Abstract
Leiomyosarcoma (LMS) of primary vascular origin is a rare entity with only potentially curative option being complete surgical resection; despite which the prognosis remains dismal. Tumour recurrence is very common, and the benefits of adjuvant therapy are undefined. A 39-year-old woman presented with 6 months’ history of abdominal pain, abdominal distension and pedal oedema. On evaluation, she was diagnosed to have chronic Budd-Chiari syndrome (BCS) secondary to a tumour arising from the inferior vena cava (IVC) on evaluation. Her liver decompensation included jaundice, gastrointestinal bleed and ascites. Following a detailed multidisciplinary team discussion, she underwent complete excision of the tumour along with a segment of the IVC with living donor liver transplantation. She remains disease-free 24 months following surgery. This is the first reported case of liver transplantation for IVC LMS causing chronic BCS.
Keywords: transplantation, surgical oncology, liver disease
Background
Primary vascular leiomyosarcoma (LMS) was first described by Perl and Virchow in 1871, and although rare, is the most common primary malignant tumour of inferior vena cava (IVC).1 2 It presents with non-specific symptoms, leading to a delay in diagnosis and poor prognosis. Based on the segment of IVC involved, the tumours are classified into three different zones, with zone II being the most common site involved.2 The optimal management of IVC LMS remains undefined due to lack of randomised trials. Sparse data in the form of case reports and short series have shown complete surgical excision to provide the best long-term survival.3–5 However, even this modality is plagued by high rates of tumour recurrence. The tumour location makes any surgery on these lesions technically challenging and fraught with complications.4 5
LMS presenting with IVC obstruction, especially those causing chronic Budd-Chiari syndrome (BCS) are considered to have a particularly poor outcome.5 We present the first ever reported case of a living donor liver transplantation, as a curative option for LMS of the IVC.
Case presentation
A 39-year-old woman, presented with 6 months’ history of abdominal pain, abdominal distension and lower extremity oedema. There was no family history of malignancy. Her body mass index was 33.4 kg/m2, and on examination, she had clinical evidence of jaundice, ascites, abdominal wall oedema with dilated veins and bilateral pedal oedema.
Investigations
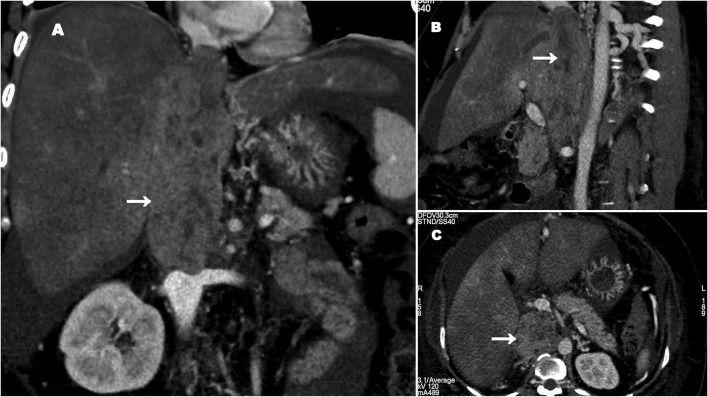
Her blood tests revealed features of decompensated liver disease ((serum bilirubin 8 mg/dL (n=0.1–1.2 mg/dL), platelets 80x109/L (n=150-450x109L), international normalised ratio (INR) 2.2 and albumin 2.1 mg/dL (n=3.5–5.5 mg/dL)). Her tumour markers were unremarkable {alpha feto protein (AFP) 4.6 ng/mL and carbohydrate antigen (CA) 19–9 32 IU/L}. Imaging in the form of a contrast-enhanced computer tomography revealed a heterogenous mass with peripheral enhancement replacing the retrohepatic IVC segment extending from 2 cm above the hepatic vein orifice to just above the renal vein orifice (figure 1). There were features of portal hypertension with complete occlusion of all three hepatic veins. Hepatic venous pressure gradient was 28 mm Hg (n=1–5 mm Hg), confirming the diagnosis of chronic BCS secondary to the tumour. Positron emission tomography showed no evidence of systemic spread.
