Abstract
Importance:
Elucidation of optimal dosing and treatment content is critical for health care providers, payers, and policy makers, as well as mechanisms of change to inform intervention delivery and training initiatives for childhood obesity.
Objectives:
To evaluate effects, following a 4-month family-based behavioral weight loss treatment (FBT), of 2 doses (HIGH or LOW) of a weight-control intervention (enhanced social facilitation maintenance [SFM+]) vs a weight-control education condition (CONTROL; matched for dose with LOW), on child anthropometrics, and to explore putative mediators of weight loss outcomes.
Design, Setting, and Participants:
For this parallel-group randomized clinical trial conducted at 2 US academic medical centers from December 2009 to March 2013, 172 parent-child dyads completed FBT and were then randomized to 8 months of SFM+ (HIGH, n = 59; LOW, n = 56) or CONTROL (n = 57). Children (aged 7–11 years) with overweight and obesity (body mass index [BMI; calculated asweight in kilograms divided by height in meters squared] ≥ 85th percentile) with at least 1 parent with overweight and obesity (BMI ≥ 25) were recruited.
Interventions:
HIGH SFM+ vs LOW SFM+ (CONTROL matched the dose of LOW).
Main Outcome Measures:
Intention-to-treat analysis using mixed-effects models estimated change in child percentage overweight (percentage above the median BMI for a child’s age and sex) for the FBT period (0–4 months) and the SFM+ period (4–12 months), and proportion of children achieving a clinically significant change in percentage overweight (≥9-unit decrease; months 0–12). Theory-based outcome mediators were also evaluated.
Results:
This study recruited 172 parent-child dyads (mean [SD] age: parents 42.3 [6.4] years; children, 9.4 [1.3] years). The omnibus treatment × time interaction for child percentage overweight was significant (F8, 618.9= 2.89; P = .004). Planned pairwise comparisons revealed that from months 4 to 12, LOW had better outcomes than CONTROL (difference, −3.34; 95% CI, −6.21 to −0.47; d = −0.40; P = .02). HIGH had better outcomes than LOW (difference, −3.37; 95% CI, −6.15 to −0.59; d = −0.38; P = .02) and CONTROL (difference, −6.71; 95% CI, −9.57 to −3.84; d = −0.77; P < .001). A greater proportion of children in HIGH (45 [82%]) vs LOW (34 [64%]) (difference, 18.00; 95%CI, 1.00–34.00; P = .03; number needed to treat = 5.56) and CONTROL (25 [48%]) (difference, 34.00; 95%CI, 16.00–51.00; P < .001; number needed to treat = 2.94) had clinically significant percentage overweight reductions. Food and activity monitoring and goal setting mediated the effect of LOW vs CONTROL (50%). Monitoring and goal setting, family and home environment, and healthy behaviors with peers mediated the effect of HIGH vs CONTROL (25%−42%).
Conclusions and Relevance:
Following FBT, specialized intervention content (SFM+) enhanced children’s weight outcomes and outperformed a credible control condition, with high dose delivery yielding the best outcomes. Sustained monitoring and goal setting, support from the family and home environment, and healthy peer interactions explained outcome differences, highlighting key treatment targets.
