVCI. A recent study investigated the grey matter volume and FC of the thalamus and the medial prefrontal cortex in patients with SVCI. This study found decreased FC in both cortical and subcortical regions, which also displayed notable grey matter volume loss, and indicated that FC deficits may be partly due to grey volume reduction.56 However, the study did not assess changes in the white matter tracts, which are the most impaired areas in SVCI.
Figure 2.
Application of diffusion tensor imaging (DTI) and functional MRI (fMRI) in subcortical vascular cognitive impairment (SVCI). DTI measures the structural connections in SVCI, whereas fMRI measures the functional connections. Both DTI and fMRI are highly sensitive techniques and display high correlations with cognitive impairments in SVCI. Due to the close link between brain structural connections and functional connections, the integration of DTI and fMRI techniques may further the investigation of SVCI pathogenesis.
Given the intrinsic integration of the white matter and grey matter, multiple studies have consistently indicated that a close and complex link exists between the structure and the function of the brain. Brain regions exhibiting significant FC are also structurally connected in DTI anatomy; regions displaying stronger FC are also more strongly connected in structure.23 24 Intact white matter integrity plays a key role in the synchronous activity and neural activation of brain functional networks, and the patterns of functional networks also reflect the structural architecture of the brain. However, the patterns of FC do not simply equate to those of structural connectivity; the relationship is also highly complex. FC does not only rely on monosynaptic connections. Instead, polysynaptic pathway connections, bidirectional circuits and common-source connections may mediate FC.64 65 Nevertheless, this close and complex link between structure and function has received increasing interest and contributed to a better understanding of the intrinsic integration of neural resources.
Given the close link between the structure and the function and the consistency in the distributions of brain abnormalities detected by DTI and fMRI in SVCI, it would not be surprising if the structural and functional properties covary across patients with SVCI. Fusing the DTI and fMRI modalities may provide new opportunities to deepen the understanding of disconnection syndromes. Actually, the combination of DTI and fMRI modalities has been applied by two studies to investigate structural and functional disconnection in alexia and AD.66 67 Alexia and AD are also considered as disconnection syndromes due to the disease patterns; a lesion caused by dysplasia, genetic factors or malnutrition (for alexia) or amyloid or tau pathology (for AD), results in a reduction in communications between brain regions that are vital to maintain normal cognitive function.11 68 Both studies showed that structural disconnection measured by DTI was associated with functional disconnection measured by fMRI, and both the structural and functional disconnections contributed to behavioural disturbance in the two conditions.66 67 The combination of DTI and fMRI modalities may be also of great value to the investigation of SVCI pathogenesis. First, DTI/fMRI fusion may contribute to the investigation of brain markers specific for SVCI. Combining DTI and fMRI measurements can reveal the entirety of the neurobiological pathways with both structural and functional abnormalities in SVCI. Second, DTI/fMRI fusion may be valuable for the identification of mixed dementia with both vascular pathology and AD pathology. Increasing evidence suggests that vascular pathology interacts with AD pathology (eg, amyloid beta pathology),69 and dementia exhibiting both pathologies is a common subtype of VCI.7 While AD pathology is measured by positron emission tomography (PET) imaging or cerebrospinal fluid analyses, vascular pathology may be undetectable when using conventional neuroimaging technique, as described above. The application of DTI/fMRI fusion may aid in the exploration of subtle structural and functional brain abnormalities, which are irrelevant to the AD pathology shown on PET imaging but associated with vascular pathology. Thus, the combination of DTI/fMRI fusion with measurements of AD pathology may be valuable for the identification of mixed pathological conditions.
