Abstract
The authors present a case of an elderly man with a history of Waldenstrom macroglobulinaemia in remission who presented with progressively worsening gait abnormalities and falls for several months. His examination was notable for bilateral lower extremity weakness and an unsteady gait. Brain and spinal MRI showed focal leptomeningeal enhancement in the brain and spinal column. Lumbar puncture was performed and cerebrospinal fluid flow cytometry demonstrated a monoclonal CD5/CD10-negative, CD20-positive B-cell lymphocyte population consistent with a diagnosis of Bing-Neel syndrome. He was started on ibrutinib, an oral Bruton’s tyrosine kinase inhibitor, and had marked improvement in his weakness and gait. Repeat imaging 2 months after starting ibrutinib showed improvement in his leptomeningeal enhancement. During subsequent follow-up, he continued to tolerate ibrutinib and had a sustained clinical response.
Keywords: cns cancer, chemotherapy, cancer intervention
Background
Bing-Neel syndrome (BNS) is a rare manifestation of Waldenstrom macroglobulinaemia (WM) characterised by central nervous system (CNS) infiltration with lymphoplasmacytic cells. The most common neurological presentations of BNS include cranial nerve palsies, sensorimotor dysfunction and ataxia.1 Given the rarity of this syndrome, there is no standardised approach to its management, though systemic chemotherapy with or without high-dose chemotherapy with autologous stem cell transplantation is often employed. Ibrutinib, an oral Bruton’s tyrosine kinase (BTK) inhibitor, was approved by the Food and Drug Administration in January 2015 for use in WM, but its use in BNS is not well established. Here, we report a case of BNS with rapid clinical and radiographic response to first-line ibrutinib.
Case presentation
A 71-year-old man with a 4-year history of WM presented to the emergency department with progressive gait abnormalities and falls. He had been in clinical remission for 9 months following first-line therapy with six cycles of bendamustine and rituximab and received one dose of planned maintenance rituximab therapy prior to this presentation. His falls were not syncopal in origin and were associated with bilateral lower extremity weakness. Physical examination was notable for a proximal muscle weakness, unsteady gait, diminished sensation to light touch bilaterally below the knees with decreased proprioception and vibratory sensation in a similar distribution.
Investigations
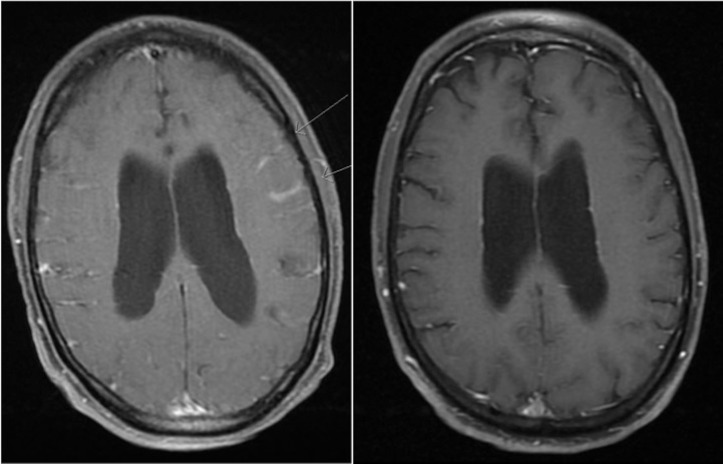
Peripheral blood IgM levels were 0.248 g/dL, consistent with previous remission levels. Brain and total spine MRI demonstrated new focal leptomeningeal enhancement in the left frontal lobe, the cauda equina and the distal thoracic spine (figure 1). Lumbar puncture was performed and cerebrospinal fluid (CSF) demonstrated elevated IgM and B2-microglobulin levels. Flow cytometry of CSF showed a monoclonal CD20+ and CD5-/CD10- B-cell lymphocyte population consistent with lymphoplasmacytic cell infiltration and he was diagnosed with BNS.
Figure 1.
T1 MRI brain axial imaging before (left) and 2 months following ibrutinib therapy (right). Leptomeningeal enhancement can be seen involving the left frontoparietal area prior to treatment.
Treatment
He was started on ibrutinib at 560 mg orally daily.
