Abstract
Metabolic feedback from the periphery to the brain results from a dynamic physiologic fluctuation of nutrients and hormones, including glucose and fatty acids, ghrelin, leptin, and insulin. The specific interactions between humoral factors and how they influence feeding is largely unknown. We hypothesized that acute glucose availability may alter how the brain responds to ghrelin, a hormonal signal of energy availability. Acute glucose administration suppressed a range of ghrelin-induced behaviors as well as gene expression changes in hypothalamic neuropeptide Y (NPY) and agouti-related peptide (AgRP) neurons after ghrelin administration. Knockdown of the energy-sensing molecule AMP-activated protein kinase (AMPK) in AgRP neurons resulted in loss of the glucose effect, and mice responded as though pretreated with saline. Conversely, 2-deoxyglucose (2-DG), which decreases glucose availability, potentiated ghrelin-induced feeding and increased hypothalamic NPY mRNA levels. AMPK knockdown did not alter the additive effect of 2-DG and ghrelin on feeding. Our findings support the idea that computation of energy status is dynamic, is informed by multiple signals, and responds to acute fluctuations in metabolic state. These observations are broadly relevant to the investigation of neuroendocrine control of feeding and highlight the underappreciated complexity of control within these systems.
Ghrelin is a hormone primarily released from the stomach that acts on its cognate receptor, the growth hormone secretagogue receptor (GHSR), to stimulate growth hormone release, food intake, and adiposity (1, 2). GHSR shows ∼90% coexpression with orexigenic agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons in the arcuate nucleus of the hypothalamus (ARC) (3). The canonical pathway for ghrelin to stimulate feeding is through increasing activity at these neurons, although ghrelin administered directly to other brain areas can also induce feeding (4). Ghrelin also drives an increase in body weight and adiposity by suppressing peripheral energy expenditure and inducing lipogenesis, an effect that occurs independently of food intake (5).
Ghrelin is released in anticipation of meals and during fasting and acts to convey a state of energy deficit to the brain. Although ghrelin’s role is generally understood as an initiator of feeding and body weight gain, in the context of sustained energy deficit its true physiological role appears to be a more complex integration of homeostatic and behavioral mechanisms. Indeed, GHSRs are found throughout the brain (6), which enables ghrelin to act in a coordinated way to protect the organism during energy deficit and while working to shift the animal toward metabolic safety. These mechanisms include maintaining blood glucose levels during fasting and severe calorie restriction (7), increasing food seeking (8), learning and reward (9), increasing fat storage independent of feeding (5), controlling the stress axis and reducing anxiety (10), and increasing cell survivability in models of neurodegeneration (11–13). These ghrelin-mediated functions provide an animal with the physiological and behavioral skills (resilience, motivation, and learning) to survive periods of relative food scarcity.
The way in which the body communicates energy deficit to the brain is not limited to a simple increase in plasma ghrelin. Numerous hormones and nutrients combine to give a metabolic signature of energy deficit to the brain. This includes, but is not limited to, high ghrelin (1), high fibroblast growth factor 21, nonesterified fatty acids and ketone bodies (14), and high asprosin (15) as well as physiological reductions in leptin (16), insulin, and glucose (17). This illustrates that a range of short- and long-term signals of energy availability provide the brain with ongoing, flexible feedback regarding nutrient status.
Glucose sensing is one way the brain gains information about acute energy state. Glucose-sensing neurons are found throughout the brain, including in the ARC. The AgRP neurons of the ARC are inhibited by glucose (18) and highly responsive to ghrelin, and ghrelin receptor signaling on these neurons mediates ghrelin’s glucoregulatory actions and contributes to its feeding effects (19). These neurons are responsible for directing a coordinated response to energy deficit that is largely driven by ghrelin signaling (20). They are well positioned to integrate other feedback signals from the periphery to provide a first point of central integration of metabolic information.
Many studies have demonstrated that chronic exposure to high-fat diet leads to the brain failing to respond to hormonal signals of energy availability such as leptin, insulin, and ghrelin. Conversely, caloric restriction is associated with increased sensitivity to both leptin and ghrelin. Whether acute fluctuations of the most available fuel source, glucose, also influence sensitivity to hormonal signals is not known. AMP-activated protein kinase (AMPK) is an energy-sensing molecule that is important in both glucose sensing and ghrelin sensing. It is regulated by glucose, and modifying AMPK sensing within the hypothalamus leads to altered sensing of metabolic states and metabolic hormones. AMPK signaling in AgRP neurons regulates the response to ghrelin (21) and glucose (22). AMPK activity in the hypothalamus is inhibited by high leptin, insulin, or glucose and by refeeding after a fast. Additionally, provision of a constitutively active form of AMPK in the medial hypothalamus (primarily arcuate, dorsomedial, and ventromedial nuclei) results in reduced sensitivity to leptin’s food-suppressing effects, indicating that AMPK activity is key in transducing signals of energy homeostasis (23). Thus, there is good evidence that AMPK acts to integrate signals of metabolic need and to promote appropriate physiological compensation.
The traditional view is that AMPK activity is regulated by AMP binding to the γ subunit, generally as a result of a high AMP/ATP ratio resulting from low available glucose to form ATP through respiration. When bound to AMPK, AMP maintains the activated state by allowing phosphorylation of Thr172, inhibiting dephosphorylation at the same site and promoting allosteric change (24). In this paradigm, both glucose and 2-deoxyglucose (2-DG) indirectly effect AMPK activity, with glucose inhibiting AMPK activity by promoting high ATP levels and 2-DG activating it by inhibiting ATP synthesis. However, in addition to this canonical mechanism, several other known or suspected modes of activation exist: binding of ligands between the α and β subunits (25), activation by the Ca2+/CaMKKβ pathway through increased intracellular calcium (26), and glucose sensing independent of adenine nucleotide levels (27). AMPK is a highly conserved and complex signaling molecule whose role in cellular signaling and energetics is not well understood, but its overarching role seems to be to promote energy homeostasis through a number of complementary pathways (24).
