Abstract
Objective
To conduct a comprehensive meta-analysis of MRI region-of-interest and voxel-based morphometry (VBM) studies in posttraumatic stress disorder (PTSD). As patients have high rates of comorbid depression an additional objective was to compare the findings to a meta-analysis of MRI studies in depression.
Method
The MEDLINE database was searched for studies from 1985 through 2016. A total of 113 studies met inclusion criteria and were included in an online database. Of these, 66 were selected for the region-of-interest meta-analysis and 13 for the VBM meta-analysis. The region-of-interest meta-analysis was conducted and compared with a meta-analysis of major depressive disorder. Within the region-of-interest meta-analysis, three subanalyses that included control groups with and without trauma were conducted.
Results
In the region-of-interest meta-analysis, patients with PTSD compared with all control subjects were found to have reduced brain volume, intracranial volume, and volumes of the hippocampus, insula, and anterior cingulate. PTSD patients compared with nontraumatized or traumatized control subjects showed similar changes. Traumatized compared with nontraumatized control subjects showed smaller volumes of the hippocampus bilaterally. For all regions, pooled effect sizes (Hedges’ g) varied from −0.84 to 0.43, and number of studies from three to 41. The VBM meta-analysis revealed prominent volumetric reductions in the medial prefrontal cortex, including the anterior cingulate. Compared with region-of-interest data from patients with major depressive disorder, those with PTSD had reduced total brain volume, and both disorders were associated with reduced hippocampal volume.
Conclusions
The meta-analyses revealed structural brain abnormalities associated with PTSD and trauma and suggest that global brain volume reductions distinguish PTSD from major depression.
Keywords: Posttraumatic Stress Disorder, Brain Imaging Techniques
Introduction
Posttraumatic stress disorder (PTSD) may result from various kinds of trauma, such as assault, rape, childhood maltreatment, terrorist attack, and combat-related stress. Individuals with PTSD suffer from distressing symptoms after direct experience or witnessing of traumatizing events (1).
Evidence from MRI studies suggests that PTSD is associated with abnormalities in brain structure. Meta-analyses of region-of-interest studies have primarily highlighted reductions in the volume of the hippocampus. These studies, however, examined small numbers of brain structures, and the study with the most brain regions analyzed (nine) was conducted more than 10 years ago (2). Voxel-based morphometry (VBM), a complementary approach to analyzing structural MRI data, surveys the entire brain to examine regional changes in brain volume. A small number of VBM meta-analyses of PTSD have been conducted, and they all used coordinate values, which are effective in summarizing consistently reported peaks, but, critically, they ignore nonsignificant data. Newer methods have been developed that allow the inclusion of three-dimensional statistical maps, obtained from the study authors, which include nonsignificant data and increase accuracy when added to coordinate data. This approach has not yet been applied in PTSD, however. Furthermore, region-of-interest and VBM meta-analyses analyze the MRI data in different ways, and these methods have not yet been formally compared in a single study.
PTSD is highly comorbid with depression, and epidemiological studies have reported that half or more individuals with PTSD have experienced a major depressive episode (3,4). If brain abnormalities are identified in PTSD, they could conceivably be associated with the comorbid major depressive disorder rather than the diagnosis of PTSD. Therefore, it is informative to compare brain changes in PTSD with those in major depressive disorder (hereafter referred to as depression) to try to determine whether the abnormalities may be related to a comorbid diagnosis of depression or are unique to PTSD.
In the present study, we first constructed a comprehensive online database of 113 MRI studies comparing patients with PTSD to control subjects. We conducted a region-of-interest meta-analysis examining the volumes of 56 brain regions. Within this meta-analysis, we also conducted three subanalyses that included control groups with and without trauma to tease apart the effects of experiencing a traumatic event and the diagnosis of PTSD. Comparing patients with PTSD to control subjects who have experienced trauma may reveal abnormalities associated with the diagnosis of PTSD, and comparing control subjects who have experienced trauma to control subjects with no trauma may highlight brain changes associated with trauma per se. To compare the PTSD region-of-interest meta-analysis to data from subjects with major depressive disorder, we used data from our previously published meta-analysis in depression (5) and made statistical comparisons between the disorders to determine which abnormalities are specific to PTSD. Finally, in a VBM meta-analysis, we examined regional gray matter volume from published coordinates; additional t-maps were provided by study authors to increase accuracy and to compare with the main region-of-interest meta-analysis.
Method
The study is divided into four parts: the construction of an online database of 113 studies that investigated structural brain abnormalities in PTSD, the main region-of-interest meta-analysis, a stratified meta-analysis comparing PTSD with depression, and a meta-analysis of VBM studies using seed-based d mapping. We followed the PRISMA checklist in reporting this meta-analysis (6).
Database of Imaging Studies in PTSD
Published studies that measured brain structure using MRI in patients with PTSD and a control group were included in the database. A MEDLINE search of studies published from 1992 through June 2016 was performed, combining Medical Subject Heading (MeSH) terms and free text searches. A total of 800 publications were identified, of which 113 met inclusion criteria and were included in the database. Further details, including search terms and a study inclusion flow chart (Figure S1), are provided in the online supplement.
PTSD Region-of-Interest Meta-Analysis
From all 89 region-of-interest studies in the database, more than 100 different brain regions were reported. Of these, we selected 69 regions that were reported by three or more studies to ensure that each meta-analysis was sufficiently powered. Because the inclusion of pediatric data may increase heterogeneity, these have been excluded from the meta-analysis; these data were included in the sensitivity analysis, however. Publications were excluded if they included sample overlap with other publications. The final number of studies included in the meta-analysis was 66.