Outcome and follow-up
Within the first week of therapy, he had significant improvement in his weakness, gait and overall ambulation which continued to improve at subsequent follow-up. Repeat imaging performed 2 months after initiating ibrutinib showed complete resolution of his brain leptomeningeal enhancement (figure 1) and significant improvement in his spinal meningeal enhancement. He was determined to have achieved a partial response at this point in time by previously described criteria.1 Three months after starting ibrutinib, he had a prolonged hospitalisation for severe Clostridium difficile infection complicated by toxic megacolon, septic shock and pulmonary embolism. His C. difficile infection was deemed to be possibly related to ibrutinib. He underwent emergent total colectomy with end ileostomy. Ibrutinib was held while the patient was critically ill and he was started on anticoagulation with apixaban. When stable for discharge and 6–8 weeks since withholding ibrutinib, he developed progressive altered mental status and repeat brain MRI showed new leptomeningeal enhancement along the frontal, parietal and temporal lobes. Ibrutinib was restarted at a lower 420 mg daily dose with subsequent improvement in his mental status. He was discharged shortly thereafter and repeat brain MRI approximately 3 months later demonstrated complete resolution of leptomeningeal enhancement. At 9-month follow-up, he continued to tolerate ibrutinib well, and had sustained a partial response by clinical and radiographic criteria.
Discussion
The BNS is a rare neurological manifestation of WM with fewer than 150 reported cases in the literature.1 It is a heterogeneous condition and patients present with symptoms of neuraxial dysfunction including headaches, cognitive deficits, paresis, cranial nerve involvement, gait disorders and psychiatric symptoms.1 In a retrospective analysis of WM at 17 hospitals in France, only 44 patients with BNS were identified over the course of 19 years.2 The median age at diagnosis was 63 years with the majority of patients progressing to BNS after a prior diagnosis of WM (64% vs 36% of patients presenting with BNS at the time of initial diagnosis). Clinical presentations were highly varied with gait disturbances (48% of patients), cranial nerve involvement (36% of patients) and cognitive deficits (27% of patients) being the most common. Treatment included systemic chemotherapy (91%) with or without rituximab, intrathecal chemotherapy (73%) and autologous stem cell transplantation (14%). Overall treatment response was 70% after first-line treatment with a median progression-free survival of 26 months and an overall survival rate of 71% at 5 years and 59% at 10 years. A recent multicentre, multinational retrospective study demonstrated similar therapeutic heterogeneity in 34 BNS patients.3 In that study, 3-year overall survival was 59% with the most common cause of death being progression of BNS (77%). These studies highlight the lack of a standardised treatment regimen for BNS and that therapy is generally based on patient comorbidities and physician preference.
The discovery of a highly expressed single point mutation in the MYD88 gene, substituting proline for leucine at position 265, was an important advancement in understanding the pathogenesis underlying WM, present in 91% of cases.4 MYD88, an adaptor protein in the toll-like receptor signalling pathway, in concert with BTK, leads to activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) through downstream effects and promotes cell survival. This signalling is constitutively activated via the L265P mutation in MYD88.4 Ibrutinib, which impedes this process through blockade of BTK, has shown efficacy in WM with a major response rate of 73% and 2-year progression-free survival and overall survival rates of 69.1% and 95.2%, respectively,5 and is now indicated for any line of therapy for this disease.6
The potential use of ibrutinib in lymphomas affecting the CNS has gained interest in recent clinical trials. Dunleavy et al reported on the incorporation of ibrutinib into a chemotherapy regimen for treatment of primary CNS lymphoma where serum and CSF levels of ibrutinib and its active metabolite, PCI-45227, were measured.7 CSF penetration, measured as the area under curve ratio of CSF to plasma concentration and corrected for human plasma protein binding, was 16.7%–100% for ibrutinib (dose 560–700 mg) and 48%–120% for its metabolite. The concentration needed for greater than 50% BTK inhibition (IC50) in CSF was maintained for 4 hours and 8.5 hours at a dose of 560 and 700 mg, respectively. In the setting of primary CNS lymphoma, the use of ibrutinib has been associated with an increased incidence of invasive aspergillosis infections, ranging from 5% to 39% of patients in those trials.8 Cabannes-Hamy et al measured ibrutinib levels in the CSF via liquid chromatography with mass tandem spectrometry in patients with BNS, confirming that it was able to penetrate the CNS at the 420 mg daily dose of ibrutinib. Both patients in this case series had objective, sustained responses for at least months.9 This finding was further supported by Mason et al who studied the use of ibrutinib in BNS with simultaneous serum and CSF measurements of ibrutinib and its metabolite.10 They found that both ibrutinib and its active metabolite crossed the blood brain barrier with quantifiable CSF levels. Further, the ibrutinib level measured in the CSF was above the IC50 for BTK inhibition. Bernard et al administered ibrutinib to patients with mantle cell lymphoma and CNS relapse at a dose of 560 mg and measured CSF ibrutinib levels well above the IC50 for BTK inhibition.11 These studies demonstrated both objective clinical responses and therapeutic CSF drug levels supporting the rationale to use ibrutinib in the treatment of BNS.