We modulated acute glucose availability using hyperglycemia or glucopenia induced by 2-DG or the ability to sense glucose in AgRP neurons by knocking down AMPK. We then investigated how these changes modified the response to ghrelin, a key signal of negative energy balance.
Methods
Mice
C57Bl/6J male mice or AgRP-cre male mice on a C57Bl/6J background (8 to 10 weeks old) were bred at Monash Animal Services. All mice were maintained on a 12-hour light: 12-hour dark cycle at 23°C and 50% to 70% humidity. Mice were fed a standard chow diet with 4.8% calories from fat (Rat and Mouse cubes; Specialty Feeds, Glen Forrest, WA, Australia). Experiments were conducted in accordance with the Monash University Animal Ethics Committee guidelines and the Australian Code for the Care and Use of Animals for Scientific Purposes Eighth Edition (2013). AgRP-cre mice were originally obtained from Jackson Laboratories and maintained by Monash Animal Services with backcrossing to C57Bl/6J every eight generations.
Surgery
All surgeries were performed under general anesthesia using isoflurane (5% induction, 2% maintenance in oxygen). Mice were anesthetized using 5% isoflurane in oxygen in an induction chamber and maintained on 2% isoflurane delivered by face mask. All mice had eye ointment (Lacri-lube; Allergan, Gordon, NSW, Australia) applied immediately after loss of consciousness. After surgery, the animals received a subcutaneous dose of 5 mg/kg meloxicam (Metacam; Boehringer Ingelheim, MacQuarie Park, NSW, Australia) in a volume of 1 mL/kg and were maintained on a heated pad until full recovery.
Drugs
IP injections
Ghrelin (PolyPeptide Group, Strasbourg, France) was administered at 0.3 mg/kg in saline. 2-DG (Sigma-Aldrich, Castle Hill, NSW, Australia) was administered at 250 mg/kg in saline. d-Glucose (Sigma-Aldrich) was given at 2.25 g/kg in distilled water (10 mL/kg) to limit hypertonicity of the injection. Control mice for glucose injections received saline to avoid hypotonicity of the injection. Saline acted as vehicle for all drugs.
Intracerebroventricular injections
Ghrelin was given at 0.33 µg/µL in artificial cerebrospinal fluid (aCSF). aCSF was used as vehicle.
Intracerebroventricular cannulations
Mice were placed in a stereotaxic frame (Stoelting, Wood Dale, IL), and the scalp was resected to reveal the skull. A 26-G cannula (Plastics One Inc., Roanoke, VA) aimed at the lateral ventricle (coordinates relative to bregma in mm: posterior −0.7, lateral ±1, ventral −2.2) was implanted. Cannulae were fixed to the skull using light-curing dental cement (G-bond and G-aenial Universal Flo; GC, Henry Schein Halas, Parkville, VIC, Australia). After surgery, mice were given Metacam as a postoperative analgesic and allowed to recover for at least 7 days before experimentation. Cannula placement was histologically verified post mortem.
Adenoviral injections
Mice were placed in a stereotaxic frame, and the scalp was resected to reveal the skull. Two holes were drilled through the skull above the ARC. A 2-µL Neuros Hamilton syringe was aimed at the ARC (coordinates relative to bregma in mm: posterior −1.7, lateral ±0.3, ventral −6.0) and carefully lowered through the brain. An automatic syringe pump (Stoelting) was used to deliver 200 nL of virus into a single side of the ARC. The infusion took place over 4 minutes, and the needle was left in place for another 4 minutes to aid diffusion into the brain tissue. The needle was then slowly removed and repositioned over the contralateral ARC, and the procedure was repeated. After the second injection, the wound was sutured, and Metacam was administered immediately after surgery and then up to twice daily as needed for the following 3 days. The mice were allowed to recover for 2 weeks before experimentation. We used a previously validated cre-dependent adeno-associated virus (AAV), which coexpresses mCherry and a dominant-negative (DN) kinase-dead α2 subunit preceded by a double-floxed inverted open reading frame (AAV-DIO-DN-AMPK). This kinase-dead subunit competes with endogenous α2, resulting in lowered arcuate α2 AMPK activity in this model (28).
Feeding experiments
All mice were fed ad libitum, and feeding experiments were conducted in the middle of the light phase. Mice were weighed, glucose or 2-DG (or saline) were injected, food was removed, and mice were returned to their home cage. Thirty minutes later, mice were injected with either ghrelin or saline, and a weighed amount of food was returned to the cage. Food intake was assessed over a 2-hour window.
Operant conditioning
Mice were housed on a reverse light/dark cycle, implanted with intracerebroventricular (ICV) cannulae, and then food restricted to ∼70% of daily caloric intake for 3 days before training began. Mice were trained to lever press for a 10% sucrose solution fixed ratio 1 schedule (5 µL per delivery) over a 90-minute session as previously published (29). Mice continued on this schedule for 7 days, when stable responding was achieved. During this time mice were acclimated to procedures for ICV and IP injections. Experimental days mirrored the food intake experiments. Mice were injected with either 2-DG or vehicle or with glucose or vehicle and returned to their home cage without food. Thirty minutes later, mice were injected ICV with glucose or aCSF in batches of five and placed briefly back into their home cage to allow for all five mice in a batch to be injected. Mice were then placed in the operant chambers, and recording started for all five mice at the same time. Mice remained in the chambers for 90 minutes, and active and inactive lever responding was recorded. Mice were returned to their home cage after the 90-minute test. All test days were interspersed by at least 2 days of nontreated operant responding to ensure mice maintained stable baseline responding.