Control groups
Of the 66 studies that were included in the region-of-interest meta-analysis, 28 selected nontraumatized control participants, 22 selected traumatized control participants, and 16 included both of these control groups. We used a strategy to balance the advantages of pooling the maximum number of studies with the need to examine potential differences in selecting a control group. In the main meta-analysis, we combined all 66 studies using the available case-control comparison; where both types of control groups were available, the nontraumatized control group was selected, as it was the most commonly available comparison group. However, in order to examine the effects of different control groups, three additional pairwise sub-meta-analyses were conducted: PTSD compared with traumatized control subjects, PTSD compared with nontraumatized control subjects, and traumatized compared with nontraumatized control subjects.
Combining study estimates
For continuous measures, we used Hedges’ g, which is the Cohen’s effect size with a correction for bias from small samples (7). Outcome measures were combined using a random-effects inverse-weighted variance model (8). The Cochran Q statistic was calculated to examine the heterogeneity between studies (9). The I2 statistic was also calculated, which is equal to the percentage of total variation between studies due to heterogeneity (10). The effect of small-study bias (which may include publication bias) was investigated for regions where at least five studies were included to ensure that the test was sufficiently powered. Small-study bias was assessed using Egger’s regression test. Brain regions with evidence of small-study bias were adjusted using a trim-and-fill method (11). For brain regions with a significant pooled effect size, we examined how robust the result was by excluding one individual effect size at a time, a leave-one-out approach.
Effect of clinical variables on hippocampal volume
The number of brain regions and clinical variables included in the database allows a potentially high number of correlations to be examined, which may lead to type I errors. Thus, the analysis was limited to the effect of clinical variables on total hippocampal volume. We selected this region because of the robust evidence of volumetric reduction in PTSD and because many studies have measured this structure, ensuring adequate statistical power. A random-effects meta-regression was implemented (METAREG command in Stata, version 9.2 [12]) to examine age at illness onset, time since trauma, Clinician-Administered PTSD Scale (CAPS) score, percentage of patients using antidepressants, percentage of patients who are drug free, and patient age.
Sensitivity analysis
To test how robust the results were to variations in the meta-analytic method, the effects of the following were examined: 1) setting the correlation coefficient between the left and right regional volumes as 0.1, 0.5, and 1 (see the online supplement for further details); 2) excluding studies that reported volumes divided by intracranial volume; 3) excluding cortical thickness measures; and 4) including 11 pediatric studies that were excluded in the main meta-analysis to reduce heterogeneity.
Stratified Region-of-Interest Meta-Analysis Comparing PTSD With Depression
Two meta-analytic approaches may be taken to examine differences between PTSD and depression: 1) meta-analysis of studies directly comparing the same brain structure in patients with PTSD and patients with depression and 2) indirect analysis comparing the pooled effect size from studies comparing patients with PTSD versus all control subjects with that from studies comparing patients with depression versus control subjects. We adopted the second approach, which has the advantage of including more studies and brain structures, because there are very few direct comparisons in the literature. First, from our previously published meta-analysis in major depressive disorder (5), we excluded pediatric samples and a study that included two patients with comorbid PTSD. To compare the results, we combined the effect sizes from PTSD patients versus all control subjects and depression patients versus control subjects and performed a stratified meta-analysis using a z test to compare across the two disorders. To reduce the number of comparisons, we focused on brain regions that were significantly different from those of control subjects in either the PTSD or depression meta-analysis. The PTSD versus all controls comparison was chosen to include a larger number of studies and increase power; however, because the depression meta-analysis did not include traumatized control subjects, we also performed an additional analysis comparing patients with PTSD versus nontraumatized control subjects to depression patients versus control subjects.
VBM Meta-Analysis Using Seed-Based d Mapping
Thirteen VBM studies from the database were included in the meta-analysis (the inclusion criteria and details of the analysis are provided in the online supplement). Coordinates signifying gray matter volume changes were extracted from each study, and t-maps from authors were analyzed using seed-based d mapping (13) (SDM, version 5.14; http://www.sdmproject.com). A jackknife sensitivity analysis was performed to assess the robustness of the results, which was achieved by excluding one study in each of the analyses.
Comparison between region-of-interest and VBM meta-analysis
Seed-based d mapping used in the VBM meta-analysis allows the selection of brain regions from a standard atlas for meta-analysis. The regions are then used to extract data from the voxel-wise meta-analysis, producing pooled estimates of effect size using Hedges’ g for the selected brain region. We used this functionality to make a comparison with the region-of-interest meta-analysis. The pooled effect size regions were compared if they were flagged as significant in either the region-of-interest or VBM meta-analysis.
Results
Database of Imaging Studies in PTSD
The database comprised 113 studies (see Table S1 in the online supplement) that included a total of 2,689 patients with PTSD, 2,250 nontraumatized control subjects and 1,646 traumatized control subjects. Table 1 summarizes the variables extracted from the studies. The bases for defining PTSD in the studies were DSM-IV (101 studies), DSM-III-R (four studies), the CAPS (five studies), ICD-10 (two studies), and the World Health Organization Composite International Diagnostic Interview (one study). In the studies’ MRI acquisition, 69% used a 1.5-T scanner and 29% used a higher magnetic field strength. The mean MRI slice thickness was 1.5 mm (SD=0.9).
