Abstract
Objective
Anaemia is associated with higher mortality among patients with non-stroke cardiovascular conditions; less is known regarding the relationship between anaemia and mortality among patients with acute ischaemic stroke.
Methods
Medical records were abstracted for n=3965 veterans from 131 Veterans Health Administration facilities who were admitted with ischaemic stroke in fiscal year 2007. Haematocrit values within 24 hours of admission were classified as ≤27%, 28%–32%, 33%–37%, 38%–42%, 43%–47% or ≥48%. Multivariate logistic regression was used to examine the relationship between anaemia and in-hospital, 30-day, 6-month and 1-year mortality, adjusting for age, medical comorbidities, modified Acute Physiology and Chronic Health Evaluation-III and stroke severity. Impact factors were calculated to standardise comparisons between haematocrit tier and other covariates.
Results
Among n=3750 patients included in the analysis, the haematocrit values were ≤27% in 2.1% (n=78), 28%–32% in 6.2% (n=234), 33%–37% in 17.9% (n=670), 38%–42% in 36.4% (n=1366), 43%–47% in 28.2% (n=1059) and ≥48% in 9.1% (n=343). Patients with haematocrit ≤27%, compared with patients in the 38%–42% range, were more likely to have died across all follow-up intervals, with statistically significant adjusted ORs (aORs) ranging from 2.5 to 3.5. Patients with polycythaemia (ie, haematocrit ≥48%) were at increased risk of in-hospital mortality (aOR=2.9; 95% CI 1.4 to 6.0), compared with patients with mid-range admission haematocrits. Pronounced differences between patients receiving and not receiving blood transfusion limited our ability to perform a propensity analysis. Impact factors in the 1-year mortality model were 0.46 (severe anaemia), 0.06 (cancer) and 0.018 (heart disease).
Conclusions
Anaemia is independently associated with an increased risk of death throughout the first year post stroke; high haematocrit is associated with early poststroke mortality. Severe anaemia is associated with 1-year mortality to a greater degree than cancer or heart disease. These data cannot address the question of whether interventions targeting anaemia might improve patient outcomes.
Keywords: ischemic stroke, anemia, polycythemia, hematocrit, mortality
Introduction
Anaemia is an independent predictor of mortality among patients with non-stroke cardiovascular conditions, such as myocardial infarction (MI) and congestive heart failure (CHF).1–3 A relationship between baseline haematocrit and increased mortality within the acute ischaemic stroke population has been reported inconsistently.4–9 Some studies have found an independent association between anaemia and mortality,5 7 9–11 whereas other studies have not confirmed this relationship.4 6 Increased mortality with higher haematocrits has been described as well, such that a non-linear, U-shaped relationship between haematocrit and poststroke mortality may exist.8 10 11
Patients with medically complex acute stroke may be especially prone to poor outcomes in the presence of low or high haematocrit values, either through conditions associated with extreme haematocrit levels or through pathophysiological consequences of haematocrit abnormalities (including decreased oxygen delivery, alterations in blood viscosity and impaired cerebral autoregulation).8 9 12–17 Previous studies examining the relationship between haematocrit and mortality in ischaemic stroke have variably adjusted for important medical conditions and neurological status that contribute to mortality.18 Inability to adjust for the burden of medical illness and stroke severity, combined with analysing haemoglobin based on a dichotomous definition of anaemia, may be responsible for the variable association between haematocrit and mortality reported in the literature. More recently, one study controlled for age, comorbidities and ischaemic stroke subtype, finding that admission haemoglobin values of less than 12.4 g/dL (approximately a haemoglobin of 37.2%)19 in men were associated with increased mortality throughout the first year post stroke.10 The authors also noted that further studies were necessary to understand the potential role of haemoglobin augmentation interventions among patients with acute ischaemic stroke presenting with anaemia.10
We used data from the Veterans Health Administration (VHA) to determine whether an association between baseline haematocrit and mortality exists throughout the first year post acute ischaemic stroke, and to assess the contribution of low and high haematocrit values to mortality during this time period, while controlling for burden of medical comorbidities and stroke severity. Given that the presence of lower measures of red blood cell volume was associated with greater mortality in prior work,1 10 we investigated whether finer tiers of haematocrit were associated with even higher mortality.1 Furthermore, we conducted a propensity analysis to understand whether receipt of blood transfusion among patients with anaemic ischaemic stroke affected postevent outcomes.
Methods
Patient population
The VHA Office of Quality Performance and the Veterans Affairs (VA) Stroke Quality Enhancement Research Initiative collaborated to conduct the first national VHA study of inpatient stroke quality. The study design and primary findings are reported elsewhere.20 In brief, VA administrative data were used to identify a study population of veterans (n=5721) who were admitted to a VA Medical Center (VAMC) in fiscal year 2007 (FY07) with a primary diagnosis of acute ischaemic stroke. A sample of 5000 medical records were selected; these included all cases from small-volume centres (≤55 patients) and a random sample of 80% of veterans at high-volume facilities (>55 patients in FY07).20 Patients were excluded if they were admitted for elective carotid endarterectomy, for postacute ischaemic stroke rehabilitation or were already admitted for a non-stroke condition when the ischaemic stroke occurred, leaving a final sample of n=3965 veterans from 131 VAMCs for whom full chart reviews were performed.
In our current analyses, patients were also excluded when haematocrit laboratory values within 24 hours of acute stroke admission were unavailable (n=94), or when we found inconsistent death dates (n=6). Additional exclusions were based on limited sample size and included women (n=95) and patients who received intravenous tissue plasminogen activator (n=32). The final analytic sample included 3750 veterans.