Data analysis
All data were analyzed using Graphpad Prism software. Student t tests and two-way ANOVAs with Tukey post hoc tests were used as appropriate. Details of the tests and significance levels are provided in the figure legends.
Results
Acute glucose suppresses ghrelin-induced feeding, operant responding, and hypothalamic mRNA expression
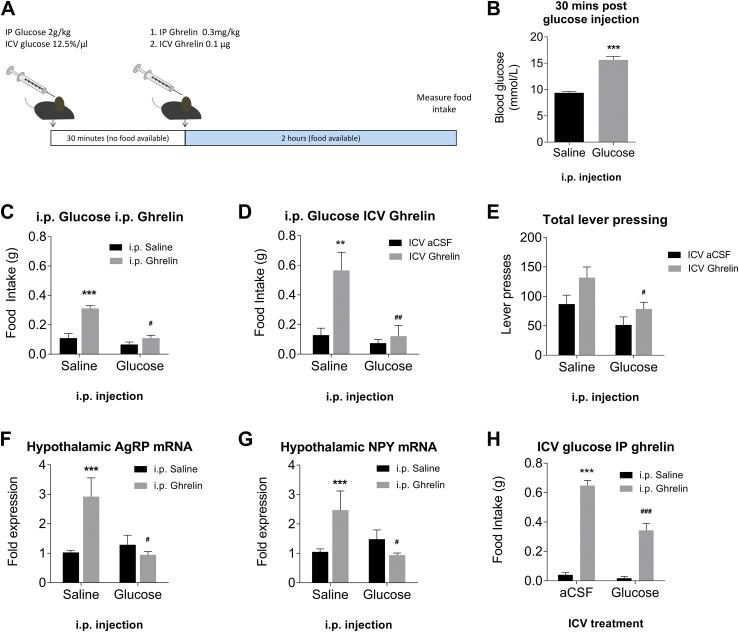
We examined if high blood glucose, as an acute signal of energy surplus, could alter the efficacy of ghrelin to induce feeding. Thirty minutes after IP injection in fed mice, blood glucose levels were almost doubled in glucose-injected mice compared with saline-injected control mice (Fig. 1B). We then administered ghrelin either IP (Fig. 1C) or ICV (Fig. 1D); mice that had received glucose did not respond to ghrelin injection with feeding, a response that was robust in saline-treated mice. Glucose pretreatment also significantly reduced ghrelin-induced operant responding for 10% sucrose solution (Fig. 1E), suggesting a possible effect on the motivational aspects of feeding. Quantitative PCR on hypothalamic explants taken 1 hour after ghrelin injection showed that glucose pretreatment completely suppressed induction of the orexigenic peptides AgRP (Fig. 1F) and NPY (Fig. 1G). To confirm that this effect was directly mediated by glucose, we administered the glucose pretreatment ICV. This also resulted in a significant reduction in ghrelin-induced feeding (Fig. 1H).
Figure 1.
Hyperglycemia suppresses physiological responses to ghrelin injection. (A) Schematic of experimental procedure. (B) Blood glucose is significantly increased after IP glucose injection. ***P < 0.01, independent measures t test (n = 34 per group). IP administration of glucose abolishes feeding response to (C) IP ghrelin injection (n = 9 to 10) or (D) ICV ghrelin injection (n = 7). (E) Glucose pretreatment significantly suppresses ghrelin-induced lever pressing in operant responding task (n = 11 to 16 per group). Glucose pretreatment suppresses induction of (F) AgRP and (G) NPY mRNA in the ARC following ghrelin injection (n = 7 to 10 per group). (H) ICV glucose attenuates ghrelin induced feeding (n = 9 to 10). **P < 0.01, ***P < 0.001 compared with saline/saline; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with saline/ghrelin (two-way ANOVA with Tukey post hoc test).