Table 1. Demographic and Clinical Data From Study Subjects in a Database of 113 MRI Studies Comparing Patients With PTSD to Control Subjectsa.
| Variable | Pooled Number of Participants in Database | Number of Studies Reporting Variable | Mean Value or Percentage per Study | Between-Study SD |
|---|---|---|---|---|
| N | N | Mean value | SD | |
| Number of PTSD patients | 2,685 | 113 | 23.8 | 16.3 |
| Number of nontraumatized control subjects | 2,250 | 73 | 30.8 | 27.8 |
| Number of traumatized control subjects | 1,646 | 64 | 25.7 | 19.7 |
| Age (years) | ||||
| Patients, mean | 108 | 36.2 | 13.6 | |
| Patients, SD | 102 | 7.3 | 3.5 | |
| Nontraumatized control subjects, mean | 72 | 34.4 | 15.6 | |
| Nontraumatized control subjects, SD | 68 | 7.0 | 4.0 | |
| Traumatized control subjects, mean | 61 | 40.2 | 11.7 | |
| Traumatized control subjects, SD | 57 | 7.7 | 3.5 | |
| PTSD patients | ||||
| Age at illness onset (years) | 24 | 24.2 | 13.7 | |
| Time since trauma (years) | 32 | 9.0 | 11.0 | |
| Duration of illness (years) | 29 | 8.0 | 7.1 | |
| Clinician-Administered PTSD Scale score | 65 | 68.2 | 12.6 | |
| N | N | Mean % | SD | |
| Medication status | ||||
| Medication free | 1,696 | 79 | 89.0 | 23.3 |
| Antidepressant | 247 | 86 | 11.1 | 21.5 |
| Mood stabilizer | 31 | 80 | 1.3 | 5.3 |
| Antipsychotic | 18 | 81 | 0.9 | 5.7 |
| Female patients | 993 | 107 | 42.0 | 35.4 |
| Female nontraumatized control subjects | 1,044 | 71 | 45.7 | 37.3 |
| Female traumatized control subjects | 518 | 59 | 33.8 | 35.1 |
PTSD Region-of-Interest Meta-Analysis
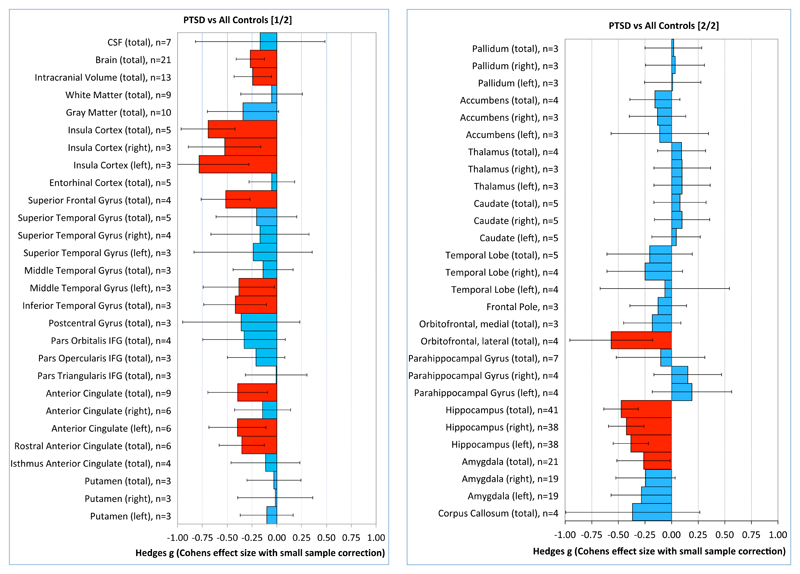
Results from the main meta-analysis comparing patients with PTSD with all control subjects are summarized in Table 2 and Figure 1. Compared with control subjects, PTSD patients had reduced brain volume, intracranial volume, and volumes of the insula (left, right, and total), superior frontal gyrus, left middle temporal gyrus, inferior temporal gyrus, anterior cingulate (left and total), rostral anterior cingulate cortex, lateral orbitofrontal cortex, and total amygdala. The left, right, and total hippocampal volumes were reduced in PTSD; however, these results were associated with significant small-sample bias. Subsequent trim-and-fill analysis for the hippocampal meta-analyses resulted in no additional imputed studies, although after the exclusion of two outlier studies (14,15) associated with large negative effect sizes (g<−2.6) for the left, right, and total hippocampus, small-sample bias was no longer significant.
Table 2. Meta-Analysis of Comparison of Patients With PTSD With All Control Subjectsa.