Figure 1.
Contrast-enhanced CT abdomen showing leiomyosarcoma of the inferior vena cava (IVC), occluding the hepatic venous orifices causing Budd-Chiari syndrome (white arrow: tumour in the IVC). (A) Coronal view. (B) Sagittal view. (C) Axial view.
Treatment
Following a detailed multidisciplinary team discussion, in view of the nature of her decompensated liver disease and the need for a complete tumour-free resection margin, she was offered an en bloc tumour excision with a segment of the IVC along with total hepatectomy and a liver transplantation.
Being a non-standardised form of treatment, and the only possible option for a likely cure, this was further discussed in detail with the patient and her relatives. The procedure was also discussed and approved by the transplant governing body of the state. Her husband volunteered to donate the right lobe of his liver. Donor safety is the most crucial aspect of living donor transplantation and donor evaluation included medical, radiological, psychological and social assessments. As a part of his assessment, the prospective donor underwent a series of interviews with the doctors and psychologist, wherein all the aspects of donation were discussed in detail. This included the perioperative period, the long-term outcomes and complications (including the risk of mortality). Only after it is ascertained that the donor understood the risks involved, and that the donation truly altruistic was the donor deemed fit to donate from a psychological point of view.
The operation involved mobilising the liver and dissecting the tumour along with a segment of the IVC off the retroperitoneum. The baseline portal pressure was 25 mm Hg. The tumour was removed en bloc along with the liver (figure 2). Cryopreserved cadaveric IVC graft was used to reconstruct the excised segment of the IVC forming a ‘neocava’. A living donor liver transplantation was performed with a right lobe in which the anterior sector (segments 5 and 8) veins were also reconstructed with cadaveric iliac vein grafts and right hepatic vein was anastomosed to the neocava. The postreperfusion portal pressure dropped to 11 mm Hg. Arterial reconstruction was by a microscopic anastomosis between the liver graft’s right hepatic artery to the recipient’s common hepatic artery. Biliary reconstruction was by a standard duct–duct anastomosis.
Figure 2.
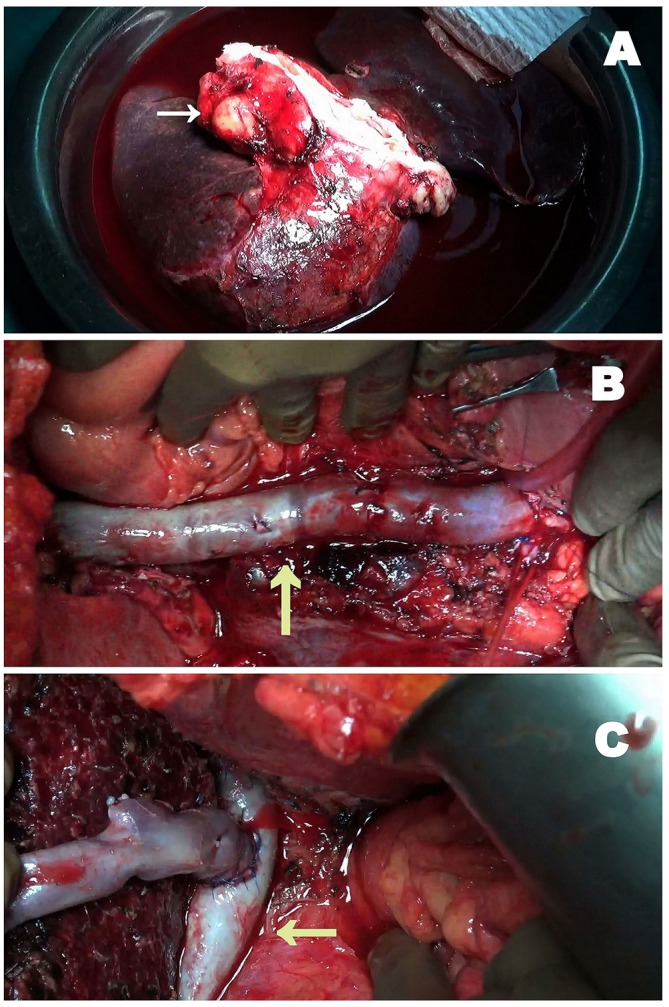
Intraoperative images of en bloc excision of leiomyosarcoma with a segment of the inferior vena cava (IVC) along with total hepatectomy and a right lobe living donor liver transplantation. (A) En bloc tumour excision with total hepatectomy (white arrow). (B): IVC reconstruction with cryopreserved IVC vein graft forming a neocava (yellow arrow: neocava). (C) Right lobe implantation onto the neocava (yellow arrow: neocava).