Acute 2-DG enhances ghrelin-induced feeding and hypothalamic mRNA expression
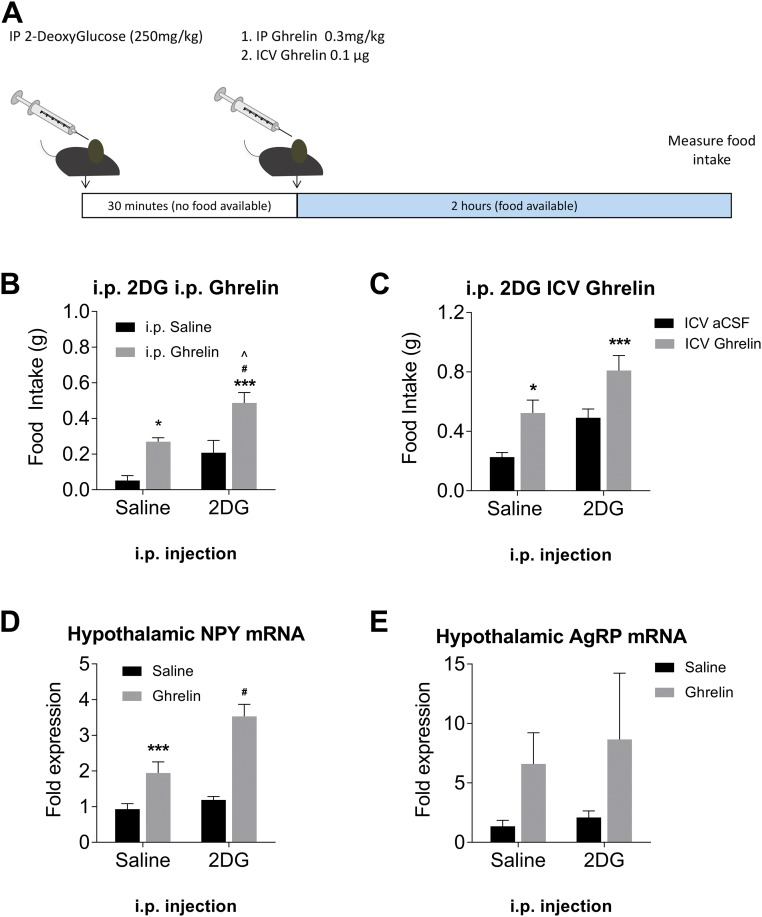
To induce low available blood glucose without withholding food and causing a range of adaptive responses, we administered 2-DG IP prior to ghrelin treatment. The protocol was the same as for the glucose experiments (Fig. 2A). Mice pretreated with 2-DG showed an increased response to ghrelin injection, eating approximately twice as much chow as mice pretreated with saline. This effect was the same for IP ghrelin (Fig. 2B) and ICV ghrelin (Fig. 2C). We again assessed hypothalamic orexigenic peptide levels 1 hour after ghrelin injection and found that for NPY (Fig. 2D) there was a significant increase in mRNA expression compared with saline-treated mice.
Figure 2.
Glucopenia enhances physiological responses to ghrelin injection. (A) Schematic of experimental procedure. IP administration of 2-DG enhances feeding response to (B) IP ghrelin injection (n = 9 to 10) or (C) ICV ghrelin injection (n = 11 to 12). Pretreatment with 2-DG suppresses induction of (D) NPY and does not significantly affect (E) AgRP mRNA in the ARC (n = 5). *P < 0.05, ***P < 0.001 compared with saline/saline; #P < 0.05 compared with saline/ghrelin; ^P < 0.05 compared with saline/2-DG (two-way ANOVA with Tukey post hoc test).
AMPK knockdown in AgRP neurons abolishes glucose’s ability to suppress feeding
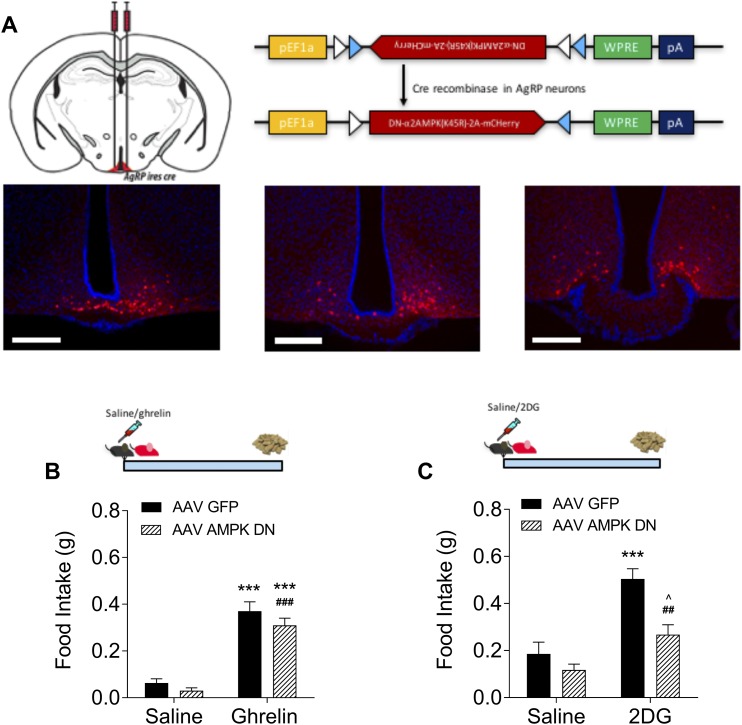
Because AMPK is a critical sensor of cellular energy availability and is implicated in ghrelin signaling, we next examined whether AMPK is functionally necessary for glucose to suppress ghrelin signaling. We used a cre-dependent AAV containing a DN construct designed to knock down AMPK expression and injected it directly into the ARC of the AgRP-cre mice (AgRPAMPK mice). The virus was tagged with an mCherry reporter, and a single injection resulted in good rostral/caudal spread of the virus (Fig. 3A). After viral knockdown, we did not see a reduction in ghrelin’s efficacy to induce feeding in AgRPAMPK mice (Fig. 3B). 2-DG indirectly activates AMPK by suppressing ATP synthesis. We examined whether loss of AMPK in AgRP neurons affected 2-DG’s ability to induce feeding in response to this metabolic emergency. Compared with AMPKGFP mice, AgRPAMPK mice showed a significantly attenuated feeding response to 2-DG injection, although they still ate significantly more than saline-injected AgRPAMPK mice (Fig. 3C).
Figure 3.