| Comparison of PTSD Patients and Control Subjects | Heterogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Studies (N) | PTSD Patients and Control Subjects (N/N) | Effect Size | 95% CI | p | Size Versus Controls (%) | I2 (%) | p | Small-Study Bias b Test, p | Leave-One-Out Analysisc, Effect Size With p<0.05 (%) |
| CSF (total) | 7 | 115/125 | –0.17 | –0.82, 0.49 | 0.61 | 98.1 | 82 | <0.01 | 0.89 | — |
| Brain (total) | 21 | 370/443 | –0.27 | –0.41, –0.13 | <0.01d | 97.8 | 5 | 0.39 | 0.22 | 100 |
| Intracranial volume (total) | 13 | 224/248 | –0.24 | –0.43, –0.05 | 0.01 | 97.3 | 10 | 0.34 | 0.11 | 100 |
| White matter (total) | 9 | 165/160 | –0.05 | –0.36, 0.26 | 0.74 | 99.3 | 47 | 0.06 | 0.02 | — |
| Gray matter (total) | 10 | 174/169 | –0.34 | –0.70, 0.02 | 0.06 | 97.1 | 61 | <0.01 | 0.74 | — |
| Insula cortex (total) | 5 | 140/184 | –0.69 | –0.96, –0.42 | <0.01d | 95.2 | 23 | 0.27 | 0.82 | 100 |
| Insula cortex (right) | 3 | 61/64 | –0.53 | –0.89, –0.16 | <0.01 | 95.1 | 0 | 0.73 | — | 67 |
| Insula cortex (left) | 3 | 61/64 | –0.78 | –1.29, –0.28 | <0.01 | 93.0 | 43 | 0.18 | — | 67 |
| Entorhinal cortex (total) | 5 | 142/147 | –0.05 | –0.28, 0.18 | 0.66 | 99.1 | 0 | 0.69 | 0.75 | — |
| Superior frontal gyrus (total) | 4 | 132/134 | –0.52 | –0.76, –0.27 | <0.01d | 96.1 | 0 | 0.51 | — | 100 |
| Superior temporal gyrus (total) | 5 | 119/131 | –0.20 | –0.61, 0.20 | 0.32 | 98.1 | 59 | 0.04 | 0.69 | — |
| Superior temporal gyrus (right) | 4 | 79/86 | –0.17 | –0.66, 0.32 | 0.50 | 98.3 | 60 | 0.06 | — | — |
| Superior temporal gyrus (left) | 3 | 79/86 | –0.24 | –0.83, 0.36 | 0.44 | 97.5 | 72 | 0.01 | — | — |
| Middle temporal gyrus (total) | 3 | 82/87 | –0.14 | –0.44, 0.16 | 0.37 | 99.3 | 0 | 0.75 | — | — |
| Middle temporal gyrus (left) | 3 | 65/66 | –0.38 | –0.74, –0.02 | 0.04 | 96.7 | 6 | 0.34 | — | 67 |
| Inferior temporal gyrus (total) | 3 | 80/86 | –0.42 | –0.74, –0.10 | <0.01 | 97.9 | 4 | 0.35 | — | 67 |
| Postcentral gyrus | 3 | 80/86 | –0.36 | –0.95, 0.23 | 0.23 | 97.6 | 70 | 0.04 | — | — |
| Inferior frontal gyrus, pars orbitalis | 4 | 146/184 | –0.33 | –0.75, 0.09 | 0.12 | 97.4 | 69 | 0.02 | — | — |
| inferior frontal gyrus, pars opercularis | 3 | 107/109 | –0.21 | –0.50, 0.08 | 0.16 | 98.9 | 12 | 0.32 | — | — |
| Inferior frontal gyrus, pars triangularis | 3 | 107/109 | –0.01 | –0.32, 0.30 | 0.97 | 100.1 | 22 | 0.28 | — | — |
| Anterior cingulate (total) | 9 | 227/278 | –0.39 | –0.69, -0.09 | 0.010 | 94.4 | 60 | 0.01 | 0.42 | 100 |
| Anterior cingulate (right) | 6 | 139/149 | –0.14 | –0.43, 0.14 | 0.32 | 96.8 | 27 | 0.22 | 0.48 | — |
| Anterior cingulate (left) | 6 | 139/149 | –0.40 | –0.69, -0.11 | 0.007 | 91.8 | 29 | 0.20 | 0.79 | 86 |
| Rostral anterior cingulate | 6 | 219/253 | –0.35 | –0.58, –0.13 | <0.01 | 96.0 | 31 | 0.20 | 0.60 | 100 |
| Isthmus anterior cingulate | 4 | 139/141 | –0.11 | –0.46, 0.23 | 0.52 | 98.7 | 51 | 0.11 | — | — |
| Putamen (total) | 3 | 100/123 | –0.03 | –0.30, 0.24 | 0.83 | 99.6 | 3 | 0.36 | — | — |
| Putamen (right) | 3 | 100/123 | –0.02 | –0.40, 0.36 | 0.93 | 99.9 | 38 | 0.20 | — | — |
| Putamen (left) | 3 | 100/123 | –0.10 | –0.37, 0.16 | 0.45 | 98.7 | 0 | 0.58 | — | — |
| Pallidum (total) | 3 | 100/123 | 0.02 | –0.25, 0.28 | 0.89 | 100.4 | 0 | 0.56 | — | — |
| Pallidum (right) | 3 | 100/123 | 0.03 | –0.24, 0.31 | 0.81 | 100.7 | 4 | 0.35 | — | — |
| Pallidum (left) | 3 | 100/123 | 0.01 | –0.25, 0.28 | 0.94 | 100.2 | 0 | 0.77 | — | — |
| Nucleaus accumbens (total) | 4 | 140/168 | –0.16 | –0.39, 0.08 | 0.19 | 97.5 | 5 | 0.37 | — | — |
| Nucleaus Accumbens (right) | 3 | 100/123 | –0.13 | –0.39, 0.14 | 0.34 | 97.7 | 0 | 0.78 | — | — |
| Nucleaus Accumbens (left) | 3 | 100/123 | –0.11 | –0.57, 0.35 | 0.64 | 98.0 | 55 | 0.11 | — | — |
| Thalamus (total) | 4 | 140/168 | 0.10 | –0.13, 0.32 | 0.40 | 100.8 | 0 | 0.84 | — | — |
| Thalamus (right) | 3 | 100/123 | 0.10 | –0.16, 0.37 | 0.45 | 101.0 | 0 | 0.51 | — | — |
| Thalamus (left) | 3 | 100/123 | 0.10 | –0.17, 0.36 | 0.47 | 101.0 | 0 | 0.84 | — | — |
| Caudate | 5 | 145/160 | 0.08 | –0.16, 0.33 | 0.52 | 101.5 | 9 | 0.35 | 0.76 | — |
| Caudate (right) | 5 | 145/160 | 0.10 | –0.16, 0.36 | 0.45 | 101.8 | 16 | 0.32 | 0.86 | — |
| Caudate (left) | 5 | 145/160 | 0.04 | –0.18, 0.27 | 0.71 | 100.9 | 0 | 0.48 | 0.68 | — |