ASL in SVCI
With the improved signal-to-noise ratio of modern high-field MRI systems, another MRI technique, ASL, has received an increasing interest in neurological disease within recent years. ASL measures CBF through the direct magnetic labelling of blood water as an ‘endogenous’ tracer at the tissue level.70 Several studies showed significant reductions in CBF mainly in frontal, parietal and temporal cortices in patients with SVCI using the ASL technique.71 72 The distribution of abnormal CBF coincides with that of DTI changes and FC changes in SVCI subjects. Reduced cerebral perfusion may result in neuronal and glial death, brain volume loss and impaired neuronal function.31 A study found that the reduced CBF in frontal cortex was associated with increased subcortical white matter lesions in patients with SVCI.71 Another study explored the relationship between CBF and cognition in patients with SVCI and showed that the reduced CBF in the frontal lobe, temporal lobe and several deep nuclei was associated with the degree of cognitive impairment in patients with SVCI.73 Notably, the CBF fluctuations of different brain areas may be concurrent, contributing to the concept of CBF connectivity based on CBF fluctuations.74 The functional networks identified using CBF connectivity display similar spatial patterns with networks based on BOLD signals, including DMN and executive control network.75 ASL has been applied to assess the CBF connectivity patterns in a variety of disorders, for example, schizophrenia, Parkinson’s disease and autism.76–78 Promisingly, ASL would also be used to detect the disconnection patterns in SVCI. Furthermore, a recent study found significant correlations between CBF and FC strength based on BOLD signals in multiple functional networks.79 The study suggested a combination of ASL and resting-state BOLD fMRI in the assessment of brain networks, since ASL providing reliable network-specific quantitative CBF measurements could be complementary to BOLD signals with a high temporal resolution. On the other hand, due to the low CBF level and delayed arterial arrival times in the white matter, the signal-to-noise ratio of the ASL image is remarkably low in the deep white matter,80 which may limit the application of the ASL technique in SVCI research. Nevertheless, ASL measures the CBF, which maintains neuronal structure and function. In the future, ASL may be combined with DTI and fMRI, which measure brain structural and functional alterations, in the investigation of SVCI pathogenesis.
Limitations of DTI and fMRI techniques
Several limitations of these techniques should also be emphasised. First, DTI and fMRI measurements are not highly specific to the type of pathogenesis. FA values in DTI are very sensitive to microstructural changes in the brain tissue but are not specific to the type of lesions. FA values are naturally decreased even in certain normal brain areas where nerve fibres cross.81 Functional alterations in fMRI also lack specificity. For example, the disrupted DMN has been shown in multiple disorders, including dementia, psychiatry, white matter disease, migraine and addiction.53 Second, neither DTI technique nor fMRI technique has an established standard in acquisition and analysis of imaging data. This may explain the inconsistent findings across studies.53 Third, these techniques, especially for fMRI, exhibit more variability than conventional neuroimaging techniques. FC is more frequently affected by multiple factors, including task demands, learning, prior experiences and consciousness.82 83 The findings are not stable enough even within studies and should be treated with caution.
Conclusions
The mechanisms underlying the ‘disconnection’ in SVCI are far from well understood. Both DTI and fMRI techniques serve as highly sensitive modalities measuring structural and functional brain abnormalities in SVCI. The microstructural damages and functional abnormalities in the anterior portion of the brain are more specific for SVCI, which further confirms the ‘disconnection syndrome’ hypothesis. The close link between brain structure and function suggests that the integration of the DTI and fMRI modalities may be valuable for the investigation of SVCI pathogenesis.
Footnotes
Contributors: QY was involved in the manuscript writing. FB was involved in the design, interpretation and writing of the review.