AMPK knockdown in AgRP neurons does not affect ghrelin sensitivity but reduces food intake in response to 2-DG. (A) Schematic of viral construct and micrographs showing targeting of virus to the ARC. Scale bar, 200 μm. (B) AgRPAMPK mice show similar sensitivity to ghrelin as AgRPGFP mice (n = 18 to 28). Main effect of ghrelin: ***P < 0.001 compared with GFP/saline; ###P < 0.001 compared with AMPK DN/saline (two-way ANOVA with Tukey post hoc test). (D) AgRPAMPK mice are less sensitive to glucoprivic feeding (n = 7 to 9). ***P < 0.001 compared with GFP/saline; ##P < 0.01 compared with DN AMPK/saline; ^P < 0.05 compared with DN AMPK/2-DG (two-way ANOVA with Tukey post hoc test).
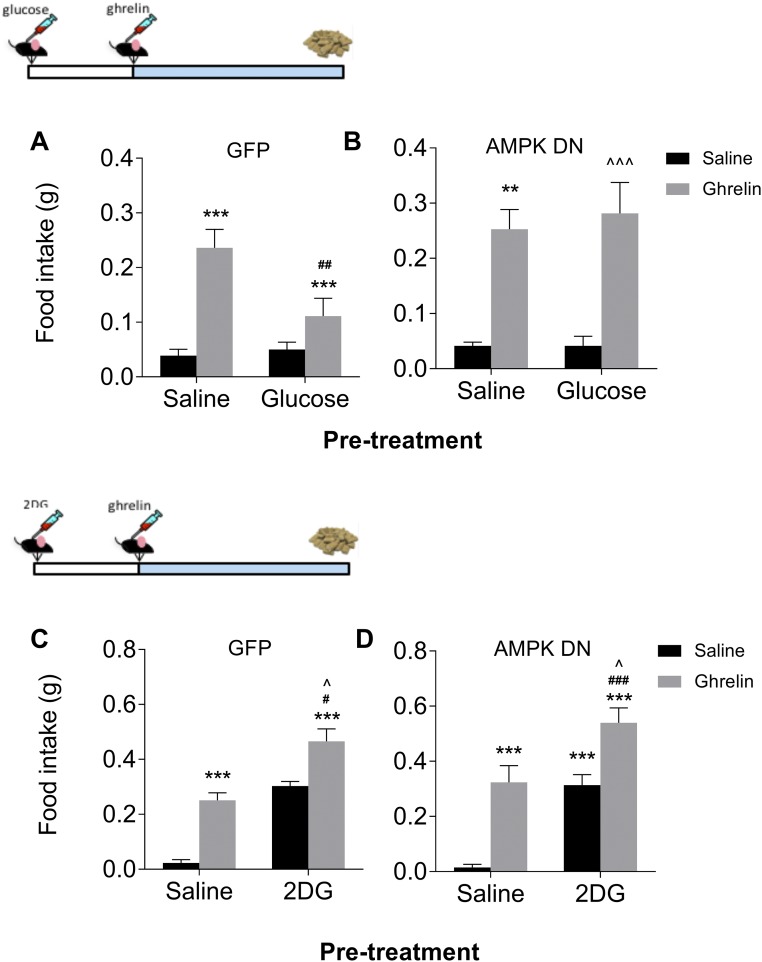
We next looked at the combined effects of glucose and ghrelin injection in mice with reduced AMPK activity in AgRP neurons. In mice injected with the control GFP virus, glucose pretreatment significantly suppressed ghrelin-induced feeding (Fig. 4A); however, this was not the case in AgRPAMPK mice, where there was no suppression of ghrelin’s feeding effects (Fig. 4B). We had previously seen an additive effect of 2-DG and ghrelin on feeding, and we wondered how AgRPAMPK mice would respond to this treatment. We did not see any effect of AMPK knockdown on the additive effects of 2-DG and ghrelin on feeding, with AMPKGFP and AgRPAMPK showing the same pattern of response (Fig. 4C and 4D).
Figure 4.
AMPK knockdown in AgRP neurons abolishes glucose-induced suppression but does not affect 2-DG–induced potentiation of ghrelin-induced feeding. (A) In AgRPGFP mice, glucose suppresses ghrelin-induced feeding (n = 8 to 10). (B) This effect is lost in AgRPAMPK mice (n = 6 to 7). Both (C) AgRPGFP (D) and AgRPAMPK mice have a similar feeding response to 2-DG (n = 7 to 9). **P < 0.01, ***P < 0.001 compared with saline/saline; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with saline/ghrelin; ^P < 0.05, ^^^P < 0.001 compared with saline/2-DG (two-way ANOVA with Tukey post hoc test).
Discussion
The way the brain computes energy status is only partially understood. It is clear from a number of studies that multiple inputs are needed to form an understanding of current energy state, and these then inform the countermeasures required to maintain energy homeostasis. The importance of individual signals may be enhanced or diminished depending on the overall humoral and neural picture. Here, we demonstrate that how the brain interprets ghrelin, the key hormonal signal of negative energy balance, can be influenced by acute glucose availability and that this is in part dependent on AMPK signaling in AgRP neurons (Fig. 5). By acutely increasing blood glucose concentrations, we abolished ghrelin-induced feeding. This effect was consistent for IP- and ICV-administered ghrelin, both failing to elicit feeding in the presence of hypergycaemia. This effect extended to reduced operant responding for 10% sucrose solution, which implies that high blood glucose may be a salient signal for modification of motivational processes. Lever pressing for a reward involves mesolimbic processing (30) but can also be driven by AgRP neuronal activation (31).