| Temporal lobe (total) | 5 | 93/102 | –0.21 | –0.61, 0.20 | 0.31 | 98.0 | 47 | 0.11 | 0.33 | — |
| Temporal lobe (right) | 4 | 82/85 | –0.25 | –0.60, 0.11 | 0.17 | 97.2 | 23 | 0.27 | — | — |
| Temporal lobe (left) | 4 | 82/85 | –0.06 | –0.67, 0.54 | 0.84 | 100.1 | 73 | 0.01 | — | — |
| Frontal pole | 3 | 107/109 | –0.13 | –0.39, 0.14 | 0.36 | 99.1 | 0 | 0.76 | — | — |
| Orbitofrontal cortex (medial division) | 3 | 107/109 | –0.18 | –0.45, 0.09 | 0.19 | 99.0 | 0 | 0.48 | — | — |
| Orbitofrontal cortex (lateral division) | 4 | 146/184 | –0.57 | –0.96, –0.18 | <0.01 | 96.7 | 64 | 0.04 | — | 100 |
| Parahippocampal gyrus (total) | 7 | 207/247 | –0.10 | –0.52, 0.31 | 0.63 | 98.9 | 78 | <0.01 | 0.27 | — |
| Parahippocampal gyrus (right) | 4 | 78/80 | 0.15 | –0.17, 0.47 | 0.35 | 102.4 | 1 | 0.39 | — | — |
| Parahippocampal gyrus (left) | 4 | 78/80 | 0.19 | –0.18, 0.57 | 0.31 | 103.6 | 25 | 0.26 | — | — |
| Hippocampus (total) | 41 | 909/991 | –0.47 | –0.64, –0.31 | <0.01d | 94.4 | 62 | <0.01 | 0.01 | 100 |
| Hippocampus (right) | 38 | 799/842 | –0.42 | –0.59, –0.26 | <0.01d | 94.5 | 58 | <0.01 | <0.01 | 100 |
| Hippocampus (left) | 38 | 799/842 | –0.38 | –0.55, –0.22 | <0.01d | 94.7 | 58 | <0.01 | 0.02 | 100 |
| Amygdala (total) | 21 | 578/652 | –0.26 | –0.51, –0.01 | 0.04 | 96.3 | 75 | <0.01 | 0.62 | 57 |
| Amygdala (right) | 19 | 499/532 | –0.24 | –0.52, 0.03 | 0.09 | 96.1 | 76 | <0.01 | 0.86 | — |
| Amygdala (left) | 19 | 499/532 | –0.28 | –0.57, 0.00 | 0.051 | 95.6 | 77 | <0.01 | 0.46 | — |
| Corpus callosum (total) | 4 | 77/111 | –0.36 | –0.99, 0.27 | 0.26 | 95.2 | 70 | 0.02 | — | — |
Boldface indicates significant differences.
Small-study bias reported for studies with Ns >4.
Leave-one-out analysis examines whether the pooled effect size becomes nonsignificant when removing one effect size at a time. A value of 100%, which is the most robust result, indicates that the pooled effect size remains significant when 100% of effect sizes are removed in turn.
Result remained significant after Bonferroni correction for multiple comparisons of 56 brain structures.
Figure 1. Meta-Analysis of Continuous Data Comparing Patients With Posttraumatic Stress Disorder and Control Subjectsa.
a Hedges’ g (Cohen effect size with small-sample correction) is shown for each structure, with 95% confidence intervals. The effect size is positive when the structure is larger in patients with PTSD compared with control subjects and negative when the structure is smaller in PTSD patients. The number of studies included in each meta-analysis is indicated for each structure.
Effect of clinical variables on hippocampal volume
Because outliers may have a disproportionate effect on meta-regression analysis, the two outliers (14,15) were removed before we investigated the effect of clinical variables on total hippocampal volume (effect sizes of outliers, −3.0 and −3.2; effect size range of the remaining 39 studies, −1.4 to 0.4; pooled effect size after two studies were excluded, −0.44, p<0.001). There was no significant effect of the following clinical variables on the difference in total hippocampal volume between patients and control subjects: CAPS score (25 studies, p=0.16), age at illness onset (nine studies, p=0.45), time since trauma (13 studies, p=0.45), percentage of patients using antidepressants (27 studies, p=0.099), percentage of patients drug free (24 studies, p=0.11), and patient age (38 studies, p=0.52).
Pairwise sub-meta-analyses
PTSD versus nontraumatized control group:
Patients with PTSD compared with nontraumatized control subjects had smaller total brain volume and volumes of gray matter, total, left, and right insula, total parahippocampal gyrus, and total, left, and right hippocampus (see Table S2 and Figure S2 in the online supplement)
PTSD versus traumatized control group:
Compared with the traumatized control group, patients with PTSD had smaller total brain volume, intracranial volume, superior frontal gyrus, total insula volume, anterior cingulate, and volumes of the lateral orbitofrontal cortex and total and right hippocampus. Contrary to the general direction of results, right and left parahippocampal gyri were significantly larger in the PTSD group compared with the traumatized control group (see Table S3 and Figure S3 in the online supplement).
