Abstract
A 67-year-old woman presented with 5 days of myalgias and fevers on completion of a 21-day course of amoxicillin for Lyme disease (Borrelia burgdorferi infection). She was found to have profound thrombocytopenia, as well as new anaemia and leucopenia. Workup revealed Babesia microti as the causative agent of her symptoms. The patient quickly improved after appropriate antimicrobial therapy directed against babesiosis was started. This case illustrates the importance of basic microbiology, including epidemiology and common vectors, when creating a differential diagnosis. Because the Ixodes scapularis tick can harbour and transmit multiple parasites simultaneously, the possibility of coinfection should be considered in any patient not responding to appropriate initial medical therapy.
Keywords: infectious diseases, travel medicine
Background
Our patient was treated for Lyme disease but later presented with symptoms of ongoing infection and was found to have babesiosis. This case illustrates the importance of recognising coinfections from a common vector. The Ixodes scapularis tick, for instance, is capable of harbouring Borrelia, Anaplasma and Babesia species. Babesia infections are not adequately covered by doxycycline, the first-line treatment for Anaplasma and Borrelia infections. Timely recognition of this coinfection is of utmost importance as it can become severe enough to require exchange transfusion therapy and is potentially fatal if left untreated.
Case presentation
A 67-year-old woman from western Wisconsin noted a circular red rash on her left thigh. Her medical history was notable for colon cancer with subsequent hemicolectomy and sleep apnoea. She was an avid gardener and spent a significant amount of time outdoors in wooded areas. The rash was thought consistent with erythema migrans, and she was prescribed a 3-week course of amoxicillin by her local provider. Amoxicillin 500 mg three times a day for 21 days was used as she had a doxycycline allergy.
Near the end of the 21-day treatment course, she developed 5 days of fevers to 39.4°C, as well as myalgias, dizziness, and fatigue. She returned to clinic for further evaluation. Laboratory studies at that visit were notable for haemoglobin of 11.3 g/dL (decreased from 13.1 g/dL 2 months prior, normal range 12.0–15.5 g/dL), platelet count of 21×103/µL (decreased from 254×103/µL, normal range 150–450×103/µL), leucocyte count of 4.1×103/µL (normal range 3.5–10.5×103/µL) with absolute neutrophil count 2.5×103/µL, erythrocyte sedimentation rate 53 mm/hour (normal range 0–30 mm/hour), sodium 131 mmol/L (normal range 135–145 mmol/L) and C reactive protein 123.4 mg/L (normal range ≤5 mg/L). Liver chemistries were within normal limits. The patient had no nuchal rigidity or rash at that time.
Given concern for sepsis versus possible ongoing tick-borne infection, blood cultures were drawn, and the patient was admitted to a local hospital. In particular, there was concern for human granulocytic anaplasmosis or babesiosis in light of the patient’s thrombocytopenia and low neutrophil count.
On the second hospital day, the patient’s haemoglobin and platelets continued to downtrend, though she remained clinically stable. She was, at that point, transferred to our facility for further workup and management.
Investigations
Laboratory investigation included a complete blood count (CBC), revealing pancytopenia and liver enzymes as shown in the table 1. Lactate dehydrogenase (LDH) was found to be elevated on initial presentation, indicative of a haemolytic process, despite a normal total bilirubin value of 0.7 mg/dL (normal range 0.1–1 mg/dL). DNA PCR returned negative for Borrelia burgdorferi, Anaplasma phagocytophilum and three species of Erlichia. Babesia microti DNA PCR was positive. The blood cultures that had been drawn at the patient’s local hospital showed no growth at 5 days.
Table 1.