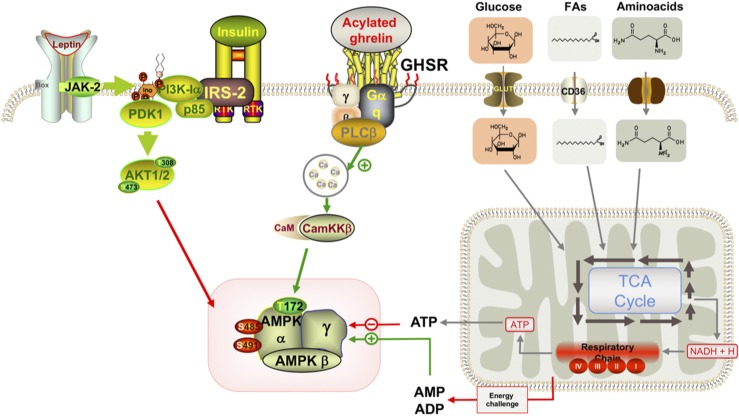
Figure 5.
Schematic representation of the availability of glucose to augment the feeding response to ghrelin. Ca, calcium; CaM, calmodulim; CamKKβ, calcium calmodulin–dependent kinase kinase β; FA, fatty acid; IRS1, insulin receptor substrate 1; JAK-2, Janus kinase 2; NADH, nicotinamide adenine dinucleotide; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphatidylinositide 3-kinase; PLC, phospholipase C; RTK, receptor tyrosine kinase; TCA, tricarboxylic acid.
Ghrelin treatment is known to induce transcription of orexigenic feeding peptides in the ARC (32), which we also demonstrated. Pretreatment with glucose suppressed this effect, showing that downstream effects of ghrelin action within the hypothalamus are sensitive to acute nutritional change. To ascertain whether glucose was acting directly on glucose-sensing neurons, we administered glucose directly into the brain. ICV administration of glucose reduced the effect but did not abolish it, as in previous experiments. This could be due to a contribution of peripheral effects or to insufficient penetration or concentration within the brain after ICV administration. Peripherally, insulin rises after an increase in blood glucose and can have central anorectic effects, although this increase is relatively small after IP administration (33). We administered glucose IP to avoid GLP-1 release and the incretin effect, which leads to a greater insulin excursion (34). With both GLP-1 and insulin able to act centrally to reduce feeding, we wanted to avoid this complicating factor. Because ICV glucose was able to recapitulate much of the suppressive effect of IP glucose, it appears our results are, to a large extent, glucose dependent. Our results clearly establish a role for a direct action of glucose on central ghrelin sensing and/or processing.
2-DG has been used experimentally to induce low available glucose, or glucopenia, for a long time. 2-DG has a hydrogen in place of the 2-hydroxyl, meaning the molecule cannot undergo further glycolysis (35). It then competitively inhibits conversion of glucose to ATP, resulting in reduced cellular ATP levels and causing a “metabolic emergency” that is centrally sensed and responded to. As expected, 2-DG itself increased feeding (36), but, more interestingly for us, ghrelin and 2-DG had an additive effect on food intake, suggesting that the reduction in available cellular energy primes the system to respond more strongly to the ghrelin signal. At a transcriptional level, we noticed changes in NPY mRNA that reflected the increases in food consumption. This reflects previous findings that NPY is critical in mediating the response to glucoprivic feeding (37). In vivo, low available blood glucose is a strong signal that things are not metabolically normal. The different methods for inducing a hypoglycemic or glucopenic event are all problematic because they all induce counter-regulatory or side effects that may then affect central processing themselves. That is to say, there is some discrepancy between available methods for experimentally inducing low glucose availability and physiological energy deficit. In response to 2-DG administration, blood glucose rises through the actions of adrenal catecholamines and adrenaline on liver, pancreas, and adipose tissues (38). In spite of these effects, the strength of 2-DG over exogenous insulin or fasting is that it provides a relatively isolated signal of glucose availability. Inducing hypoglycemia by giving insulin, which is an anorectic signal, would confound the concept of hypoglycemia as the driving force. Fasting is the ultimate model of energy deficit, but the humoral blueprint of the fasted state is broader than just low glucose and high ghrelin, and it is, in fact, this complexity we are trying to unravel.
AgRP neurons are crucial effector neurons for regulating body weight and feeding; loss of AgRP neuronal function underlies ghrelin resistance after high-fat diet feeding (39). Therefore, we speculated that AgRP neurons might be responsible for mediating the effects we saw. AMPK is a well-known nutrient sensor, important in signaling glucose availability and downstream effects of ghrelin, and thus is a logical target for mediating a combined effect of these two factors on feeding. We created AgRPAMPK mice by using a cre-dependent AAV to selectively reduce the amount of available AMPK in AgRP neurons. The DN construct encodes for a kinase dead (K45R) a2 subunit, which competes with the endogenous α2 subunit, resulting in reduced AMPK activity in AgRP neurons. These mice showed no changes in ghrelin sensitivity under normal conditions, indicating that AMPK activity in AgRP neurons is not critical for transducing the circulating ghrelin signal into a feeding response in normal mice fed ad libitum. Others have shown that Compound C given ICV is capable of reducing ghrelin-induced feeding (40). ICV administration reaches a much broader population than AgRP neurons, and Compound C is not selective for AMPK (41). However, we did see changes in the way mice responded to ghrelin when pretreated with glucose. AgRPGFP mice showed an attenuated response to ghrelin when pretreated with glucose, similar to C57Bl/6 mice, suggesting that acute glucose availability suppresses ghrelin’s ability to signal hunger. In AgRPAMPK mice this was no longer the case, and we saw a complete reinstatement of ghrelin-induced feeding. This indicates that AMPK is needed for glucose to be able to shut down the response to circulating ghrelin.