Traumatized control group versus nontraumatized control group:
Compared with the nontraumatized control subjects, the traumatized control group showed significant reductions in total, left, and right hippocampal volumes (see Table S4 and Figure S4 in the online supplement)
Sensitivity Analysis
When the correlation coefficient between left and right regions was changed from 0.8 to 0, 0.5, or 1.0, there was no change in any of the results. When three studies that divided brain volumes by intracranial volume were excluded, there was no change in any of the results. When the cortical thickness measures were excluded, the total parahippocampal gyrus volume decrease observed in the PTSD versus nontraumatized controls comparison was no longer significant and the reduction of the lateral orbitofrontal cortex volume in the PTSD versus traumatized controls comparison was no longer significant. When 11 pediatric studies were included in the meta-analysis, there were a number of new results, which are detailed in the online supplement.
Comparison of PTSD region-of-interest meta-analysis with depression
Ten brain structures in both the present PTSD versus all controls region-of-interest meta-analysis and the previously published depression versus controls region-of-interest meta-analysis (5) showed significant differences compared with control subjects (Table 3) Two of these regions significantly differed between PTSD and depression. Compared with depressed patients and control subjects, PTSD patients had significantly reduced total brain volume. Compared with PTSD patients and control subjects, depressed patients had significantly reduced volume of the thalamus. Both PTSD and depression patients had significantly smaller hippocampal volume compared with control subjects, with no difference between the patient groups in this brain region. In an analysis using the smaller PTSD versus nontraumatized controls contrast, PTSD patients had reduced total brain volume compared with depression patients, although this difference fell short of significance (p=0.07) (see Table S8 in the online supplement).
Table 3. Statistical Comparison of the Present PTSD Meta-Analysis With a Previous Meta-Analysis of Major Depressive Disordera.
| PTSD Patients Versus Control Subjects | Depression Patients Versus Control Subjects | PTSD Versus Depression | ||||||
|---|---|---|---|---|---|---|---|---|
| Region | Studies (N) | Effect Size | p | Studies (N) | Effect Size | p | Effect Size | p |
| CSF | 7 | –0.17 | 0.61 | 7 | 0.53 | 0.001 | –0.70 | 0.058 |
| Brain | 21 | –0.27 | <0.001 | 25 | –0.05 | 0.27 | –0.21 | 0.016 |
| Intracranial volume | 13 | –0.24 | 0.012 | 20 | –0.12 | 0.056 | –0.13 | 0.26 |
| Gray matter | 10 | –0.34 | 0.063 | 7 | –0.13 | 0.19 | –0.21 | 0.30 |
| Anterior Cingulate | 9 | -0.39 | 0.010 | 6 | -0.21 | 0.22 | -0.19 | 0.41 |
| Putamen | 3 | –0.03 | 0.83 | 8 | –0.25 | 0.009 | 0.22 | 0.20 |
| Caudate | 5 | 0.08 | 0.52 | 12 | –0.20 | 0.017 | 0.28 | 0.062 |
| Thalamus | 4 | 0.10 | 0.40 | 7 | –0.34 | 0.012 | 0.43 | 0.014 |
| Hippocampus | 41 | –0.47 | <0.001 | 32 | –0.48 | <0.001 | 0.01 | 0.92 |
| Amygdala | 21 | –0.26 | 0.04 | 17 | –0.02 | 0.89 | –0.24 | 0.18 |
For the comparison between PTSD patients and depression patients, boldface indicates significant differences. In the second to last column negative effect sizes indicate that the region is smaller in PTSD patients, and positive effect sizes indicate that the region is smaller in depression patients. Further details regarding the depression meta-analysis can be found in reference 5.
VBM meta-analysis using seed-based d-mapping
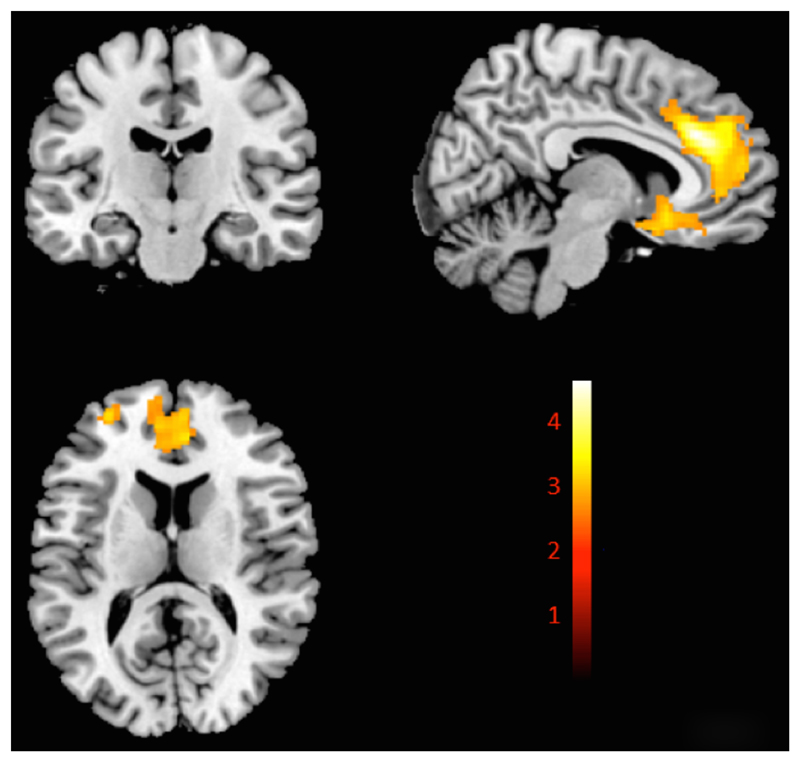
The VBM main meta-analysis (Figure 2; see also Table S9 in the online supplement) revealed that PTSD patients compared with all control subjects exhibited significant gray matter volume reductions in a large cluster in the medial prefrontal cortex encompassing the left and right anterior cingulate and extending to the subgenual prefrontal cortex. Reductions in volume were also observed in the left superior frontal gyrus and other smaller clusters, including the amygdala bilaterally, extending to the head of the hippocampi. The jackknife sensitivity analysis indicated that the large clusters were robust (see Figure S8 in the online supplement) The meta-analysis was repeated for PTSD versus nontraumatized control subjects (see Table S10 and Figures S9 and S10 in the online supplement), PTSD versus traumatized control subjects (see Table S11 and Figures S11 and S12 in the online supplement), and PTSD versus all control subjects, including pediatric data (see Table S12 and Figures S13 and S14 in the online supplement).