Data
Date were collected through retrospective chart review of medical records using remote electronic record data only (ie, no paper records were used). Chart reviews were performed by abstractors from the West Virginia Medical Institute who were especially trained for the study. Among the 307 data elements, 90% had an interobserver agreement ≥70% (please refer to online supplementary 1 for a representative sample of select covariates). Data collected for this study included demographic factors, medical comorbidities (eg, medical history, Charlson Index),21 stroke severity score (as measured by the retrospective National Institutes of Health Stroke Scale (NIHSS))22 and admission data (eg, vital signs, medication, laboratory tests, active medical conditions). Race/ethnicity data were obtained from the VHA’s Functional Status and Outcomes Database (FSOD), and supplemented by the VHA inpatient/outpatient files and the Centers for Medicare and Medicaid (CMS) if race/ethnicity was missing in the FSOD.
svn-2018-000149supp001.pdf (80.1KB, pdf)
Independent variable
The primary outcome of interest was all-cause mortality, as measured from the VA Vital Status File (VSF), containing dates of deaths from all VA beneficiaries. Death information in the VA VSF originates from a variety of VA and non-VA sources (eg, CMS). The VA VSF is relatively complete and accurate when compared with information contained in the National Death Index.23 We examined in-hospital, 30-day, 6-month and 1-year mortality.
Primary dependent variable
The primary dependent variable was haematocrit values within 24 hours of hospital admission, prespecified as an ordinal variable based on prior research (not involving the ischaemic stroke population) using categories of ≤27% (severe anaemia), 28%–32% (moderate anaemia), 33%–37% (mild anaemia), 38%–42% (mid-range; referent), 43%–47% (mid-range) and ≥48% (polycythaemia).2 Haematocrit was not dichotomised as anaemia based on the WHO criteria (ie, haemoglobin <13.7 g/dL in men and <12.6 g/dL in women),19 given previously reported non-linear associations between mortality and anaemia in non-stroke conditions1 2 24 and ischaemic stroke.6 9 11 We were unable to characterise the type of anaemia (eg, iron deficiency, anaemia of chronic disease) with the available data.
Covariates
Potential covariates associated with mortality were selected a priori based on prior research.9 17 21 25 26 Demographic variables, admission laboratory values (eg, platelet count), admission vital sign data (eg, oxygen saturation) and medical comorbidities were considered.21 In addition, the modified Acute Physiology and Chronic Health Evaluation (APACHE)-III25 (factoring out the contribution of haematocrit) and NIHSS scores were included.22
Statistical analysis
All statistical analyses used SAS V.9.2. We conducted bivariate analyses to describe the demographic and clinical characteristics of the study sample across each of the six categories of haematocrit, and we assessed differences using χ2 tests for categorical variables and analysis of variance tests for continuous variables.
Multivariate logistic regression models were used to examine the association between haematocrit and mortality, after adjusting for covariates. A ratio of 10 outcome events per variable was maintained during model construction.27 28 A p value of <0.05 was used to demarcate statistical significance. The final multivariate logistic regression models included confounders selected a priori and identified in bivariate analyses as important predictors of mortality. The final mortality models included age (in 10-year increments), medical history of heart disease, cancer, chronic obstructive pulmonary disease (COPD), kidney disease requiring dialysis and atrial fibrillation, and admission hypoxia (oxygen saturation <90%), pneumonia and thrombocytopaenia (platelet count <100 cells/mm3),26 modified APACHE-III (as a measure of acute severity of disease), Charlson Index (as a measure of chronic disease comorbidity) and NIHSS (as a measure of stroke severity). No adjustments were made for multiple comparisons. As missing data were rare, imputations were not made for missing data. Adjusted ORs (aORs) and 95% CIs are presented. For each regression model, discrimination (C-statistics) and calibration (Hosmer-Lemeshow goodness of fit (HLGOF)) statistics were calculated to gauge model performance and fit, respectively.29 30 Finally, survival curves were calculated using the Kaplan-Meier method and were compared using log-rank statistics.
Impact factors were calculated to make a standardised comparison between each category of haematocrit, medical comorbidities and NIHSS, and their contribution to mortality throughout the first year post stroke (online supplementary 2).29 30 To assess the contribution of blood transfusions on risk of mortality, a propensity analysis was conducted.
svn-2018-000149supp002.pdf (152.1KB, pdf)
Results
The mean age of n=3750 veterans in the study was 67.9 years, and 67% were white. The distribution of haematocrit categories at admission (table 1) included approximately 2% with severe anaemia (haematocrit ≤27%) and 9% with polycythaemia (haematocrit ≥48%). The bivariate relationship between haematocrit levels and demographic, clinical characteristics and mortality is shown in tables 1 and 2.
Table 1.