A common clinical dilemma and concern is the use of ibrutinib in the setting of systemic anticoagulation. Retrospective studies, however, have not demonstrated a noticeable increase in risk for secondary major bleeding events in patients treated with a combination of ibrutinib and vitamin K antagonists or direct oral anticoagulants.12 The general paucity of documented major bleeding clinical endpoints with systemic anticoagulation and BTK inhibition suggests that this potential risk is small and does not outweigh the benefits in patients where dual therapy is indicated. However, anticoagulation and ibrutinib should continue to be held for any intraspinal or other major procedures.
In conclusion, we present a case that adds support to the use and efficacy of ibrutinib in the treatment of BNS. This case is unique in illustrating the quick radiographic and clinical response to ibrutinib, and the ability to rechallenge and recapture response after holding the drug for an extended period. Given that conventional therapies in BNS have significant toxicities, short duration of response and associated poor overall survival, the use of ibrutinib is an appealing therapeutic option. As randomised prospective trials are challenging in this rare disease, long-term follow-up will be needed to assess durability of responses with single-agent ibrutinib in BNS.
Learning points.
Bing-Neel syndrome (BNS) is a rare neurological complication of Waldenstrom macroglobulinaemia (WM) with no standardised treatment.
Ibrutinib, an oral Bruton’s tyrosine kinase inhibitor, has been approved for treatment of WM and has shown efficacy in the treatment of lymphomas affecting the central nervous system.
Clinicians should consider ibrutinib as a potential first-line treatment for BNS.
Footnotes
Contributors: AT was directly involved in the care of the patient. He wrote the manuscript, collected the radiographic images and implemented critical revisions. DS was directly involved in the care of the patient and helped write the manuscript. SW supervised the management of the patient and provided revisions to the manuscript. JR supervised the management of the patient, completed a portion of the literature review and provided numerous critical revisions to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the U.S. Government. This is a U.S. Government work. There are no restrictions on its use.
Competing interests: There were no financial conflicts of interests among any of the authors.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Minnema MC, Kimby E, D’Sa S, et al. Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. Haematologica 2017;102:43–51. 10.3324/haematol.2016.147728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015;94:1587–94. 10.3324/haematol.2015.133744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo JJ, D’Sa S, Lunn MP, et al. Central nervous system involvement by Waldenström macroglobulinaemia (Bing-Neel syndrome): a multi-institutional retrospective study. Br J Haematol 2016;172:709–15. 10.1111/bjh.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012;367:826–33. 10.1056/NEJMoa1200710 [DOI] [PubMed] [Google Scholar]
- 5.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med 2015;372:1430–40. 10.1056/NEJMoa1501548 [DOI] [PubMed] [Google Scholar]
- 6.Gertz MA. Waldenström macroglobulinemia: 2017 update on diagnosis, risk stratification, and management. Am J Hematol 2017;92:209–17. 10.1002/ajh.24557 [DOI] [PubMed] [Google Scholar]
- 7.Dunleavy K, Lai C, Roschewski M, et al. Phase I study of dose-adjusted-teddi-r with ibrutinib in untreated and relapsed/Refractory Primary CNS Lymphoma. Blood 2015;126:472. [Google Scholar]
- 8.Grommes C, Younes A. Ibrutinib in PCNSL: The Curious Cases of Clinical Responses and Aspergillosis. Cancer Cell 2017;31:731–3. 10.1016/j.ccell.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabannes-Hamy A, Lemal R, Goldwirt L, et al. Efficacy of ibrutinib in the treatment of Bing-Neel syndrome. Am J Hematol 2016;91:E17–E19. 10.1002/ajh.24279 [DOI] [PubMed] [Google Scholar]
- 10.Mason C, Savona S, Rini JN, et al. Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing Neel syndrome. Br J Haematol 2017;179:339–41. 10.1111/bjh.14218 [DOI] [PubMed] [Google Scholar]
- 11.Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood 2015;126:1695–8. 10.1182/blood-2015-05-647834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun S, Vincelette ND, Acharya U, et al. Risk of atrial fibrillation and bleeding diathesis associated with ibrutinib treatment: a systematic review and pooled analysis of four randomized controlled trials. Clin Lymphoma Myeloma Leuk 2017;17:31–7. 10.1016/j.clml.2016.09.010 [DOI] [PubMed] [Google Scholar]