Trend of laboratory studies
| 2 months prior to admission | Hospital day 1 | Hospital day 2 | Hospital day 3 (atovaquone and azithromycin started) | Hospital day 4 | Hospital day 5 | 1 week post discharge | |
| Haemoglobin (g/dL) | 13.1 | 11.3 | 10.0 | 9.0 | 9.7 | 10.0 | 11.4 |
| Platelets ×103/µL | 254 | 4.1 | 4.1 | 3.1 | 3.3 | 3.7 | 6.6 |
| Leucocytes (x103/µL) | 8.2 | 21 | 21 | 17 | 23 | 42 | 295 |
| Alanine aminotransferase (U/L) | 32 | 34 | 50 | 64 | 64 | 44 | |
| Aspartate aminotransferase (U/L) | 39 | 31 | 53 | 54 | 51 | 25 |
Differential diagnosis
Tick-borne infections, particularly Anaplasma and Babesia, were high on the differential given the patient’s exposure to wooded areas and recent treatment for Lyme disease. The patient’s abnormalities in CBC are not typical of Borrelia infections; therefore, a coinfection was of greater concern than recrudescent Lyme disease. Erlichia, though it can cause cytopenias, is transmitted by a different species of tick, Amblyomma americanum, and is less commonly seen in the Midwestern USA.
Viral infection was also under consideration. HIV and hepatitis C can present with similar cytopenias.
Malaria is another cause of haemolytic anaemia and fevers, though our patient had no recent travel outside of the country, so this diagnosis was of much lower likelihood.
Other possibilities included drug-induced cytopenias, as the patient had been on recent antimicrobial therapy or autoimmune cytopenias (including systemic lupus erythematosus (SLE)-induced cytopenia).
Treatment
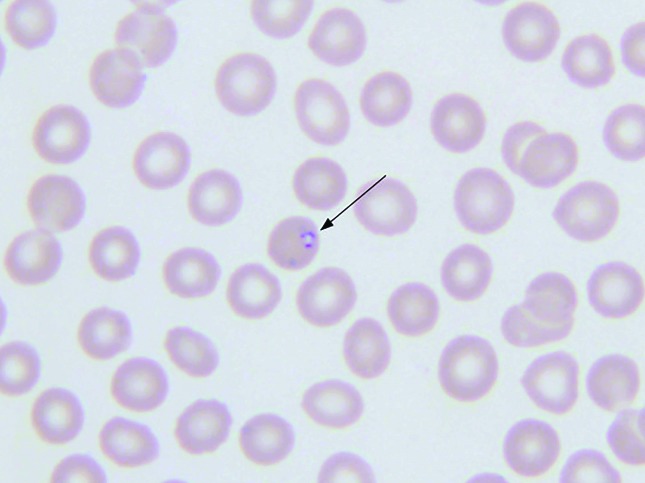
On arrival to our hospital, the patient was febrile to 38.4°C. Her laboratory studies were notable for haemoglobin of 9.0 g/dL with LDH of 265 U/L (normal range 122–222 U/L), indicating likely haemolysis. Platelet count was decreased to 17×103/µL, with an associated decline in leukocytes to 3.1×103/µL. Liver enzymes were found to be newly elevated (Aspartate aminotransferase (AST) 53 U/L (normal 8–43 U/L) and alanine aminotransferase (ALT) 50 U/L (normal 7–45 U/L)). PCR testing was negative for Anaplasma and Borrelia but positive for Babesia microti. Further laboratory testing was completed including a thin blood smear from the date of hospital transfer that revealed a parasite load of 0.4% (see figure 1).
Figure 1.
Blood smear demonstrating intracellular Babesia species.
Antimicrobial therapy with azithromycin 250 mg by mouth (PO) daily and atovaquone 750 mg PO twice daily was initiated for a planned 10-day course. By hospital day five, the patient had been afebrile >24 hours and was deemed stable for dismissal. Her platelet count had doubled to 42 000×103/µL and transaminase levels stabilised. She continued to report night sweats, although her initial myalgias and dizziness had resolved.
Outcome and follow-up
The patient was seen by her primary care provider 1 week after hospital discharge, at which time she overall reported improvement in her symptoms, though did continue to have some fatigue. Laboratory studies demonstrated an increase in all three cell lines, as well as normalisation of her liver chemistries, as indicated in the above table.