Characteristics of patients with ischaemic stroke*
| Overall sample (n=3750) | Admission haematocrit value | P values | ||||||
| ≤27% (n=78) |
28%–32% (n=234) |
33%–37% (n=670) |
38%–42% (n=1366) | 43%–47% (n=1059) | ≥48% (n=343) |
|||
| Age, year (mean, SD) | 67.9 | 70.4 | 62.8 | 66.7 | 65.4 | 65.5 | 64.2 | <0.0001 |
| Race, white | 67.4 | 57.7 | 62.8 | 66.7 | 65.4 | 70.8 | 71.7 | 0.0048 |
| Medical history | ||||||||
| Prior stroke or TIA | 29.0 | 28.2 | 28.6 | 32.8 | 31.8 | 24.7 | 24.5 | 0.0003 |
| Intracranial haemorrhage | 0.8 | 0.0 | 0.9 | 1.0 | 0.7 | 0.8 | 0.9 | 0.9225 |
| Hypertension | 79.1 | 73.1 | 80.8 | 78.8 | 81.3 | 76.9 | 78.1 | 0.0855 |
| Diabetes | 39.9 | 53.9 | 47.9 | 48.2 | 40.4 | 34.2 | 30.6 | <0.0001 |
| Hyperlipidaemia | 48.6 | 37.2 | 41.5 | 47.9 | 52.4 | 46.9 | 47.2 | 0.0025 |
| Atrial fibrillation | 10.2 | 10.3 | 13.7 | 14.8 | 10.6 | 6.5 | 8.5 | <0.0001 |
| CAD | 28.5 | 30.8 | 34.6 | 31.9 | 30.3 | 24.7 | 22.2 | <0.0001 |
| Myocardial infarction | 10.8 | 11.5 | 9.8 | 12.4 | 10.9 | 9.8 | 10.8 | 0.6849 |
| CABG | 10.7 | 7.7 | 13.3 | 13.1 | 11.3 | 9.1 | 7.6 | 0.0198 |
| PTCA† | 5.8 | 6.4 | 6.0 | 4.6 | 5.8 | 6.3 | 6.4 | 0.7729 |
| Carotid stenosis | 5.4 | 6.4 | 7.3 | 6.0 | 5.6 | 4.7 | 4.1 | 0.4886 |
| CEA/CAS | 2.4 | 1.3 | 3.9 | 2.8 | 2.5 | 2.1 | 1.2 | 0.3214 |
| CHF | 12.1 | 18.0 | 18.0 | 17.2 | 11.1 | 9.2 | 10.2 | <0.0001 |
| PAD | 8.7 | 15.4 | 12.0 | 9.9 | 8.6 | 7.5 | 6.7 | 0.0293 |
| Current smoking | 35.9 | 25.4 | 22.4 | 25.5 | 33.4 | 42.6 | 56.3 | <0.0001 |
| GI/GU bleeding | 0.5 | 1.3 | 1.3 | 0.9 | 0.4 | 0.1 | 0.3 | 0.0772 |
| COPD/asthma/bronchitis | 15.7 | 12.8 | 21.4 | 17.6 | 16.0 | 12.8 | 16.3 | 0.0096 |
| Kidney disease | 1.1 | 2.6 | 4.3 | 1.5 | 0.9 | 0.5 | 0.6 | <0.0001 |
| Kidney disease requiring dialysis | 0.6 | 0.0 | 2.6 | 0.5 | 0.7 | 0.1 | 0.9 | 0.0030 |
| HIV | 0.8 | 1.3 | 1.3 | 1.5 | 0.8 | 0.4 | 0.3 | 0.0881 |
| Cancer | 8.1 | 12.8 | 18.4 | 10.9 | 8.0 | 5.1 | 3.8 | <0.0001 |
| Liver disease | 1.9 | 1.3 | 3.0 | 1.9 | 1.8 | 1.7 | 2.0 | 0.8367 |
| Peptic ulcer disease | 1.6 | 1.3 | 1.3 | 1.8 | 1.8 | 1.3 | 1.2 | 0.9154 |
| DVT/PE | 1.7 | 5.1 | 3.9 | 1.2 | 2.1 | 0.9 | 1.8 | 0.0041 |
| Dementia | 7.8 | 10.3 | 14.1 | 11.2 | 8.0 | 5.1 | 4.1 | <0.0001 |
| Non-stroke admission diagnoses and procedures‡ | ||||||||
| Pneumonia | 1.6 | 6.4 | 3.9 | 3.0 | 1.1 | 0.7 | 0.9 | <0.0001 |
| Acute myocardial infarction | 0.9 | 0.0 | 0.9 | 1.5 | 1.0 | 0.6 | 0.9 | 0.45 |
| Acute renal failure | 1.9 | 7.7 | 5.1 | 3.1 | 1.3 | 0.8 | 1.5 | <0.0001 |
| Blood transfusion during admission | 2.0 | 41.0 | 7.7 | 1.0 | 0.7 | 0.3 | 0.9 | <0.0001 |
| Charlson Index, mean (SD) | 4.8 (2.0) | 5.5 (2.3) | 6.0 (2.2) | 5.6 (2.0) | 4.8 (1.9) | 4.2 (1.8) | 4.1 (1.9) | <0.0001 |
| Modified APACHE-III, mean (SD)§ | 17.5 (7.2) | 18.7 (17.9) | 19.7 (12.3) | 18.9 (11.5) | 17.2 (10.10) | 16.6 (10.3) | 17.0 (8.5) | <0.0001 |
| Stroke severity | ||||||||
| NIHSS<10 | 88.4 | 83.3 | 82.1 | 88.7 | 87.6 | 91.1 | 88.6 | 0.0013 |
| NIHSS (mean, SD) | 6.0 | 7.3 | 2.2 | 5.8 | 6.1 | 5.7 | 6.3 | 0.0010 |
| Preambulatory status | <0.0001 | |||||||
| Ambulatory | 93.3 | 82.1 | 86.3 | 88.7 | 95.4 | 95.1 | 95.9 | |
| Non-ambulatory | 5.8 | 12.8 | 12.0 | 9.4 | 4.3 | 4.4 | 2.9 | |
| Unknown | 0.9 | 5.1 | 1.7 | 1.9 | 0.3 | 0.5 | 1.2 | |
| Admission laboratory values | ||||||||
| Platelet count <100 109 per liter | 1.8 | 6.4 | 5.6 | 2.2 | 1.4 | 0.9 | 1.8 | <0.0001 |
| White blood cell count, cells ×109/L, mean (SD) | 8.6 (4.9) | 9.7 (11.7) | 8.0 (3.8) | 8.2 (6.1) | 8.3 (3.9) | 8.8 (4.2) | 9.7 (5.4) | <0.0001 |
| Serum BUN, mg/dL, mean (SD) | 20.0 (11.6) | 32.1 (22.5) | 27.5 (17.2) | 23.2 (13.1) | 19.1 (9.7) | 17.2 (7.9) | 17.9 (12.0) | <0.0001 |