Figure 2. Seed-Based d Mapping (SDM) Meta-Analysis of 13 Voxel-Based Morphometry Studies Showing Significant Decreased Gray Matter Volumes in the PTSD Group Compared With All Control Subjectsa.
a Color bar indicates SDM z scores. Peak change coordinates, 6, 32, 28. SDM z score=4.5. Detailed coordinates are listed in Table S8 in the online supplement. The t-map of this image is available at www.ptsdmri.uk.
Comparison between region-of-interest and VBM meta-analysis of PTSD
To compare the region-of-interest and VBM meta-analyses, regions were included if they were flagged as significant in either the region-of-interest (Table 2) or the VBM meta-analysis (Figure 2) and the brain region was available using the SDM extract tool, which gives pooled effect sizes for a region. A total of eight bilateral regions were compared (see Table S13 in the online supplement). The region-of-interest meta-analysis reported volume reductions in the PTSD group in the insula bilaterally, the left anterior cingulate, and the hippocampus bilaterally, but not the right anterior cingulate or amygdala. Conversely, the VBM meta-analysis reported reductions in the anterior cingulate and amygdala bilaterally but not the insula or hippocampus.
The database, forest plots for each meta-analysis, and three-dimensional image files from the SDM analysis are available at http://www.ptsdmri.uk (see Figure S15 in the online supplement).
Discussion
This study is, to our knowledge, the most comprehensive meta-analysis of MRI studies in PTSD to date, in terms of both number of brain regions and number of included studies. New techniques used in this study include use of t-maps in the VBM analysis, a direct comparison between VBM and region-of-interest techniques, a statistical comparison between PTSD and depression, and a freely available online database and meta-analysis. In the main region-of-interest meta-analysis, PTSD patients compared with all control subjects had volumetric reductions of the brain, intracranial volume, insula, superior frontal gyrus, temporal gyri, anterior cingulate, rostral anterior cingulate, hippocampus, and amygdala. PTSD patients compared with nontraumatized control subjects showed a similar pattern of reduced volumes, including total brain, insula, and hippocampus. PTSD patients compared with traumatized control subjects showed a similar pattern of volumetric reductions, including total brain, intracranial volume, insula, anterior cingulate and hippocampus. This subanalysis showed a volumetric increase of the parahippocampal gyri bilaterally in PTSD, a finding related to higher early trauma scores (16). Traumatized control subjects compared with nontraumatized control subjects showed only smaller volumes of the hippocampus. When compared with a meta-analysis of patients with depression, the distinguishing feature of PTSD was a reduction in total brain volume. We found a different pattern of regions highlighted in the region-of-interest analysis compared with the VBM meta-analysis.
Hippocampus
From the region-of-interest meta-analysis, the reductions in hippocampal volume in PTSD patients versus traumatized control subjects and PTSD patients versus nontraumatized control subjects was in agreement with previous MRI meta-analytic studies (2,17–20) This finding is also consistent with previous neurocognitive studies (21,22) showing poorer memory performance in PTSD patients. Hippocampal volume reduction may be a generalized marker of mental health disorders, as it has been associated with chronic hypercortisolemia (23,24), which is related to chronic stress (25). Our finding of no difference in hippocampal volume in PTSD compared with depression supports this idea, as hypercortisolemia frequently occurs in patients with depression (23,26). Further evidence for a generalized marker comes from MRI meta-analyses reporting reduced hippocampal volume in bipolar disorder (27) and in schizophrenia (28). Reduced hippocampal volume in traumatized versus nontraumatized control subjects indicates that exposure to a traumatic event itself, even if the exposed person does not develop PTSD, may be associated with volume reduction.
Intracranial and Brain Volume
Intracranial volume was reduced in PTSD patients in the main region-of-interest analysis as well as in PTSD patients compared with traumatized control subjects. Total intracranial volume stabilizes in early adolescence (ages 11 to 14) and provides an estimate of premorbid brain volume (29). Thus, smaller intracranial volume in PTSD may indicate abnormal brain development before or during early adolescence. Consequently, reduced intracranial volume may be a risk factor for PTSD and may be associated with trauma susceptibility. Total brain volume was also significantly smaller in PTSD patients compared with all control subjects, nontraumatized control subjects, and traumatized control subjects but not in the comparison between traumatized and nontraumatized control subjects. These results suggest that brain volume reductions in PTSD patients are related to the disorder itself rather than the exposure to trauma. In addition, our comparison with depression suggests that total brain volume reduction is a more specific marker for PTSD than is hippocampal volume.