Discussion
B. microti, the most common species of Babesia causing babesiosis in the USA, is a protozoan organism carried by the I. scapularis tick.1 This is the same species of tick that transmits B. burgdorferi (the bacteria causative of Lyme disease) and A. phagocytophilium (the bacteria causative of human granulocytic anaplasmosis).2 Similar to malaria, babesiosis is caused by parasitic invasion of erythrocytes, causing a haemolytic anaemia.3 Babesia species can be identified on blood smear as intraerythrocytic rings, or in the dividing form of a tetrad, commonly termed a ‘Maltese Cross’.4 The dividing tetrad form is a rare finding on blood smear but is pathognomonic for Babesia infection and is helpful, along with epidemiological information, to distinguish babesiosis from malaria.4
B. microti is endemic to the Northeast and Midwest USA. Per reports from the Centers for Disease Control and Prevention, there were 1762 cases of babesiosis in 2013 between 27 states in the USA.5 This is much less common than Lyme disease, with a reported 27 203 cases in 2013.6 Of the reported babesiosis cases in 2013, 95% were from the states of Connecticut, Massachusetts, Minnesota, New Jersey, New York, Rhode Island and Wisconsin.5
Our patient was from western Wisconsin. Reported cases of babesiosis have increased in that area over the past several years.7 This has been attributed in part to an increased area in which Babesia is endemic, as evidenced by increased identification of B. microti during tick sampling.8 Also, the ease and availability of diagnostic testing has increased with the introduction of B. microti DNA PCR.7 This has been shown to be more sensitive than traditional blood smear for identification of parasites.1
When patients present with a tick bite or suspected tick-borne infection, it is important to recognise that a single tick can serve as a common vector for multiple infections. Early identification of coinfections allows for the correct treatment to be administered. Although doxycycline provides coverage for Anaplasma and Borrelia infections, it is not effective against Babesia species.2 8
Simultaneous infection of babesiosis and Lyme disease has been described in the past. In a prospective corhort study, Krause et al.9 compared 214 patients with Lyme disease to 26 patients with simultaneous Lyme disease and babesiosis. The majority of infections occurred between May and September. Similar to our case, coinfected individuals were more likely to report associated flu-like symptoms, such as fatigue, headaches, chills/sweats, anorexia, nausea and conjunctivitis. The combination of fever, chills and headache was thought to be the predictive concurrent infection, noted in almost half (44%) of patients with coinfection compared with 13% of those with isolated Lyme disease. Similar findings have been suggested for human granulocytic anaplasmosis and Lyme disease coinfections, although this association is not consistent across all published studies.10 Additionally, patients simultaneously infected with Lyme disease and babesiosis have been reported to be more likely to have splenomegaly on physical exam and are more commonly found to be anaemic and/or thrombocytopenic on an initial CBC.
Our patient presented with symptoms of babesiosis almost 3 weeks after completing antimicrobial therapy for Lyme disease. A delay in babesiosis diagnosis was also suggested by Krause et al; however, duration of delay was not reported. Case reports by Marcus et al and Surgers et al, both describe patients presenting with an erythematous rash 3–4 weeks prior to a worsening of symptoms leading to the diagnosis of babesiosis, similar to our patient.11 12 The cause for the delay in our case is not known; however, it is possible that patients have mild parasite load during their initial evaluation, not severe enough to cause systemic symptoms to warrant further evaluation.
Based on this information, it is suggested that concurrent babesiosis infection be considered in patients with Lyme disease who remain febrile after 48 hours of appropriate antimicrobial therapy or those with unexplained anaemia and/or thrombocytopenia.10
Per guidelines from the Infectious Disease Society of America (IDSA), adult patients with non-severe babesiosis should be treated with a combination of oral atovaquone and azithromycin for 7–10 days.2 The IDSA currently recommends that patients with more severe infection be treated with quinine and intravenous clindamycin.2 This second regimen was the initial therapy for babesiosis, first used in 1982, but is often poorly tolerated due to side effects.13 It therefore is not first line except in cases of severe infection or when the patient cannot tolerate atovaquone and azithromycin.2 13 More recent data from a retrospective review of 62 cases of babesiosis suggests that atovaquone and azithromycin can be an effective regimen even for patients with severe disease, though this population notably only included 11 patients who required intensive care unit admission with six requiring exchange transfusion.14
Urgent exchange transfusion should be considered for patients with severe babesiosis, including those with a parasite load ≥10%, organ dysfunction including renal, hepatic or pulmonary involvement or a high burden of haemolysis.2 Those at risk for severe infection include patients with HIV, asplenia, immunosuppressive drug therapy and those with comorbid lung or liver disease.2 15 Because our patient’s parasite load was 0.4% and she remained clinically stable throughout admission, atovaquone and azithromycin were used and were well tolerated. In general, treatment response should be expected within the first 48 hours of starting antiparasitic therapy, as was true with our patient.