| Serum creatinine, mg/dL, mean (SD) | 1.33 (0.9) | 1.88 (1.9) | 1.86 (1.5) | 1.53 (1.0) | 1.27 (0.7) | 1.18 (0.5) | 1.21 (0.6) | <0.0001 |
| Albumin, g/dL, mean (SD) | 3.74 (0.8) | 3.27 (0.8) | 3.39 (0.7) | 3.52 (0.8) | 3.79 (0.8) | 3.89 (0.8) | 3.88 (0.9) | <0.0001 |
| Glucose, mg/dL, mean (SD) | 140 (68) | 147 (75) | 149 (74) | 138 (67) | 141 (66) | 140 (71) | 138 (67) | 0.3138 |
| LDL-c, mg/dL, mean (SD) | 105.6 (36.6) | 84.4 (39.6) | 88.6 (29.3) | 93.7 (36.7) | 93.7 (36.7) | 112.6 (37.6) | 111.9 (36.6) | <0.0001 |
| INR, mean (SD) | 1.19 (0.9) | 1.25 (0.5) | 1.35 (1.1) | 1.26 (1.1) | 1.16 (0.8) | 1.15 (0.9) | 1.21 (1.1) | 0.0300 |
| Admission vital signs | ||||||||
| SBP, mm Hg, mean (SD) | 151 (28.2) | 139 (28.8) | 145 (29.2) | 146 (28.5) | 152 (28.0) | 155 (28.6) | 156 (26.8) | <0.0001 |
| Heart rate, beats/min, mean (SD) | 77 (16.4) | 83 (18.4) | 78 (15.2) | 75 (15.8) | 76 (16.4) | 78 (16.5) | 81 (17.6) | <0.0001 |
| Oxygen saturation, % saturation, mean (SD) | 96.7 (2.6) | 96.1 (4.8) | 96.5 (3.1) | 96.9 (2.8) | 96.8 (2.4) | 96.7 (2.4) | 96.2 (2.5) | 0.0003 |
| Hypoxia (<90%), % | 2.32 | 5.13 | 4.70 | 2.24 | 2.12 | 1.98 | 2.04 | 0.0917 |
*Values are expressed as % unless otherwise indicated.
†PTCA, with or without stenting.
‡Represents active medical conditions at time of stroke admission (eg, concomitant stroke and pneumonia).
§Modified APACHE-III calculated without haematocrit values.
APACHE-III, Acute Physiology and Chronic Health Evaluation-III; BUN, blood, urea, nitrogen; CABG, coronary artery bypass graft; CAD, coronary artery disease; CAS, carotid artery stent; CEA, carotid artery endarterectomy; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DVT/PE, deep venous thrombosis/pulmonary embolism; GI/GU, gastrointestinal/genitourinary; INR, international normalised ratio; LDL-c, low-density lipoprotein-c; NIHSS, National Institutes of Health Stroke Scale; PAD, peripheral artery disease; PTCA, percutaneous transluminal coronary angioplasty; SBP, systolic blood pressure; TIA, transient ischaemic attack.
Table 2.
Haematocrit level and mortality
| Admission haematocrit value | P values | |||||||
| Overall sample (n=3750) | ≤27% (n=78) |
28%–32% (n=234) |
33%–37% (n=670) |
38%–42% (n=1366) | 43%–47% (n=1059) |
≥48% (n=343) |
||
| Mortality (%) | ||||||||
| In-hospital | 3.6 | 9.0 | 6.4 | 4.6 | 2.7 | 2.4 | 5.3 | 0.0002 |
| 30-day | 7.8 | 21.8 | 14.5 | 9.3 | 7.4 | 4.9 | 7.3 | <0.0001 |
| 90-day | 14.5 | 35.9 | 31.6 | 21.5 | 12.6 | 8.6 | 9.6 | <0.0001 |
| 1-year | 18.8 | 43.6 | 38.5 | 26.9 | 16.9 | 12.6 | 10.8 | <0.0001 |
In multivariate logistic regression analyses, both low and high levels of haematocrit were significantly associated with mortality. Compared with patients with mid-range haematocrit levels (38%–42%), patients with severe anaemia (≤27%) had aORs of 2.5–3.5 for mortality across all time points; all associations were statistically significant (table 3). Patients with mild and moderate anaemia also experienced increased mortality for 6-month and 1-year outcomes (table 3; figure 1). Mid-range haematocrits of 43%–47% were not associated with increased mortality. Polycythaemia was associated only with in-hospital mortality (aOR=2.89; 95% CI 1.39 to 6.00). The C-statistics for the models predicting mortality were excellent (C-statistic >0.8 for all models). The HLGOF statistics for all models were non-significant (HLGOF >0.05), indicating that model fit was good. The impact factors for variables in the 1-year mortality model were as follows: severe anaemia (0.46), NIHSS (0.23), history of cancer (0.06) and heart disease (0.018) (online supplementary 2).29
Table 3.