INTRODUCTION
Childhood overweight and obesity affect nearly one-third of children in the United States and are associated with severe medical and psychosocial comorbidities that track into adulthood, resulting in high health care costs.1,2The US Preventive Services Task Force (USPSTF) reviews of childhood weight loss interventions have concluded that comprehensive behavioral interventions can improve weight status for up to 12 months.3–5 A dose-response pattern has been consistently documented3,4,6–8 (eg, in the 2017 report, having ≥26 contact hours was associated with greater weight loss), providing support that greater intervention intensity is associated with improved weight outcomes.4Randomized clinical trials (RCTs) are needed to empirically test dose-response effects of behavioral interventions on weight outcomes, as well as to investigate the effects of treatment content. Also, despite the advances in treatment, little is known about the operative mechanisms and mediators of change that can inform intervention delivery and training initiatives.6–8
We previously conducted a pioneering study9 to examine specialized treatment content to improve weight outcomes in children with overweight and obesity and their families. Parent-child dyads consisting of 1 child and parent received family-based behavioral weight loss treatment (FBT) and then were randomized to 1 of the following: (1) behavioral skills maintenance treatment (BSM), a cognitive-behavioral approach that builds on standard behavioral weight loss skills to improve self-regulation and promote relapse prevention; (2) social facilitation maintenance treatment (SFM), a socioecological approach that emphasizes parental facilitation of children’s peer networks and improvement of children’s body image, as well as their responses to teasing, to facilitate maintenance of behaviors consistent with improved weight outcomes; or (3) a no-continued-treatment control condition.9 Social facilitation maintenance, but not BSM, significantly improved children’s long-term weight loss outcomes, as did enlistment of peer support and abilities to cope with teasing compared with control.10–12 Simulation results indicated that if the self-regulatory skills in BSM (eg, ongoing weight, diet, and activity monitoring) were incorporated into SFM [SFM+], weight outcomes could be further improved.10
In addition to treatment content designed to improve weight outcomes, more frequent sessions (ie, higher treatment dose) may also improve outcomes by allowing more time to practice the retrieval of new skills across contexts (eg, home, peer, school, and community).6,13–15 Contextual learning theory posits that promoting generalization of behaviors across settings encourages mastery of the newly learned behaviors into habits (eg, healthy energy balance behaviors) and decreases relapse.16,17 Accordingly, we sought to empirically test whether SFM+ content and dose enhance weight loss outcomes.
The current trial compared 2 doses of SFM+ (HIGH [32 weekly sessions] and LOW [16 every-other-week sessions]) to a credible weight management education program (CONTROL [16 every-other-week sessions]) following FBT. We hypothesized that both doses of SFM+ would result in better child weight loss outcomes than CONTROL, with the higher SFM+ dose having greater effects than the lower SFM+ dose. We also hypothesized that greater adherence to SFM+ specific strategies for generalizing healthy behaviors and social support across contexts would mediate better weight outcomes.
METHODS
Patients
Children (aged 7–11 years) with overweight or obesity (body mass index [BMI; calculated as weight in kilograms divided by height in meters squared] ≥85th percentile for age and sex) and at least 1 parent with overweight or obesity (BMI ≥25) were recruited through media, advertisements, and physician referrals. At least 1 parent or guardian participated with the child at 1 of 2 US university-based clinics (St Louis, Missouri, or Seattle, Washington). Exclusion criteria included child or parent participation in other weight loss treatment, use of weight-affecting medications, or psychiatric and medical conditions that would hinder participation. Parents and children provided written informed consent and assent, respectively. Each site’s institutional review board approved the study. The trial protocol is available in Supplement 1.
Procedures
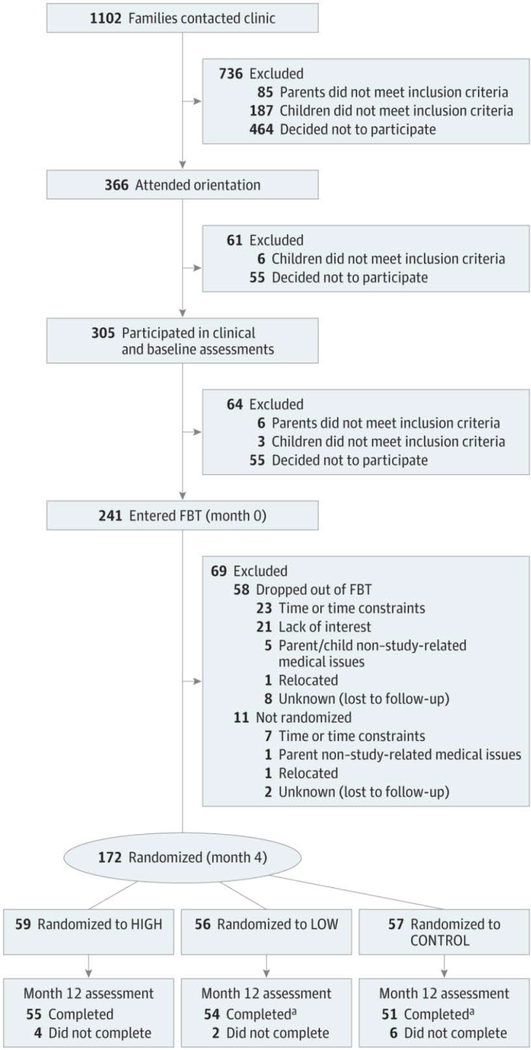
Figure 1 presents participant recruitment, participation, and retention. We used a parallel-group RCT design conducted from December 2009 to March 2013. Parent-child dyads (n = 241) entered FBT (months 0–4). Following FBT, random allocation was conducted using computer-generated random numbers by an independent data coordinating center. Within each clinic site, a dynamic randomization scheme18 was applied to child age, sex, race/ethnicity (non-Hispanic white vs other), change in percentage overweight during FBT (dichotomized by group median [10.44]), and child baseline social problems as measured by the Child Behavior Checklist (dichotomized by group median [t-score of 53]). The dyads then participated in SFM+ or CONTROL (months 4–12). Relative weight outcomes were assessed at months 0, 2, 4, 8, and 12. Additional assessments to identify behavioral changes that mediated changes in relative weight were conducted at months 6, 8, and 10. Although originally planned as a 24-month trial, only outcomes up to 12 months are reported herein due to differential implementation of continued contact during months 12 through 24 across sites and cohorts..