Outcome and follow-up
Her postoperative period was uneventful, and she was discharged home on the 14thpostoperative day. Post-transplant immunosuppression was with steroids, tacrolimus and mycophenolate mofetil. One month following the surgery, everolimus was added, keeping in mind its tumour-static activity. The combined tacrolimus–everolimus levels were maintained at 6–8 ng/mL. Histopathology of the excised specimen demonstrated R0 (complete) margin-free resection of IVC LMS (grade 2 tumour with mitosis and differentiation score of 2 each and no necrosis). An oncology consult was obtained, and it was decided not to offer the patient adjuvant therapy. On follow-up, she remains well and disease-free 24 months following the surgery. The donor was discharged home on the sixth postoperative day, making an full recovery with no short-term or long-term morbidity.
Discussion
LMS are rare tumours of the IVC constituting less than 0.5% of all adult sarcomas.5 Despite being slow-growing tumours confined to the IVC, they carry a poor prognosis due to a delay in diagnosis and tumour location, often making radical surgery treacherous. Radical surgical resection is possibly the mainstay of treatment for IVC LMS and demands expertise. There are reports of en bloc resection of the tumour segment with {Polytetrafluoroethylene (PTFE), Dacron patch} or without vascular reconstruction, multivisceral resection requiring venovenous bypass and hypothermic perfusion of liver and liver autotransplantation.6–8 An autotransplantation was not an option for our patient, as the liver had already decompensated, and was itself a standalone indication of transplantation. The postoperative morbidity associated with IVC resection for sarcoma is high (>20%), and the poor prognostic factors include BCS, suprahepatic tumours, lower extremity oedema, IVC occlusion and decompensated liver disease.5 9 10 All of which were present in our patient. The IVC graft-related morbidity include graft thrombosis, sepsis and the risk of pulmonary embolism.10 Local recurrences occur in 13%–33% of patients, whereas distant metastases are seen in 30%–50% of patients.10 Liver transplantation for primary or secondary liver sarcomas have been fraught with unacceptable rates of recurrence and remain a poor choice of treatment.11 None of the patients in the study had IVC LMS; making our report a unique one.
Being a large volume live donor liver transplantation unit, it was intuitive for us to offer the patient a liver transplantation. This is as mentioned above is a non-standardised form of therapy, with unclear benefit. Fortunately, the patient has shown a disease-free survival of over 24 months following the liver transplantation, justifying our treatment algorithm.
The current role of adjuvant therapy to improve the overall survival is questionable, although reports have shown a marginal decrease in local recurrence with radiation therapy.9 12 According to a pooled analysis of 377 cases, the overall survival rate after curative surgical resection at 1 year and 5 years were 92% and 57%, and the disease-free survival at 1 year and 5 years were 55% and 5%, respectively.10 BCS is seen in up to 25% of patients either due to direct tumour infiltration or thrombosis of hepatic veins, and outcomes are particularly poor due to the associated liver dysfunction.5 10 12 Margin status, complete radical resection and size of the tumour are important prognostic factors that determine the overall survival, and macroscopic margin positivity is associated with poor outcomes.10 12
Our patient presented with chronic BCS and liver decompensation; she underwent living donor liver transplantation as it was the only option available to restore her liver function and achieve a complete tumour clearance. To the best of our knowledge, this is the first report of living donor liver transplantation for IVC LMS with decompensated chronic BCS. This successful case report needs to be tempered against the sobering thought of life-long immunosuppression and its undefined yet deleterious effects on tumour recurrence which need to be discussed in detail at the time of preoperative evaluation.