Association between haematocrit and mortality
| In-hospital mortality | 30-day mortality | 6-month mortality | 1-year mortality* | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age† | 1.63 (1.26 to 2.11) | 1.870 (1.56 to 2.25) | 1.56 (1.36 to 1.78) | 1.47 (1.305 to 1.65) |
| Haematocrit (%) | ||||
| ≤27 | 3.46 (1.30 to 9.22) | 2.47 (1.20 to 5.05) | 2.54 (1.39 to 4.63) | 2.58 (1.46 to 4.55) |
| 28–32 | 1.66 (0.75 to 3.67) | 1.37 (0.82 to 2.30) | 2.19 (1.49 to 3.23) | 2.05 (1.43 to 2.95) |
| 33–37 | 1.67 (0.94 to 3.00) | 0.97 (0.65 to 1.45) | 1.42 (1.06 to 1.91) | 1.30 (1.00 to 1.70) |
| 38–42 | 1.00 | 1.00 | 1.00 | 1.00 |
| 43–47 | 1.17 (0.63 to 2.16) | 0.83 (0.55 to 1.27) | 0.84 (0.61 to 1.15) | 0.94 (0.72 to 1.23) |
| ≥48 | 2.89 (1.39 to 6.00) | 1.17 (0.64 to 2.14) | 0.84 (0.51 to 1.39) | 0.68 (0.43 to 1.07) |
| Platelet count 10 9 per liter | 2.92 (1.00 to 8.50) | 3.75 (1.69 to 8.31) | 2.63 (1.33 to 5.19) | 2.40 (1.26 to 4.56) |
| Charlson Index | 0.97 (0.82 to 1.14) | 1.019 (0.91 to 1.14) | 1.17 (1.08 to 1.28) | 1.21 (1.12 to 1.31) |
| Modified APACHE-III‡ | 1.04 (1.02 to 1.07) | 1.04 (1.02 to 1.06) | 1.02 (1.01 to 1.04) | 1.03 (1.01 to 1.04) |
| NIHSS | 1.13 (1.11 to 1.15) | 1.145 (1.12 to 1.16) | 1.12 (1.11 to 1.14) | 1.11 (1.09 to 1.13) |
| Atrial fibrillation | 1.03 (0.57 to 1.86) | 1.13 (0.75 to 1.69) | 1.41 (1.04 to 1.92) | 1.44 (1.08 to 1.91) |
| Cancer | 2.28 (1.16 to 4.50) | 2.06 (1.27 to 3.33) | 1.84 (1.27 to 2.67) | 1.91 (1.36 to 2.69) |
| Heart disease§ | 1.38 (0.87 to 2.18) | 1.43 (1.03 to 1.97) | 1.07 (0.84 to 1.37) | 1.11 (0.89 to 1.38) |
| COPD/asthma/bronchitis | 0.93 (0.52 to 1.66) | 1.23 (0.84 to 1.80) | 0.95 (0.71 to 1.28) | 0.96 (0.74 to 1.26) |
| Kidney disease requiring dialysis¶ | NA | 0.80 (0.23 to 2.77) | 0.81 (0.33 to 1.99) | 0.80 (0.35 to 1.83) |
| Oxygen saturation <90% | 1.30 (0.44 to 3.83) | 1.65 (0.72 to 3.82) | 1.66 (0.80 to 3.44) | 1.25 (0.61 to 2.54) |
| Pneumonia | 1.54 (0.56 to 4.25) | 3.12 (1.49 to 6.55) | 4.45 (2.27 to 8.90) | 4.27 (2.16 to 8.45) |
*Mortality model discrimination and calibration: in-hospital (C=0.91; HLGOF=0.97); 30-day (C=0.83; HLGOF=0.82); 6-month (C=0.86; HLGOF=0.73); 1-year (C=0.88; HLGOF=0.43).
†Age, in 10-year increments.
‡Modified APACHE-III calculated without haematocrit values.
§Heart disease is a composite of history of coronary heart disease, myocardial infarction, coronary artery bypass graft surgery, percutaneous transluminal coronary angioplasty/coronary artery stenting, congestive heart failure, carotid artery stenosis and peripheral artery disease.
¶ORs for kidney disease requiring dialysis could not be generated due to the absence of in-hospital mortality events among patients with this comorbidity.
APACHE-III, Acute Physiology and Chronic Health Evaluation-III; C, C-statistic; COPD, chronic obstructive pulmonary disease; HLGOF, Hosmer-Lemeshow goodness of fit; NIHSS, National Institutes of Health Stroke Scale; NA, not available.
Figure 1.
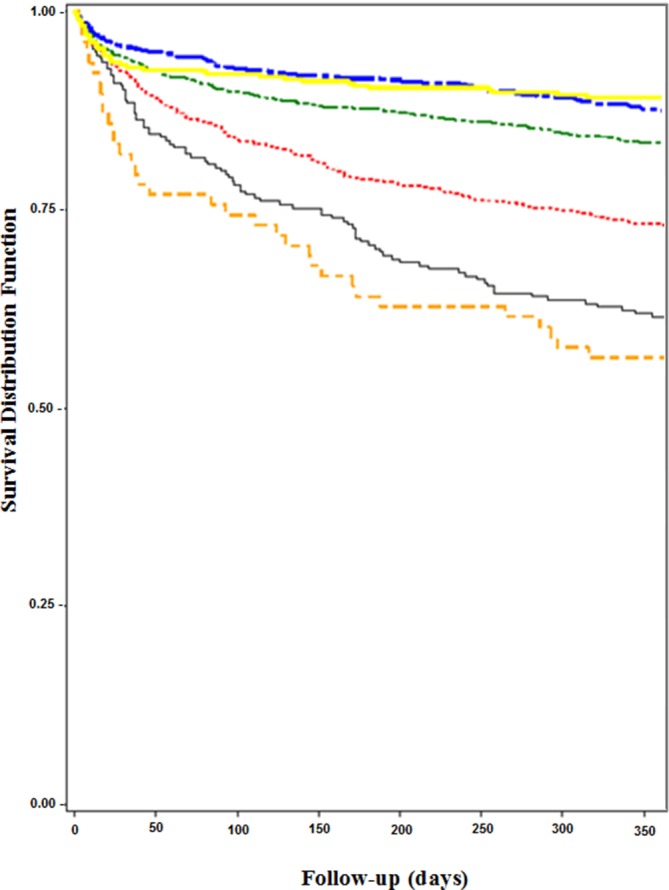
Kaplan-Meier curve demonstrating 1-year survival by admission haematocrit for men with ischaemic stroke. Tiers of haematocrit include ≤27% (orange) (severe anaemia), 28%–32% (grey) (moderate anaemia), 33%–37% (red) (mild anaemia), 38%–42% (green) (referent), 43%–47% (blue) and ≥48% (yellow) (polycythaemia). Severe anaemia was associated with the highest mortality, beginning during the index ischaemic stroke hospitalisation, whereas moderate and mild anaemia were associated with increased mortality by 6 months after hospital discharge. Polycythaemia was associated with mortality only during the index hospitalisation.