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81671665), Jiangsu Provincial Key Medical Talents (grant number ZDRCA2016085) and the Natural Science Foundation of Jiangsu Province (grant number BK20160071).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hachinski V, Iadecola C, Petersen RC, et al. . National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–41. 10.1161/01.STR.0000237236.88823.47 [DOI] [PubMed] [Google Scholar]
- 2. Fratiglioni L, Launer LJ, Andersen K, et al. . Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54:S10–15. [PubMed] [Google Scholar]
- 3. Plassman BL, Langa KM, Fisher GG, et al. . Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125–32. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang ZX, Zahner GE, Román GC, et al. . Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 2005;62:447–53. 10.1001/archneur.62.3.447 [DOI] [PubMed] [Google Scholar]
- 5. Yamada M, Mimori Y, Kasagi F, et al. . Incidence of dementia, Alzheimer disease, and vascular dementia in a Japanese population: Radiation Effects Research Foundation adult health study. Neuroepidemiology 2008;30:152–60. 10.1159/000122332 [DOI] [PubMed] [Google Scholar]
- 6. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8:1006–18. 10.1016/S1474-4422(09)70236-4 [DOI] [PubMed] [Google Scholar]
- 7. Gorelick PB, Scuteri A, Black SE, et al. . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672–713. 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Román GC, Erkinjuntti T, Wallin A, et al. . Subcortical ischaemic vascular dementia. Lancet Neurol 2002;1:426–36. 10.1016/S1474-4422(02)00190-4 [DOI] [PubMed] [Google Scholar]
- 9. Bracco L, Campani D, Baratti E, et al. . Relation between MRI features and dementia in cerebrovascular disease patients with leukoaraiosis: a longitudinal study. J Neurol Sci 1993;120:131–6. 10.1016/0022-510X(93)90263-X [DOI] [PubMed] [Google Scholar]
- 10. Geschwind N. Disconnexion syndromes in animals and man. I. Brain 1965;88:237–94. 10.1093/brain/88.2.237 [DOI] [PubMed] [Google Scholar]
- 11. Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain 2005;128:2224–39. 10.1093/brain/awh622 [DOI] [PubMed] [Google Scholar]
- 12. de Mendonça A, Ribeiro F, Guerreiro M, et al. . Clinical significance of subcortical vascular disease in patients with mild cognitive impairment. Eur J Neurol 2005;12:125–30. 10.1111/j.1468-1331.2004.00892.x [DOI] [PubMed] [Google Scholar]
- 13. Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke 2009;40:S40–S44. 10.1161/STROKEAHA.108.533638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thal DR, Grinberg LT, Attems J. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 2012;47:816–24. 10.1016/j.exger.2012.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke 2015;17:2–6. 10.5853/jos.2015.17.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dey AK, Stamenova V, Turner G, et al. . Pathoconnectomics of cognitive impairment in small vessel disease: A systematic review. Alzheimers Dement 2016;12:831–45. 10.1016/j.jalz.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 17. O’Brien JT, Erkinjuntti T, Reisberg B, et al. . Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. 10.1016/S1474-4422(03)00305-3 [DOI] [PubMed] [Google Scholar]
- 18. Hsu YH, Huang CF, Lo CP, et al. . Frontal assessment battery as a useful tool to differentiate mild cognitive impairment due to subcortical ischemic vascular disease from Alzheimer disease. Dement Geriatr Cogn Disord 2016;42:331–41. 10.1159/000452762 [DOI] [PubMed] [Google Scholar]
- 19. Jagust WJ, Zheng L, Harvey DJ, et al. . Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol 2008;63:72–80. 10.1002/ana.21296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nitkunan A, Barrick TR, Charlton RA, et al. . Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke 2008;39:1999–2005. 10.1161/STROKEAHA.107.507475 [DOI] [PubMed] [Google Scholar]