Insula and Anterior Cingulate
In the region-of-interest meta-analysis, insula volume was smaller in PTSD patients compared with both nontraumatized and traumatized control subjects, suggesting that insula reduction may underlie the pathophysiology of PTSD (30)_. Reductions in anterior cingulate volume were found in the region-of-interest and VBM meta-analysis. A number of functional MRI studies have reported abnormalities of the anterior cingulate in PTSD (31–33), including a study of monozygotic twins (34) that suggested that hyperresponsiveness of the dorsal anterior cingulate is a familial risk factor for PTSD.
Comparison of Region-of-Interest Meta-Analysis and VBM Meta-Analysis
We found relatively poor agreement when comparing the VBM and region-of-interest meta-analyses, and this has implications for neuroimaging meta-analyses of other disorders. We have considered a number of reasons for these differences. First, VBM involves smoothing, which is known to bias sensitivity to brain regions the size of the smoothing kernel (≈10 mm). Second, region-of-interest studies typically report on a subset of brain regions and are likely to suffer from publication bias. Third, where seed-based d mapping uses coordinate data, the effect size is biased toward zero in brain regions where there are no significant clusters. Lastly, VBM analyses typically adjust for global brain volumes, whereas region-of-interest studies use absolute volumes.
Limitations
The literature search included MEDLINE and manual searches, as previous experience (5) has indicated that MEDLINE has high coverage of MRI studies in clinical populations, and the present study included more publications than previous meta-analyses. The use of one bibliographic database is a limitation, however. Significant heterogeneity was detected for many of the brain structures in the PTSD group (see Table 2), which we have also observed in a number of other MRI meta-analyses (5, 35–36). This is likely to be caused by variations in patient characteristics and MRI data, and a random-effects model was utilized to account for such heterogeneity. Publication bias was detected for total hippocampal volume; we attempted to remedy this using a trim-and-fill method, although no new studies were imputed. Although the present meta-analysis summarizes small heterogeneous samples, large-scale studies using standardized MRI acquisition protocols and epidemiological neuroimaging samples, such as UK Biobank, are likely to tease apart the different structural abnormalities associated with risk for PTSD, experience of trauma, and development of PTSD. We compared PTSD and depression because they are highly comorbid; however, PTSD co-occurs with other anxiety disorders and substance use disorders (37) and these comorbidities could be associated with some of our findings.
Supplementary Material
References
- 1.Shiromani P, Keane T, LeDoux JE. Post-Traumatic Stress Disorder: Basic Science and Clinical Practice. New York: Humana Press; 2009. [Google Scholar]
- 2.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Başoğlu M, Kiliç C, Salcioğlu E, et al. Prevalence of posttraumatic stress disorder and comorbid depression in earthquake survivors in Turkey: an epidemiological study. J Trauma Stress. 2004;17:133–141. doi: 10.1023/B:JOTS.0000022619.31615.e8. [DOI] [PubMed] [Google Scholar]
- 5.Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder: meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 7.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Orlando, Fla: Academic Press; 1985. [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead A. Meta-Analysis of Controlled Clinical Trials. Chichester, UK: Wiley; 2002. [Google Scholar]
- 10.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Duval S, Tweedie RA. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 12.Sharp S. Meta-analysis regression. Stata Tech Bull. 1998;7:16–22. ( http://econpapers.repec.org/article/tsjstbull/y_3a1998_3av_3a7_3ai_3a42_3asbe23.htm) [Google Scholar]
- 13.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges DW, Allen S, Tate DF, et al. Reduced hippocampal volume in alcohol and substance naïve Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16:219–224. doi: 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Baldaçara L, Zugman A, Araújo C, et al. Reduction of anterior cingulate in adults with urban violence–related PTSD. J Affect Disord. 2014;168:13–20. doi: 10.1016/j.jad.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 19.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 20.O’Doherty DCM, Chitty KM, Saddiqui S, et al. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141:105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stricker NH, Lippa SM, Green DL, et al. Elevated rates of memory impairment in military service-members and veterans with posttraumatic stress disorder. J Clin Exp Neuropsychol. 2017;39:768–785. doi: 10.1080/13803395.2016.1264575. [DOI] [PubMed] [Google Scholar]
- 23.Axelson DA, Doraiswamy PM, McDonald WM, et al. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- 24.Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 26.Mello AF, Juruena MF, Pariante CM, et al. [Depression and stress: is there an endophenotype?] Rev Bras Psiquiatr. 2007;29(suppl 1):S13–S18. doi: 10.1590/s1516-44462007000500004. (Portuguese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hibar DP, Westlye LT, van Erp TGM, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21:1710–1716. doi: 10.1038/mp.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haijma SV, Van Haren N, Cahn W, et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedges DW, Woon FLM. Premorbid brain volume estimates and reduced total brain volume in adults exposed to trauma with or without posttraumatic stress disorder: a meta-analysis. Cogn Behav Neurol. 2010;23:124–129. doi: 10.1097/WNN.0b013e3181e1cbe1. [DOI] [PubMed] [Google Scholar]
- 30.Herringa R, Phillips M, Almeida J, et al. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res. 2012;203:139–145. doi: 10.1016/j.pscychresns.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MJ, Chey J, Chung A, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res. 2008;42:268–277. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 34.Shin LM, Bush G, Milad MR, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry. 2011;168:979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempton MJ, Geddes JR, Ettinger U, et al. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 36.Kempton MJ, Stahl D, Williams SCR, et al. Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophr Res. 2010;120:54–62. doi: 10.1016/j.schres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 37.Brady KT, Killeen TK, Brewerton T, et al. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61(suppl 7):22–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.