Learning points.
Inferior vena cava (IVC) leiomyosarcoma (LMS) are rare tumours carrying a poor prognosis.
Aggressive complete resection is the only curative option for long-term survival, and the present role of adjuvant therapy remains undefined.
Management of IVC LMS associated with chronic Budd-Chiari syndrome and decompensation is a unique challenge, and the prognosis is particularly poor in this subset.
Liver transplantation may offer long-term survival in these patients, however, its pros and cons should be discussed preoperatively, before an informed treatment algorithm is embarked on.
Footnotes
Contributors: AR, VG, SMR: contributed to conception and design, acquisition, analysis and interpretation of data. VG, AR, MR: drafted the article and revised it critically for important intellectual content. MR: gave the final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Approved by the institutional ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Perl L, Virchow R. Ein fall von sarkom der vena cava inferior. Virchow’s Arch 1871;53:378–83. 10.1007/BF01957198 [DOI] [Google Scholar]
- 2.Mastoraki A, Leotsakos G, Mastoraki S, et al. Challenging diagnostic and therapeutic modalities for leiomyosarcoma of inferior vena cava. Int J Surg 2015;13:92–5. 10.1016/j.ijsu.2014.11.051 [DOI] [PubMed] [Google Scholar]
- 3.Kieffer E, Alaoui M, Piette JC, et al. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg 2006;244:289–95. 10.1097/01.sla.0000229964.71743.db [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cananzi FC, Mussi C, Bordoni MG, et al. Role of surgery in the multimodal treatment of primary and recurrent leiomyosarcoma of the inferior vena cava. J Surg Oncol 2016;114:44–9. 10.1002/jso.24244 [DOI] [PubMed] [Google Scholar]
- 5.Mingoli A, Cavallaro A, Sapienza P, et al. International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res 1996;16:3201–5. [PubMed] [Google Scholar]
- 6.Fernandez HT, Kim PT, Anthony TL, et al. Inferior vena cava reconstruction for leiomyosarcoma of Zone I-III requiring complete hepatectomy and bilateral nephrectomy with autotransplantation. J Surg Oncol 2015;112:481–5. 10.1002/jso.24041 [DOI] [PubMed] [Google Scholar]
- 7.Mann GN, Mann LV, Levine EA, et al. Primary leiomyosarcoma of the inferior vena cava: a 2-institution analysis of outcomes. Surgery 2012;151:261–7. 10.1016/j.surg.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 8.Ravaioli M, Serenari M, Cescon M, et al. Liver and Vena Cava En Bloc Resection for an Invasive Leiomyosarcoma Causing Budd-Chiari syndrome, under veno-venous bypass and liver hypothermic perfusion: liver hypothermic perfusion and veno-venous bypass for inferior vena cava leiomyosarcoma. Ann Surg Oncol 2017;24:556–7. 10.1245/s10434-016-5285-1 [DOI] [PubMed] [Google Scholar]
- 9.Laskin WB, Fanburg-Smith JC, Burke AP, et al. Leiomyosarcoma of the inferior vena cava: clinicopathologic study of 40 cases. Am J Surg Pathol 2010;34:873–81. 10.1097/PAS.0b013e3181ddf569 [DOI] [PubMed] [Google Scholar]
- 10.Wachtel H, Gupta M, Bartlett EK, et al. Outcomes after resection of leiomyosarcomas of the inferior vena cava: a pooled data analysis of 377 cases. Surg Oncol 2015;24:21–7. 10.1016/j.suronc.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 11.Husted TL, Neff G, Thomas MJ, et al. Liver transplantation for primary or metastatic sarcoma to the liver. Am J Transplant 2006;6:392–7. 10.1111/j.1600-6143.2005.01179.x [DOI] [PubMed] [Google Scholar]
- 12.Teixeira FJR, do Couto Netto SD, Perina ALF, et al. Leiomyosarcoma of the inferior vena cava: Survival rate following radical resection. Oncol Lett 2017;14:3909–16. 10.3892/ol.2017.6706 [DOI] [PMC free article] [PubMed] [Google Scholar]