- 21. O’Sullivan M, Morris RG, Huckstep B, et al. . Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 2004;75:441–7. 10.1136/jnnp.2003.014910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Damoiseaux JS, Rombouts SA, Barkhof F, et al. . Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 2006;103:13848–53. 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagmann P, Cammoun L, Gigandet X, et al. . Mapping the structural core of human cerebral cortex. PLoS Biol 2008;6:e159 10.1371/journal.pbio.0060159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khalsa S, Mayhew SD, Chechlacz M, et al. . The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. Neuroimage 2014;102(Pt 1):118–27. 10.1016/j.neuroimage.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 25. van Eimeren L, Grabner RH, Koschutnig K, et al. . Structure-function relationships underlying calculation: a combined diffusion tensor imaging and fMRI study. Neuroimage 2010;52:358–63. 10.1016/j.neuroimage.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 26. Schmahmann JD, Smith EE, Eichler FS, et al. . Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 2008;1142:266–309. 10.1196/annals.1444.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed 2002;15:435–55. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- 28. Rovaris M, Iannucci G, Cercignani M, et al. . Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: study with whole-brain tissue histogram analysis. Radiology 2003;227:731–8. 10.1148/radiol.2273020721 [DOI] [PubMed] [Google Scholar]
- 29. Takao H, Abe O, Yamasue H, et al. . Gray and white matter asymmetries in healthy individuals aged 21-29 years: a voxel-based morphometry and diffusion tensor imaging study. Hum Brain Mapp 2011;32:1762–73. 10.1002/hbm.21145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhan W, Zhang Y, Mueller SG, et al. . Characterization of white matter degeneration in elderly subjects by magnetic resonance diffusion and FLAIR imaging correlation. Neuroimage 2009;47(Suppl 2):T58–T65. 10.1016/j.neuroimage.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banerjee G, Wilson D, Jäger HR, et al. . Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment. Biochim Biophys Acta 2016;1862:926–38. 10.1016/j.bbadis.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 32. Xu Q, Zhou Y, Li YS, et al. . Diffusion tensor imaging changes correlate with cognition better than conventional MRI findings in patients with subcortical ischemic vascular disease. Dement Geriatr Cogn Disord 2010;30:317–26. 10.1159/000320491 [DOI] [PubMed] [Google Scholar]
- 33. Sabri O, Ringelstein EB, Hellwig D, et al. . Neuropsychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke 1999;30:556–66. 10.1161/01.STR.30.3.556 [DOI] [PubMed] [Google Scholar]
- 34. Chimowitz MI, Awad IA, Furlan AJ. Periventricular lesions on MRI. Facts and theories. Stroke 1989;20:963–7. 10.1161/01.STR.20.7.963 [DOI] [PubMed] [Google Scholar]
- 35. Fein G, Van Dyke C, Davenport L, et al. . Preservation of normal cognitive functioning in elderly subjects with extensive white-matter lesions of long duration. Arch Gen Psychiatry 1990;47:220–3. 10.1001/archpsyc.1990.01810150020004 [DOI] [PubMed] [Google Scholar]
- 36. Tuladhar AM, van Norden AG, de Laat KF, et al. . White matter integrity in small vessel disease is related to cognition. Neuroimage Clin 2015;7:518–24. 10.1016/j.nicl.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SH, Park JS, Ahn HJ, et al. . Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: correlates with cognitive and motor deficits. J Neuroimaging 2011;21:317–24. 10.1111/j.1552-6569.2010.00527.x [DOI] [PubMed] [Google Scholar]
- 38. Lin L, Xue Y, Duan Q, et al. . Microstructural white matter abnormalities and cognitive dysfunction in subcortical ischemic vascular disease: an atlas-based diffusion tensor analysis study. J Mol Neurosci 2015;56:363–70. 10.1007/s12031-015-0550-5 [DOI] [PubMed] [Google Scholar]
- 39. Kim YJ, Kwon HK, Lee JM, et al. . White matter microstructural changes in pure Alzheimer’s disease and subcortical vascular dementia. Eur J Neurol 2015;22:709–16. 10.1111/ene.12645 [DOI] [PubMed] [Google Scholar]
- 40. Zhou Y, Qun-Xu, Qin LD, et al. . A primary study of diffusion tensor imaging-based histogram analysis in vascular cognitive impairment with no dementia. Clin Neurol Neurosurg 2011;113:92–7. 10.1016/j.clineuro.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 41. Jung NY, Han CE, Kim HJ, et al. . Tract-specific correlates of neuropsychological deficits in patients with subcortical vascular cognitive impairment. J Alzheimers Dis 2016;50:1125–35. 10.3233/JAD-150841 [DOI] [PubMed] [Google Scholar]