Figure 1.
Study Participant Flow
CONTROL indicates weight management education condition; FBT, family-based behavioral weight loss treatment; HIGH, enhanced social facilitation maintenance (32 sessions of enhanced social facilitation maintenance); and LOW, enhanced social facilitation maintenance (16 sessions of enhanced social facilitation maintenance).
aFor 1 assessment, only child data were collected.
Family-based Behavioral Weight-Loss Treatment
All families received standard FBT, delivered in 16 weekly, 30-minute family sessions and 45-minute separate parent and child groups. Family-based behavioral weight loss treatment targeted behavior change for children and parents using standard techniques for dietary and activity modification (eg, reinforcement, stimulus control, preplanning, and relapse prevention).9,19 The Traffic Light Plan19 provided an easy system for children and families to identify healthier vs less healthy food and activity options. Parent-child dyads were weighed at each session.
SFM+ Interventions and Control Condition
Overview.
After FBT, parent-child dyads were randomized to HIGH, LOW, or CONTROL. LOW and HIGH had similar content but LOW was 16 every-other-week sessions and HIGH was 32 weekly sessions. All information, handouts, and materials in HIGH were also provided to families in LOW, but HIGH received additional opportunities to engage in and practice skills through more intervention contact. HIGH and LOW content was delivered in 30-minute family sessions plus 45-minute separate child and parent group sessions. The focus was on helping families establish a social and physical environment across all contexts of their lives to promote healthy behaviors and weight-control success.9,20 LOW matched the dose (16 every-other-week sessions) and attention (75 minutes/session) of CONTROL, but CONTROL was delivered in group-only sessions. Parent-child dyads in LOW and HIGH were weighed each session; consistent with the education-only focus of the CONTROL condition, staff did not weigh CONTROL children.
SFM+ Intervention.
Enhanced social facilitation maintenance (HIGH and LOW) was a multicomponent intervention designed to optimize the durability and generalizability of eating and physical activity changes from FBT via practice across multiple social and environmental contexts (eg, within the home, school, work, restaurants, with friends). In addition, SFM+ bolstered skills introduced in FBT to manage negative peer interactions (eg, teasing) that hinder healthy behaviors21–25 and focused on building supportive family and peer environments conducive to healthy weight-control behaviors (eFigure 1 in Supplement 2).
CONTROL Condition.
CONTROL was a weight management education intervention. Families received novel information on nutrition and exercise beyond what FBT provided, (eg, details about benefits of fiber). CONTROL families participated in hands-on activities, including cooking, stretching, and grocery store tours. Use of self-regulatory skills was not discussed or encouraged; if families requested skills instruction, they were referred to their FBT materials.
Interventionist Training and Supervision
All interventionists had earned bachelor’s degrees or higher and were trained prior to delivering family and group sessions. Families were randomly assigned to interventionists during both FBT and SFM+; a family never received FBT and SFM+ family sessions from the same interventionist. Per-session preparation for all interventions consisted of protocol meetings to review materials and problem solve implementation challenges. Additionally, FBT and SFM+ interventionists participated in 2-day trainings prior to respective treatment initiation and received weekly supervision and monthly cross-site supervision calls.
Measures
Baseline demographic variables included child and parent race, ethnicity, age, and sex; child’s BMI percentile category26; and parents’ BMI, occupation, and education (Table 1), with parent occupation and education used in the Barratt Simplified Measure of Social Status27 to approximate family socioeconomic status (SES; higher values = higher SES). Children’s weight and height were assessed at months 0, 4, 8, and 12.