When we tested 2-DG in the AgRPAMPK mice, we found that they were less sensitive to the feeding effects of 2-DG compared with AgRPGFP mice. The hyperglycemic response to 2-DG is present in decerebrate rats where the hindbrain is surgically disconnected from the forebrain, so the counter-regulatory response to 2-DG is clearly generated more broadly than the hypothalamus (38). Decerebrate rats do not voluntarily eat, so the hypothalamic connection is clearly needed to facilitate glucoprivic feeding. Our results show that, although glucoprivic feeding is present in AgRPAMPK mice, it is attenuated, so there is a role for AgRP neurons in mediating the full orexigenic response. This is supported by previous findings that NPY knockout mice show reduced feeding after 2-DG administration (37). However, there were no differences in the way AgRPAMPK and AgRPGFP responded to the combined treatment of 2-DG and ghrelin, with the two groups showing remarkably similar patterns of responding. This seems to indicate that AMPK signaling in AgRP neurons is not required for the additive effects of ghrelin and 2-DG on feeding. Considering the widespread nature of the GHSR and the ubiquitous nature of AMPK, this is not surprising.
Whether the additive effects of 2-DG and ghrelin on feeding are AMPK dependent more globally is unanswered, but aspects of AMPK biology suggest it may still be important. Recently, a new role for AMPK in glucose sensing independent of AMP/ATP ratio has been described (27). Perhaps the most interesting aspect of this mechanism is that low glucose availability both activates AMPK and primes it for further action, thus putting gain into the system (27). This may then be exploited by other AMPK-utilizing substrates if energy levels continue to fall or if the brain receives information that this is the case, as with exogenous ghrelin administration. Whether this mechanism may be important in non-AgRP cells remains to be elucidated.
Glucoprivic potentiation of ghrelin-induced feeding occurs independently of AMPK signaling in AgRP neurons. Given the state of metabolic emergency that 2-DG induces in the brain, having a broader response to this insult seems biologically prudent. Indeed, this reflects a repeating theme in the neural control of feeding behavior, where mechanisms that suppress feeding are weaker or less global than those that potentiate it. This is likely due to the evolutionary context where reduced food availability was a far larger problem than food surplus. Overall, the current data show that acute energy availability, in the form of glucose, influences how the brain responds to ghrelin, another signal of energy status. These findings provide further evidence for the idea that the brain computes a “metabolic snapshot” readout from a range of available information sources and then acts in an integrated way to restore energy balance.
Neuroendocrine feedback during an energy-deficient state involves a number of hormonal and nutrient changes. This has been underappreciated, and future studies need to consider the relevant physiological context when examining hormonal actions in the brain. To begin to address these complex questions, we studied the interaction between glucose and ghrelin in a broader regulatory context. Here we provide evidence that short-term regulation of the hormonal signal ghrelin is under regulation by acute blood and brain glucose levels. Hyperglycemia, as a signal of immediate energy surplus, is able to suppress the ability of ghrelin to induce feeding, motivated responding, and hypothalamic mRNA production. We show that the suppression of feeding is dependent on appropriate AMPK action in AgRP neurons, reflecting a key role for AgRP neurons to sense and respond to incoming information of energy surplus. This concept that an intact cellular energy-sensing machinery is required for AgRP neurons to accurately function as frontline metabolic sensors with the capacity to orchestrate a broad physiological response to metabolic disturbances is developing. Recently, we showed that mice lacking carnitine O-acetyltransferase in AgRP neurons are unable to finely regulate a number of metabolic processes (42).
The dogma of AMPK as a “barometer” of AMP/ATP is a limited view of this complex signaling molecule. We and others have suggested that ghrelin signaling directly engages AMPK to regulate feeding circuits (43, 44); however, our current data suggest that this may not be case. We propose that instead AMPK facilitates cross talk between ghrelin and other signals of current energy availability, setting the metabolic tone of the AgRP cell, which is then able to potentiate or inhibit ghrelin signaling depending on energy status. Although outside the scope of this study, we hypothesize that insulin and leptin may similarly use AMPK to gauge the strength of the physiological response. Indeed, others have noted the variability of the effectiveness of these peptides on suppression of feeding (45), and we propose that differences in baseline AMPK tone may be one explanation for this observation. AMPK is a highly conserved, multifaceted signaling molecule that is important in a number of cellular signaling processes relating to metabolic homeostasis. We show that AMPK in AGRP neurons is required for full appreciation of current metabolic state and offer further evidence that the brain reads metabolic state as the sum of available signals and responds to this integrated picture.