- 42. Shim YS, Yoon B, Shon YM, et al. . Difference of the hippocampal and white matter microalterations in MCI patients according to the severity of subcortical vascular changes: neuropsychological correlates of diffusion tensor imaging. Clin Neurol Neurosurg 2008;110:552–61. 10.1016/j.clineuro.2008.02.021 [DOI] [PubMed] [Google Scholar]
- 43. Chen TF, Lin CC, Chen YF, et al. . Diffusion tensor changes in patients with amnesic mild cognitive impairment and various dementias. Psychiatry Res 2009;173:15–21. 10.1016/j.pscychresns.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 44. Zarei M, Damoiseaux JS, Morgese C, et al. . Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke 2009;40:773–9. 10.1161/STROKEAHA.108.530832 [DOI] [PubMed] [Google Scholar]
- 45. Fu JL, Zhang T, Chang C, et al. . The value of diffusion tensor imaging in the differential diagnosis of subcortical ischemic vascular dementia and Alzheimer’s disease in patients with only mild white matter alterations on T2-weighted images. Acta Radiol 2012;53:312–7. 10.1258/ar.2011.110272 [DOI] [PubMed] [Google Scholar]
- 46. Keller CJ, Bickel S, Honey CJ, et al. . Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci 2013;33:6333–42. 10.1523/JNEUROSCI.4837-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Logothetis NK, Pauls J, Augath M, et al. . Neurophysiological investigation of the basis of the fMRI signal. Nature 2001;412:150–7. 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- 48. Richiardi J, Altmann A, Milazzo AC, et al. . Brain networks. Correlated gene expression supports synchronous activity in brain networks. Science 2015;348:1241–4. 10.1126/science.1255905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry 2013;74:340–7. 10.1016/j.biopsych.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heise V, Filippini N, Trachtenberg AJ, et al. . Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. Neuroimage 2014;98:23–30. 10.1016/j.neuroimage.2014.04.081 [DOI] [PubMed] [Google Scholar]
- 51. Nichols LM, Masdeu JC, Mattay VS, et al. . Interactive effect of apolipoprotein e genotype and age on hippocampal activation during memory processing in healthy adults. Arch Gen Psychiatry 2012;69:804–13. 10.1001/archgenpsychiatry.2011.1893 [DOI] [PubMed] [Google Scholar]
- 52. Shaw EE, Schultz AP, Sperling RA, et al. . Functional connectivity in multiple cortical networks is associated with performance across cognitive domains in older adults. Brain Connect 2015;5:505–16. 10.1089/brain.2014.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology 2014;272:29–49. 10.1148/radiol.14132388 [DOI] [PubMed] [Google Scholar]
- 54. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008;1124:1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 55. Sun YW, Qin LD, Zhou Y, et al. . Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav Brain Res 2011;223:388–94. 10.1016/j.bbr.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 56. Zhou X, Hu X, Zhang C, et al. . Aberrant functional connectivity and structural atrophy in subcortical vascular cognitive impairment: relationship with cognitive impairments. Front Aging Neurosci 2016;8:14 10.3389/fnagi.2016.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim HJ, Cha J, Lee JM, et al. . Distinctive resting state network disruptions among Alzheimer’s dDisease, subcortical vascular dementia, and mixed dementia patients. J Alzheimers Dis 2016;50:709–18. 10.3233/JAD-150637 [DOI] [PubMed] [Google Scholar]
- 58. Yi L, Wang J, Jia L, et al. . Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state fMRI study. PLoS One 2012;7:e44758 10.1371/journal.pone.0044758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yi LY, Liang X, Liu DM, et al. . Disrupted topological organization of resting-state functional brain network in subcortical vascular mild cognitive impairment. CNS Neurosci Ther 2015;21:846–54. 10.1111/cns.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biswal B, Yetkin FZ, Haughton VM, et al. . Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 61. Li C, Zheng J, Wang J, et al. . An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer’s disease. Curr Alzheimer Res 2009;6:525–30. 10.2174/156720509790147142 [DOI] [PubMed] [Google Scholar]
- 62. Li C, Zheng J, Wang J, et al. . Comparison between Alzheimer’s disease and subcortical vascular dementia: attentional cortex study in functional magnetic resonance imaging. J Int Med Res 2011;39:1413–9. 10.1177/147323001103900428 [DOI] [PubMed] [Google Scholar]