Table 1.
Participant Demographic Characteristics at Baseline and Relative Weight at Baseline and Randomization
| Mean (SD) |
||||
|---|---|---|---|---|
| Variable | All Groups (n = 172)a | HIGH (n = 59)a | LOW (n = 56)a | CONTROL (n = 57)a |
| Child age, y | 9.4 (1.3) | 9.5 (1.3) | 9.4 (1.2) | 9.5 (1.3) |
| Parent age, y | 42.3 (6.4) | 42.3 (6.7) | 42.1 (6.5) | 42.5 (6.0) |
| Child race/ethnicity, % | ||||
| White non-Hispanlc | 63.4 | 61.0 | 64.3 | 64.9 |
| White Hispanic | 7.6 | 5.1 | 7.1 | 10.5 |
| African American | 22.1 | 23.7 | 23.2 | 19.3 |
| Other or multiple races | 7.0 | 10.2 | 5.4 | 5.3 |
| Child sex (female, %) | 61.6 | 62.7 | 64.3 | 57.9 |
| Family SESb | 44.0 (10.2) | 42.2 (10.5) | 44.4 (10.3) | 45.4 (9.8) |
| Parent BMI (baseline, month 0) | 37.9 (9.4) | 38.5 (10.2) | 39.3 (10.4) | 36.0 (7.0) |
| Child BMI percentile category (baseline, month 0) | ||||
| Overweight (85.0th-94.9th percentile), % | 9.9 | 11.9 | 8.9 | 8.8 |
| Obesity (100%−120% of the 95th percentile), % | 40.1 | 30.5 | 46.4 | 43.9 |
| Severe obesity (≥120% of the 95th percentile), % | 50.0 | 57.6 | 44.6 | 47.4 |
| Child percentage overweight | ||||
| Baseline (month 0) | 64.2 (25.2) | 67.0 (25.8) | 64.0 (26.2) | 61.3 (23.7) |
| Randomization (month 4) | 50.8 (26.1) | 54.1 (26.8) | 50.8 (25.7) | 47.3 (25.7) |
| Change over FBT (months 0–4) | -13.4 (8.1) | -12.9 (7.7) | -13.2 (9.2) | -14.0 (7.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CONTROL, weight management education condition; FBT, family-based behavioral weight loss treatment; HIGH, 32 sessions of enhanced social facilitation maintenance; SES, socioeconomic status.
The sample sizes reflect those at randomization.
Barratt Simplified Measure of Social Status, parent report: scores could range from 8 to 66, with higher numbers indicating higher SES
Anthropometrics were calculated from weight (calibrated electronic scale to nearest 0.1 kg in light clothing without shoes) and height (via stadiometer to the nearest 0.1 cm) measurements following detailed protocols. Using Centers for Disease Control and Prevention norms from 2000,26 percentage overweight (percentage that the child’s BMI was above median for age and sex) was computed as the primary outcome measure given its sensitivity to change throughout the BMI range.28 This sensitivity is particularly salient in our study, as 50% of children had severe obesity (ie, BMI ≥120% of the 95th percentile). Although not possible to keep assessors blind to condition, use of a standard protocol for obtaining participants’ height and weight (eg, standardized instruction to participants, calculating the mean of multiple measurements) ensured objective and reliable measurement of the primary outcome. Assessors were blinded to prior heights and weights, further protecting against assessment bias.
Based on a meta-analysis7 indicating that BMI decreases of 1.3 are associated with improvements in cardiovascular risk factors, we created a prediction equation to convert change in BMI to change in percentage overweight with data collected during FBT. The prediction equation was highly accurate (R2 = .995) and showed that a BMI reduction of 1.3 was equivalent to a reduction of 9.2 percentage overweight units. Therefore, as a secondary outcome, we selected a decrease of 9 units or more in percentage overweight from baseline as an a priori cut point for determining clinically significant reductions in body weight. Body mass index z score (via the lambda-mu-sigma [LMS] method)26 is correlated with percentage overweight and often reported in childhood obesity trials, so it was included for comparison purposes (eg, for future meta-analyses).29
Mediator variables were assessed throughout SFM+ and CONTROL (months 6, 8, and 10). Child behaviors were assessed by self-report and parent report to evaluate mechanisms of change specific to SFM+ (eTable 1 in Supplement 2). Knowledge of information presented in CONTROL but not SFM+ was also assessed, although it was not expected to serve as a mediator because CONTROL was anticipated to yield better specific knowledge scores but not better outcomes than LOW or HIGH.