Blood was transfused in n=73 (2.0%) of patients at admission; n=57 (78.1%) occurred in patients with haematocrits <38% (table 1). Patients receiving a blood transfusion were more likely to die during the hospitalisation (17.8% vs 3.3%), at 30 days (32.9% vs 7.3%), 6 months (49.3% vs 13.8%) and 1 year (61.6% vs 18.0%) (p<0.0001 for all). In conducting a propensity analysis, given the pronounced differences between patients who did and those who did not receive blood transfusions, a stable propensity model could not be developed.
Discussion
After adjusting for relevant medical conditions, measures of acute severity of disease and burden of chronic medical comorbidities, and stroke severity, mild and moderate anaemia were only associated with increased 6-month and 1-year mortality among men. Severe anaemia was associated with increased mortality from hospital admission throughout the first year post stroke. Furthermore, severe anaemia at admission was associated with 1-year mortality to a similar degree as stroke severity and to a greater degree than a history of either heart disease or cancer. Polycythaemia was associated only with in-hospital mortality, confirming a U-shaped relationship between measures of red blood cell volume and postischaemic stroke outcomes.8 10 11
The prevalence of anaemia and the association between haematocrit and mortality (all-cause and non-cardiovascular) have been described among those with non-stroke conditions.1–3 Increased in-hospital and 30-day postacute MI mortality has been reported with worsening degrees of anaemia.3 Similarly, among patients ≥65 years of age admitted with CHF, haematocrit ≤27% was associated with increased 1-year mortality (HR=1.40; 95% CI 1.02 to 1.92)2; this association was largely attributed to the burden of medical comorbidities, suggesting that anaemia may be a marker, rather than a prime driver, of increased mortality in this population.1
The prevalence of WHO-defined anaemia among patients with ischaemic stroke has ranged from 15% to 25%; the association between haematocrit and mortality has been variably described.4 7–9 11 During an ischaemic stroke hospitalisation, admission anaemia has been associated with the combined endpoint of in-hospital mortality and discharge to hospice in those with NIHSS<10 (aOR=4.14; 95% CI 1.47 to 11.90).9 After adjusting for age, sex, medical comorbidities, prior disability, NIHSS and stroke subtype (ie, ischaemic or haemorrhagic), anaemia has been associated with higher mortality at 1 month (aOR=1.90; 95% CI 1.05 to 3.43) and 1 year (aOR=1.72; 95% CI 1.00 to 2.93).11 Elsewhere, increased 6-month poststroke mortality (aOR=4.70; 95% CI 1.1 to 8.2) was reported after adjusting for age, Scandinavian Stroke Score and a combined variable of heart failure and/or impaired renal function and/or troponin T>0.01 01 µg/L.7 Additional studies have noted that admission anaemia among patients with first atherosclerosis-related stroke was associated with higher 3-year mortality (aOR=2.22; 95% CI 1.13 to 4.39) after controlling for age, coronary artery disease, chronic renal insufficiency and hyperlipidaemia.5 Others have found that baseline haematocrit was not associated with poststroke mortality; rather, a decrease in haematocrit value from baseline was associated with increased mortality (aOR=1.12; 95% CI 1.01 to 1.23; p=0.027).6 More recently, in a sample of 3298 male patients with ischaemic stroke, after controlling for age, prestroke modified Rankin Scale score, comorbidities, Oxfordshire Community Stroke Project classification and prior antithrombotic use, a haemoglobin less than 12.4 g/dL (approximately a haemoglobin of 37.2%) was associated with increased odds of death throughout the first year post stroke.10 In examining poststroke mortality among patients presenting with haematocrit values corresponding to a haemoglobin less than 12.4 g/dL (ie, ≤27%, 28%–32% and 33%–37%), we report an even greater odds of mortality with the lowest measures of red blood cell volume.8 10
Higher haematocrit levels have previously been associated with increased stroke risk, especially haematocrits >45%.10 31 32 Among patients with first ever stroke, the literature reports that women were more likely than men to have haematocrits >50% (6.6% vs 2.8%; p<0.0001). After adjusting for age, vascular risk factors, chronic atrial fibrillation, the presence of total anterior circulation infarction and haematocrit categories, haematocrits 46%–50% and >50% independently predicted 28-day mortality in women, but not men.8 Others reported that patients with ischaemic stroke in the highest haemoglobin quartiles (≥15.3 g/dL in men and ≥14.2 g/dL in women) had higher 1-month and 1-year mortality rates than patients in the middle quartiles.11 Elsewhere, higher inpatient and 1-month mortality was noted among patients within the highest quintile (≥15.6 g/dL).10 The authors postulated that higher haemoglobin values could be seen in dehydration and COPD. In our cohort, admission creatinine and blood/urea/nitrogen were higher than the normal reference range, possibly reflecting hypovolaemia at the time of presentation, whereas COPD occurred more often in patients with lower haematocrits.
Anaemia may reflect the underlying health status of patients with stroke, given the advanced age and burden of comorbid illnesses that are seen in patients with stroke with anaemia compared with patients with more mid-range (38%–47%) haematocrit. Oxygen delivery to the brain is optimal at a haematocrit of 40%,33 and mid-range haematocrit has been associated with better stroke outcomes (eg, discharge to hospice vs inpatient rehabilitation unit; improved 3-month modified Rankin score).4 6 Across studies, anaemic poststroke patients with cardiac disease, malignancy, renal impairment, older age and worse stroke severity had higher mortality.3 5 7 9 11 Similar to these studies, we found older age, higher comorbid disease burden and worse stroke severity occurred commonly among patients with lower haematocrits. Anaemia may confer increased mortality risk beyond its association with medical illness, perhaps through its influence on cerebral perfusion pressure and flow dynamics both to and within the ischaemic penumbra.