- 63. Li C, Zheng J, Wang J. An fMRI study of prefrontal cortical function in subcortical ischemic vascular cognitive impairment. Am J Alzheimers Dis Other Demen 2012;27:490–5. 10.1177/1533317512455841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Adachi Y, Osada T, Sporns O, et al. . Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex 2012;22:1586–92. 10.1093/cercor/bhr234 [DOI] [PubMed] [Google Scholar]
- 65. Shen K, Bezgin G, Hutchison RM, et al. . Information processing architecture of functionally defined clusters in the macaque cortex. J Neurosci 2012;32:17465–76. 10.1523/JNEUROSCI.2709-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Molko N, Cohen L, Mangin JF, et al. . Visualizing the neural bases of a disconnection syndrome with diffusion tensor imaging. J Cogn Neurosci 2002;14:629–36. 10.1162/08989290260045864 [DOI] [PubMed] [Google Scholar]
- 67. Wang Z, Wang J, Zhang H, et al. . Interhemispheric functional and structural disconnection in alzheimer’s disease: a combined resting-state fMRI and DTI study. PLoS One 2015;10:e0126310 10.1371/journal.pone.0126310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brier MR, Thomas JB, Ances BM. Network dysfunction in Alzheimer’s disease: refining the disconnection hypothesis. Brain Connect 2014;4:299–311. 10.1089/brain.2014.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke 2003;34:335–7. 10.1161/01.STR.0000054050.51530.76 [DOI] [PubMed] [Google Scholar]
- 70. Hendrikse J, Petersen ET, Golay X. Vascular disorders: insights from arterial spin labeling. Neuroimaging Clin N Am 2012;22:259–69. 10.1016/j.nic.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 71. Schuff N, Matsumoto S, Kmiecik J, et al. . Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 2009;5:454–62. 10.1016/j.jalz.2009.04.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gao YZ, Zhang JJ, Liu H, et al. . Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer’s disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Curr Neurovasc Res 2013;10:49–53. 10.2174/156720213804806016 [DOI] [PubMed] [Google Scholar]
- 73. Sun Y, Cao W, Ding W, et al. . Cerebral blood flow alterations as assessed by 3D ASL in cognitive impairment in patients with subcortical vascular cognitive impairment: a marker for disease severity. Front Aging Neurosci 2016;8:211 10.3389/fnagi.2016.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen JJ, Jann K, Wang DJ. Characterizing resting-state brain function using arterial spin labeling. Brain Connect 2015;5:527–42. 10.1089/brain.2015.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liang X, Zou Q, He Y, et al. . Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A 2013;110:1929–34. 10.1073/pnas.1214900110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu J, Zhuo C, Qin W, et al. . Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res 2015;63:28–35. 10.1016/j.jpsychires.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 77. Fernández-Seara MA, Mengual E, Vidorreta M, et al. . Resting state functional connectivity of the subthalamic nucleus in Parkinson’s disease assessed using arterial spin-labeled perfusion fMRI. Hum Brain Mapp 2015;36:1937–50. 10.1002/hbm.22747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jann K, Hernandez LM, Beck-Pancer D, et al. . Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav 2015;5:n/a 10.1002/brb3.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jann K, Gee DG, Kilroy E, et al. . Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. Neuroimage 2015;106:111–22. 10.1016/j.neuroimage.2014.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu WC, Lin SC, Wang DJ, et al. . Measurement of cerebral white matter perfusion using pseudocontinuous arterial spin labeling 3T magnetic resonance imaging – an experimental and theoretical investigation of feasibility. PLoS One 2013;8:e82679 10.1371/journal.pone.0082679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 2013;73:239–54. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- 82. Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A 2009;106:10841–6. 10.1073/pnas.0903253106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol 2009;19:1023–7. 10.1016/j.cub.2009.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]