Statistical Power
Sample size calculations were based on an ability to detect a significant condition (HIGH vs LOW vs CONTROL) by time (randomization at month 4, month 8, and 12-month follow-up) interaction within the mixed-effects model with statistical power of 95% and α error rate of .05. Assuming a standardized difference in child percentage overweight at 12-month follow-up between HIGH and CONTROL of 0.67, a standardized difference between LOW and CONTROL of 0.25, and a standardized difference between HIGH and LOW of 0.42 (which were based on the anticipated weight change trajectory differences from 4 to 12 months across groups: difference of −8 in percentage overweight for HIGH vs CONTROL; −3 for LOW vs CONTROL; and −5 for HIGH vs LOW), and a correlation in percentage overweight between adjacent time points of 0.85, a total sample size of 148 participants (approximately 49/group) was required. Based on our previous trial,9 we anticipated an attrition rate of approximately 37% from starting FBT to the 12-month assessment. Thus, we needed to enroll 240 dyads to retain 165 participants (approximately 55/group) to have sufficient power to detect expected differences at the 12-month assessment.
Statistical Analyses
The primary analyses were intention-to-treat, defined as using all available data from randomized participants (n = 172 dyads), and were performed using linear mixed-effects (LME) models. Treatment effects were modeled by the interaction of the fixed effects of condition (HIGH, LOW, CONTROL) and time (month 0, 2, 4, 8, and 12). Time was treated as a categorical variable, which allowed for the inclusion of predefined contrasts to test whether 1 group differed from another in amount of child relative weight change from months 4 to 12 and from months 0 to 12. Correlations in relative weight over time were modeled using an autoregressive covariance structure. Site and site×time effects on relative weight were modeled as random effects to generalize treatment effects beyond the 2 clinical sites. Missing data in the primary outcome were observed to be missing completely at random (Little’s missing completely at random test χ214 = 21.23; P = .10). The LME models used maximum likelihood estimation, thus using data from all randomized dyads regardless of missing follow-up data. In sensitivity analyses, models were restricted to the 160 dyads (93% of randomized sample) who completed month 12 assessments to determine whether results were similar for a “completers” approach.
To assess clinical effects, generalized LME models with a logit link determined whether conditions differed in the proportion of children who obtained greater than or equal to 9-unit reductions in percentage overweight from months 0 to 12. Number needed to treat (NNT) was calculated to provide clinically meaningful effect sizes, indicating the number of participants who must receive HIGH to yield 1 more success (ie, 1 more child to achieve ≥9-unit reduction in percentage overweight) with this reduction compared with LOW or CONTROL; the same was calculated for LOW vs CONTROL.30
Potential mediators were identified using LME models in which the mediator variable was entered as the repeatedly measured outcome variable. For these LME models, continuous time (months 4, 6, 8, 10, and 12) was log-transformed to model decelerating changes over SFM+ (HIGH or LOW) or CONTROL, which provided superior model fit compared with a linear time variable (eTable 1 in Supplement 2). For LME models with a significant condition × time interaction (P < .05), formal mediation was evaluated using the SPSS PROCESS macro (IBM Corp) (eFigure 2 in Supplement 2).31 In these mediation models, the mediator variable was the individual-varying slope score estimated from the LME; this variable represents change in the mediator from months 4 to 12. The primary estimate from the mediation model is the indirect effect, which is defined as the product of the effect of treatment on the mediator variable and the association between the mediator and the outcome variable. Mediation is evident when the 95% CI, constructed using 5000 bootstrapped resamples, around the indirect effect does not contain 0. The mediation effect size is quantified using percentage mediated (ratio of the indirect effect to the total treatment effect on child relative weight change).
RESULTS
Preliminary Analyses
Of the 241 parent-child dyads who began FBT, 172 (71.4%) completed FBT and were randomized (Figure 1). No adverse events were reported. There were no significant differences in demographic or weight measures between those randomized vs not randomized, except for parent age (t238 = 2.55; P = .01), with randomized parents being older (mean [SD] age, 42.8 [6.3] vs 40.5 [6.3] years, respectively).