Based on the current results and the extant literature, it seems prudent to evaluate patients with stroke for treatable causes of anaemia, and to minimise hospital-acquired anaemia during an acute ischaemia stroke admission. From the available data, haemoglobin augmentation strategies cannot be advocated for poststroke patients at this time.34 While haemoglobin augmentation strategies have been associated with favourable outcomes in patients with MI and CHF, in patients with chronic kidney disease, diabetes and anaemia, darbepoetin alfa administration was associated with increased stroke risk (5.0% vs 2.6%, HR: 1.92; 95% CI 1.38 to 2.68, p<0.001).33 Mathematical modelling data support a transfusion threshold of haematocrit <30% among patients with acute ischaemic stroke.13 When applying this transfusion threshold to our cohort, 180 (4.8%) patients would have received a blood transfusion. However, receipt of blood transfusion during a hospitalisation for acute ischaemic stroke was neither correlated with improved in-hospital nor 90-day mortality.35 As there were such pronounced differences between patients with anaemia who did and did not receive blood transfusion, a stable propensity model could not be generated.35 Additional work is required to evaluate whether interventions that treat anaemia or polycythaemia reduce poststroke mortality.
The strengths of our study include the large sample size and the ability to control for several important medical conditions, stroke severity and admission vital sign data. Limitations include an entirely male (veteran) population, and the results may not be generalisable to women. Of note, prior studies have found that the prevalence of anaemia is similar between men and women, but higher haematocrit levels were associated with mortality only among women.8 24 Second, we cannot comment on the aetiology of anaemia and how causes of anaemia may influence outcomes. Few of our patients (0.5% of the entire sample) had gastrointestinal or genitourinary bleeding; it is therefore likely that most of the anaemia in this cohort was not secondary to acute blood loss. Third, we can only examine the relationship between admission haematocrit and mortality, and not trends in haematocrit observed during hospitalisation or after discharge. Studies have reported that anaemia acquired during an acute ischaemic stroke hospitalisation was more predictive of poorer outcomes than baseline haematocrit.6 Fourth, we are unable to ascertain the cause of death in our cohort, although sepsis, acute organ failure and cancer would be expected to account for a higher proportion of death among patients with severe anaemia compared with more normal haematocrit values. In previous work examining death certificate data, we reported that most poststroke mortality among patients with and without anaemia was attributable to the incident stroke, rather than to concomitant medical comorbidities.36 Finally, we cannot comment on stroke subtype. One study reported no association between elevated haematocrit and large-vessel stroke,36 whereas others found an association between intracranial atherosclerosis and stroke.5
Recognising the presence and importance of a non-linear, U-shaped association between admission haematocrit and all-cause poststroke mortality may have implications at both the patient level, regarding evaluation and management, as well as at the facility level, when understanding risk-adjusted outcomes at discrete time points.37 Additional work is required to understand whether intervening on anaemia via haemoglobin augmentation strategies (eg, blood transfusion) and polycythaemia improves poststroke trajectories of care.
Footnotes
Contributors: JJS: study concept and design; analysis and interpretation. LJM, BJF: analysis and interpretation. JC, LSW: study concept and design; critical revision of the manuscript for important intellectual content. DMB: study concept and design; analysis and interpretation; critical revision of the manuscript for important intellectual content.
Funding: This work was supported by the Department of Veterans Affairs, VHA, Office of Quality and Performance, and Health Services Research & Development Service Quality Enhancement Research Initiative Service Directed Project 12-178 and Career Development Award 11-262, and the Department of Veterans Affairs, Health Services Research & Development, Stroke Quality Enhancement Research Initiative (QUERI) Rapid Response Project 09-184. The views expressed in this article are those of the authors and do not necessarily represent the view of the Department of Veterans Affairs.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The VA Institutional Review Boards in West Haven, Connecticut, and Indianapolis, Indiana, approved this study.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Kosiborod M, Curtis JP, Wang Y, et al. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med 2005;165:2237–44. 10.1001/archinte.165.19.2237 [DOI] [PubMed] [Google Scholar]
- 2. Kosiborod M, Smith GL, Radford MJ, et al. The prognostic importance of anemia in patients with heart failure. Am J Med 2003;114:112–9. 10.1016/S0002-9343(02)01498-5 [DOI] [PubMed] [Google Scholar]
- 3. Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 2001;345:1230–6. 10.1056/NEJMoa010615 [DOI] [PubMed] [Google Scholar]
- 4. Diamond PT, Gale SD, Evans BA. Relationship of initial hematocrit level to discharge destination and resource utilization after ischemic stroke: a pilot study. Arch Phys Med Rehabil 2003;84:964–7. [DOI] [PubMed] [Google Scholar]
- 5. Huang WY, Chen IC, Meng L, et al. The influence of anemia on clinical presentation and outcome of patients with first-ever atherosclerosis-related ischemic stroke. J Clin Neurosci 2009;16:645–9. 10.1016/j.jocn.2008.08.014 [DOI] [PubMed] [Google Scholar]