In summary, providers caring for patients with a diagnosis of tick-borne infection should be cognizant of possible coinfections when a patient does not respond to initial therapy. In cases where babesiosis is diagnosed, high alert should be kept for complications including severe haemolysis, and renal, hepatic or pulmonary failure. Urgent exchange transfusion should be considered in such instances.
Learning points.
The Ixodes scapularis tick can harbour and transmit Borrelia, Anaplasma, and Babesia species.
Concurrent babesiosis infection should be considered in patients with Lyme disease who remain febrile after 48 hours of appropriate antimicrobial therapy or those with unexplained anaemia and/or thrombocytopenia. Delayed presentations of babesisos have been reported. Patients with babesiosis should be treated with a combination of oral atovaquone and azithromycin for 7–10 days. Infectious Disease Society of America guidelines suggest that patients with severe infection should be treated with quinine and intravenous clindamycin, although more recent data suggest that atovaquone and azithromycin may be effective in this population as well.
Patients at risk for severe Babesia infection include those aged greater than 50 years and immunocompromised individuals.
Patients with severe babesiosis should be transferred to a facility where exchange transfusion is an available therapeutic option as infectious complications can be fatal.
Acknowledgments
We would like to acknowledge Larry J Prokop, MLS, for his help in conducting an exhaustive literature search for prior reports of babesiosis and Lyme disease coinfections.
Footnotes
Contributors: All the authors contributed in conception, design of study, acquisition of data, analysis and/or interpretation of data and approval of the version of the manuscript to be published. KH contributed in drafting the manuscript. MB contributed in revising the manuscript critically for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Teal AE, Habura A, Ennis J, et al. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol 2012;50:903–8. 10.1128/JCM.05848-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1089–134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 3.Knapp KL, Rice NA. Human Coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015;2015:1–11. 10.1155/2015/587131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akel T, Mobarakai N. Hematologic manifestations of babesiosis. Ann Clin Microbiol Antimicrob 2017;166:6 10.1186/s12941-017-0179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, 2015. Parasites - Babesiosis 2013 Data Archive. https://www.cdc.gov/parasites/babesiosis/data-statistics/2013.html (updated 13 Jan 2015).
- 6.Centers for Disease Control and Prevention, 2017. Lyme disease data tables. https://www.cdc.gov/lyme/stats/tables.html
- 7.Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant Babesiosis in Southwestern Wisconsin. WMJ 2015;114:152–7. [PubMed] [Google Scholar]
- 8.Chabria S, Ogbuagu O. Fatal multiple deer tick-borne infections in an elderly patient with advanced liver disease. BMJ Case Rep 2015;2015:bcr2014208182 10.1136/bcr-2014-208182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause PJ, Telford SR, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996;275:1657–60. [PubMed] [Google Scholar]
- 10.Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 2016;315:1767–77. 10.1001/jama.2016.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus LC, Steere AC, Duray PH, et al. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Ann Intern Med 1985;103:374–6. [DOI] [PubMed] [Google Scholar]
- 12.Surgers L, Belkadi G, Foucard A, et al. Babesiosis and Lyme disease co-infection in a female patient returning from the United States. Med Mal Infect 2015;45(11-12):490–2. 10.1016/j.medmal.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000;343:1454–8. 10.1056/NEJM200011163432004 [DOI] [PubMed] [Google Scholar]
- 14.Kletsova EA, Spitzer ED, Fries BC, et al. Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann Clin Microbiol Antimicrob 2017;16:26 10.1186/s12941-017-0198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am 2015;29:357–70. 10.1016/j.idc.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]