Across conditions, for all relative weight measures, there was significant change during prerandomization FBT from months 0 to 4 (all P < .001). Treatment conditions were well balanced on demographics, weight-status variables at months 0 and 4 (randomization), and FBT changes from months 0 to 4 (Table 1). The 3 conditions (HIGH, LOW, and CONTROL) attended a similar number of FBT sessions. As designed, participants in HIGH attended more maintenance sessions (mean [SD], 23.5 [7.8]) than LOW (12.5 [3.7]) or CONTROL (10.4 [3.9]) from months 4 to 12.
Condition Effects on Child Relative Weight Over Time
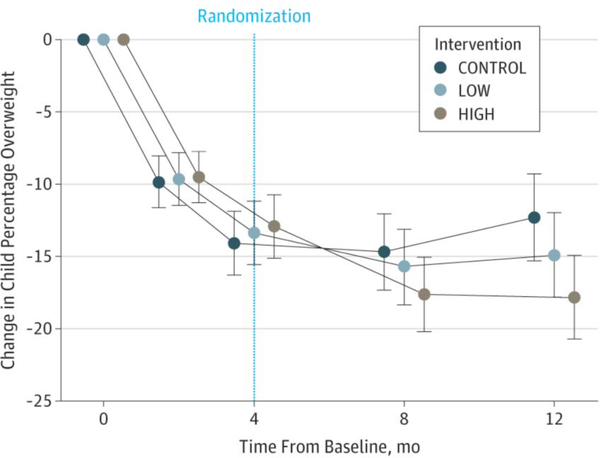
The omnibus condition × time interaction on child percentage overweight was significant (F8,618.9 = 2.89; P = .004) and was followed by planned pairwise comparisons. Figure 2A plots differential changes in child percentage overweight from baseline to month 12 across the 3 conditions.
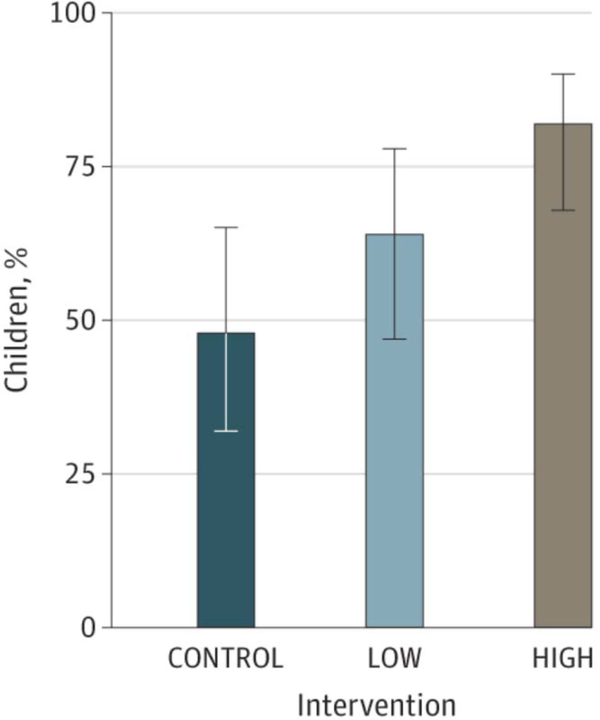
Figure 2.
Treatment Effects on Percentage Overweight and Proportion of Children Achieving Clinically Meaningful Weight-Loss Targets.
CONTROL indicates weight management education condition; HIGH, enhanced social facilitation maintenance (32 sessions of enhanced social facilitation maintenance); and LOW, enhanced social facilitation maintenance (16 sessions of enhanced social facilitation maintenance). A, Mean and 95% CI for reductions in percentage overweight are shown. B, percentages and 95% CI in percentage overweight are shown.
Figure 2A. Change in percentage overweight from baseline.
Figure 2B. Percentage of children achieving clinically meaningful weight loss targets (percentage overweight ≥8 units from 0–12 months)
Primary Outcome.
Table 2 provides the between-group differences in percentage overweight from months 4 to 12. Following FBT, children in HIGH had superior reductions with a 3.37 decrease compared with LOW (95% CI, −6.15 to −0.59; d = −0.38; P = .02), and a 6.71 decrease compared with CONTROL (95% CI, −9.57 to −3.84; d = −0.77; P < .001). Children in LOW showed intermediate reductions with a 3.34 decrease compared with children in CONTROL (95% CI, −6.21 to −0.47; d = −0.40; P = .02). Similar results were obtained with 12-month assessment completers (eTable 2 in Supplement 2) and for BMI z score (eTable 3 in Supplement 2). eTable 4 in Supplement 2 presents the means and standard deviations across time for percentage overweight and BMI z score.