Acknowledgments
Financial Support: This study was supported by Australian National Health and Research Council (NHMRC) Grant 1084600 (to D.C.S.), NHMRC fellowship Grant 1084344 (to Z.B.A.), National Institutes of Health Grants R01 DK108797 and NINDS R21 NS097922 (to D.K.), NHMRC Grant 1072364 (to S.H.L.), and by the Victorian Government Operational Infrastructure Support Programme. S.H.L. and R.S. are supported by an Australian NHMRC early career research fellowship. A.J.L. is an NHMRC Principal Research Fellow (1116930).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 2-DG
2-deoxyglucose
- AAV
adeno-associated virus
- aCSF
artificial cerebrospinal fluid
- AgRP
agouti-related peptide
- AMPK
AMP-activated protein kinase
- ARC
arcuate nucleus of the hypothalamus
- DN
dominant-negative
- GHSR
growth hormone secretagogue receptor
- ICV
intracerebroventricular
- NPY
neuropeptide Y
References
- 1. Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. [DOI] [PubMed] [Google Scholar]
- 2. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJS, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LHT. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. [DOI] [PubMed] [Google Scholar]
- 3. Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–316. [DOI] [PubMed] [Google Scholar]
- 4. Lockie SH, Dinan T, Lawrence AJ, Spencer SJ, Andrews ZB. Diet-induced obesity causes ghrelin resistance in reward processing tasks. Psychoneuroendocrinology. 2015;62:114–120. [DOI] [PubMed] [Google Scholar]
- 5. Perez-Tilve D, Heppner K, Kirchner H, Lockie SH, Woods SC, Smiley DL, Tschöp M, Pfluger P. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011;25(8):2814–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73(9):915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–388. [DOI] [PubMed] [Google Scholar]
- 10. Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, Andrews ZB. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72(6):457–465. [DOI] [PubMed] [Google Scholar]
- 11. Hornsby AKE, Redhead YT, Rees DJ, Ratcliff MSG, Reichenbach A, Wells T, Francis L, Amstalden K, Andrews ZB, Davies JS. Short-term calorie restriction enhances adult hippocampal neurogenesis and remote fear memory in a Ghsr-dependent manner. Psychoneuroendocrinology. 2016;63:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kent BA, Beynon AL, Hornsby AKE, Bekinschtein P, Bussey TJ, Davies JS, Saksida LM. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology. 2015;51:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS, Andrews ZB. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J Neurosci. 2016;36(10):3049–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. [DOI] [PubMed] [Google Scholar]
- 15. Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, Sarkar P, Rendon DA, Gaber MW, LeMaire SA, Coselli JS, Milewicz DM, Sutton VR, Butte NF, Moore DD, Chopra AR. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165(3):566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. [DOI] [PubMed] [Google Scholar]
- 17. Ruud J, Steculorum SM, Brüning JC. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun. 2017;8:15259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chalmers JA, Jang JJ, Belsham DD. Glucose sensing mechanisms in hypothalamic cell models: glucose inhibition of AgRP synthesis and secretion. Mol Cell Endocrinol. 2014;382(1):262–270. [DOI] [PubMed] [Google Scholar]
- 19. Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2013;3(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mani BK, Zigman JM. Ghrelin as a survival hormone. Trends Endocrinol Metab. 2017;28(12):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. 2008;366(2):388–392. [DOI] [PubMed] [Google Scholar]
- 22. Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LGD, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford MLJ, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117(8):2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–574. [DOI] [PubMed] [Google Scholar]
- 24. Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26(3):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282(45):32539–32548. [DOI] [PubMed] [Google Scholar]
- 26. Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. [DOI] [PubMed] [Google Scholar]
- 27. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. [DOI] [PubMed] [Google Scholar]
- 28. Kong D, Dagon Y, Campbell JN, Guo Y, Yang Z, Yi X, Aryal P, Wellenstein K, Kahn BB, Sabatini BL, Lowell BB. A postsynaptic AMPK→p21-activated kinase pathway drives fasting-induced synaptic plasticity in AgRP neurons. Neuron. 2016;91(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown RM, Short JL, Cowen MS, Ledent C, Lawrence AJ. A differential role for the adenosine A2A receptor in opiate reinforcement vs opiate-seeking behavior. Neuropsychopharmacology. 2009;34(4):844–856. [DOI] [PubMed] [Google Scholar]
- 30. Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151(10):4745–4755. [DOI] [PubMed] [Google Scholar]
- 33. Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–E1332. [DOI] [PubMed] [Google Scholar]
- 34. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. [DOI] [PubMed] [Google Scholar]
- 35. Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224(2):963–969. [PubMed] [Google Scholar]
- 36. Smith GP, Epstein AN. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol. 1969;217(4):1083–1087. [DOI] [PubMed] [Google Scholar]
- 37. Sindelar DK, Ste Marie L, Miura GI, Palmiter RD, McMinn JE, Morton GJ, Schwartz MW. Neuropeptide Y is required for hyperphagic feeding in response to neuroglucopenia. Endocrinology. 2004;145(7):3363–3368. [DOI] [PubMed] [Google Scholar]
- 38. DiRocco RJ, Grill HJ. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science. 1979;204(4397):1112–1114. [DOI] [PubMed] [Google Scholar]
- 39. Briggs DI, Lockie SH, Benzler J, Wu Q, Stark R, Reichenbach A, Hoy AJ, Lemus MB, Coleman HA, Parkington HC, Tups A, Andrews ZB. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology. 2014;155(7):2411–2422. [DOI] [PubMed] [Google Scholar]
- 40. López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S, Deoliveira RM, Castañeda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Diéguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7(5):389–399. [DOI] [PubMed] [Google Scholar]
- 41. Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408(3):297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reichenbach A, Stark R, Mequinion M, Denis RRG, Goularte JF, Clarke RE, Lockie SH, Lemus MB, Kowalski GM, Bruce CR, Huang C, Schittenhelm RB, Mynatt RL, Oldfield BJ, Watt MJ, Luquet S, Andrews ZB. AgRP neurons require carnitine acetyltransferase to regulate metabolic flexibility and peripheral nutrient partitioning. Cell Reports. 2018;22(7):1745–1759. [DOI] [PubMed] [Google Scholar]
- 43. Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7(5):377–388. [DOI] [PubMed] [Google Scholar]
- 44. Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals [published correction appears in Nature. 2009;459:736]. Nature. 2008;454(7206):846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woods SC, Langhans W. Inconsistencies in the assessment of food intake. Am J Physiol Endocrinol Metab. 2012;303(12):E1408–E1418. [DOI] [PMC free article] [PubMed] [Google Scholar]