- 6. Kellert L, Martin E, Sykora M, et al. Cerebral oxygen transport failure?: decreasing hemoglobin and hematocrit levels after ischemic stroke predict poor outcome and mortality: STroke: RelevAnt Impact of hemoGlobin, Hematocrit and Transfusion (STRAIGHT)--an observational study. Stroke 2011;42:2832–7. 10.1161/STROKEAHA.110.606665 [DOI] [PubMed] [Google Scholar]
- 7. Nybo M, Kristensen SR, Mickley H, et al. The influence of anaemia on stroke prognosis and its relation to N-terminal pro-brain natriuretic peptide. Eur J Neurol 2007;14:477–82. 10.1111/j.1468-1331.2006.01591.x [DOI] [PubMed] [Google Scholar]
- 8. Sacco S, Marini C, Olivieri L, et al. Contribution of hematocrit to early mortality after ischemic stroke. Eur Neurol 2007;58:233–8. 10.1159/000107946 [DOI] [PubMed] [Google Scholar]
- 9. Sico JJ, Concato J, Wells CK, et al. Anemia is associated with poor outcomes in patients with less severe ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:271–8. 10.1016/j.jstrokecerebrovasdis.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 10. Barlas RS, Honney K, Loke YK, et al. Impact of Hemoglobin Levels and Anemia on Mortality in Acute Stroke: Analysis of UK Regional Registry Data, Systematic Review, and Meta-Analysis. J Am Heart Assoc 2016;5:e003019 10.1161/JAHA.115.003019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanne D, Molshatzki N, Merzeliak O, et al. Anemia status, hemoglobin concentration and outcome after acute stroke: a cohort study. BMC Neurol 2010;10:22 10.1186/1471-2377-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allport LE, Parsons MW, Butcher KS, et al. Elevated hematocrit is associated with reduced reperfusion and tissue survival in acute stroke. Neurology 2005;65:1382–7. 10.1212/01.wnl.0000183057.96792.a8 [DOI] [PubMed] [Google Scholar]
- 13. Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modelling study. Br J Anaesth 1997;79:346–51. 10.1093/bja/79.3.346 [DOI] [PubMed] [Google Scholar]
- 14. Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med 2005;118:1288.e11–1288.e19. 10.1016/j.amjmed.2005.06.039 [DOI] [PubMed] [Google Scholar]
- 15. Irace C, Ciamei M, Crivaro A, et al. Hematocrit is associated with carotid atherosclerosis in men but not in women. Coron Artery Dis 2003;14:279–84. 10.1097/01.mca.0000071769.74379.49 [DOI] [PubMed] [Google Scholar]
- 16. Leal-Noval SR, Múñoz-Gómez M, Murillo-Cabezas F. Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Curr Opin Crit Care 2008;14:156–62. 10.1097/MCC.0b013e3282f57577 [DOI] [PubMed] [Google Scholar]
- 17. Mohr JC, Grotta DW, Weir JC, et al. Pathophysiology, Diagnosis, and Management. 5 ed, 2011. [Google Scholar]
- 18. Smith EE, Shobha N, Dai D, et al. Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With the Guidelines-Stroke Program. Circulation 2010;122:1496–504. 10.1161/CIRCULATIONAHA.109.932822 [DOI] [PubMed] [Google Scholar]
- 19. Organization WH. Nutritional Anemia: a report of a WHO Scientific Group - World Health Organization Technical Report Service. [Google Scholar]
- 20. Bravata DMO, Vogel DL, B Williams LS. The Quality of VA Inpatient Stroke Care, FY2007: Final National and Medical Center Results, VHA Office of Quality and Performance (OQP) Special Study, 2009. [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke 2000;31:858–62. 10.1161/01.STR.31.4.858 [DOI] [PubMed] [Google Scholar]
- 23. Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:1–8. 10.1186/1478-7954-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med 2005;165:2214–20. 10.1001/archinte.165.19.2214 [DOI] [PubMed] [Google Scholar]
- 25. Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991;100:1619–36. [DOI] [PubMed] [Google Scholar]
- 26. Sico JJ, Phipps MS, Concato J, et al. Thrombocytopenia and in-hospital mortality risk among ischemic stroke patients. J Stroke Cerebrovasc Dis 2013;22:e99–e102. 10.1016/j.jstrokecerebrovasdis.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 27. Concato J, Peduzzi P, Holford TR, et al. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol 1995;48:1495–501. 10.1016/0895-4356(95)00510-2 [DOI] [PubMed] [Google Scholar]
- 28. Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–10. 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 29. Feinstein AR. Principles of Medical Statistics. 1 ed. London: Chapman Hall/CRC 2001. [Google Scholar]
- 30. LaValley MP, Regression L. Circulation 2008;117:2395–9. [DOI] [PubMed] [Google Scholar]
- 31. Pinsky M. Cerebral Blood Flow: Mechanisms of Ischemia, Diagnosis, and Therapy. 1 ed New York: Springer, 2002. [Google Scholar]
- 32. Tohgi H, Yamanouchi H, Murakami M, et al. Importance of the hematocrit as a risk factor in cerebral infarction. Stroke 1978;9:369–74. 10.1161/01.STR.9.4.369 [DOI] [PubMed] [Google Scholar]
- 33. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019–32. 10.1056/NEJMoa0907845 [DOI] [PubMed] [Google Scholar]
- 34. Kellert L, Schrader F, Ringleb P, et al. The impact of low hemoglobin levels and transfusion on critical care patients with severe ischemic stroke: STroke: RelevAnt Impact of HemoGlobin, Hematocrit and Transfusion (STRAIGHT)--an observational study. J Crit Care 2014;29:236–40. 10.1016/j.jcrc.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 35. Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J 2011;32:1704–8. 10.1093/eurheartj/ehr031 [DOI] [PubMed] [Google Scholar]
- 36. Wade JP, Taylor DW, Barnett HJ, et al. Hemoglobin concentration and prognosis in symptomatic obstructive cerebrovascular disease. Stroke 1987;18:68–71. 10.1161/01.STR.18.1.68 [DOI] [PubMed] [Google Scholar]
- 37. Fonarow GC, Alberts MJ, Broderick JP, et al. Stroke outcomes measures must be appropriately risk adjusted to ensure quality care of patients: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2014;45:1589–601. 10.1161/STR.0000000000000014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2018-000149supp001.pdf (80.1KB, pdf)
svn-2018-000149supp002.pdf (152.1KB, pdf)