Table 2.
Between-Group Difference in Child Percentage Overweight (Change From Month 4 to Month 12)
| Comparison | Estimate (95% Cl)a | P Value | Cohen db |
|---|---|---|---|
| HIGH vs CONTROL | -6.71 (−9.57 to −3.84) | <.001 | -0.77 |
| LOW vs CONTROL | -3.34 (−6.21 to −0.47) | .02 | -0.40 |
| HIGH vs LOW | -3.37 (−6.15 to −0.59) | .02 | -0.38 |
Abbreviations: CONTROL, weight management education condition; HIGH, 32 sessions of enhanced social facilitation maintenance; LOW, 16 sessions of enhanced social facilitation maintenance.
A negative estimate indicates that the treatment condition identified first had larger weight reduction over the period specified than the treatment condition identified second.
Cohen d is calculated as (mean1 – mean2)/SDperiod
Secondary Outcome.
HIGH had the largest proportion of children (n = 45 [82%]) who achieved greater than or equal to a 9-unit decrease in percentage overweight from months 0 to 12 (Table 3 and Figure 2B). Specifically, the proportion of children in HIGH was 34 percentage points higher compared with CONTROL (95% CI, 16 to 51; P < .001; NNT = 2.94), and 18 percentage points higher compared with LOW (95% CI, 1 to 34; P = .03; NNT = 5.56). There was a difference of 16 percentage points (95% CI, −3 to 35; P = .10; NNT = 6.25) between LOW and CONTROL. The results were similar for achievement of a BMI z score reduction of 0.25 or more from baseline (eTable 3 in Supplement 2).
Table 3.
Between-Group Difference in Percentage of Children Achieving Clinically Meaningful Weight Loss Target (Month 0 to Month 12)a
| Comparison | Percentage Point Difference (95% Cl) | P Value | Number Needed to Treat |
|---|---|---|---|
| HIGH vs CONTROL | 34 (16 to 51) | <.001 | 2.94 |
| LOW vs CONTROL | 16 (−3 to 35) | .10 | 6.25 |
| HIGH vs LOW | 18 (1 to 34) | .03 | 5.56 |
Abbreviations: CONTROL, weight management education condition; HIGH, 32 sessions of enhanced social facilitation maintenance; LOW, 16 sessions of enhanced social facilitation maintenance.
Defined as greater than or equal to 9-unit reduction in percentage overweight from baseline.
Mediators of Weight Outcome.
Three parent-reported variables (monitoring and goal setting to support child; family and home environment to support child; healthy child behaviors with peers) showed differential change by condition (condition × time, all P < .05) and were subjected to mediation analyses. Table 4 provides a full summary of the mediation models using these 3 variables. In separate models, all 3 were significant mediators of the effect of HIGH vs CONTROL on child percentage overweight, with monitoring and goal setting, family and home environment, and healthy behaviors with peers accounting for 42%, 27%, and 25%, respectively, of the superior effect of HIGH. Monitoring and goal setting mediated the effect of LOW vs CONTROL, accounting for 50% of the superior effect of LOW.
Table 4.
Mediators of Maintenance Treatment Differences in Percentage Overweight Outcomes
| Mediator Variable and Treatment Contrast | Path Aa | Path Bb | Path Cc | Path Cd | Path AB (95% Cl)e | % Mediatedf |
|---|---|---|---|---|---|---|
| Monitoring and Goal Setting to Support Child | ||||||
| CONTROL vs LOW | 0.29g | -3.07g | -0.90 | -1.79g | -0.89 (−1.79 to-0.29) | 50 |
| CONTROL vs HIGH | 0.48g | -2.93g | -1.92g | -3.33g | -1.42 (−2.57 to-0.59) | 42 |
| Family and Home Environment to Support Child | ||||||
| CONTROL vs LOW | 0.09 | -2.42 | -1.51 | -1.72g | -0.21 (−0.78 ta 0.05) | NA |
| CONTROL vs HIGH | 0.15g | -5.7Sg | -2.38g | -3.25g | -0.88 (−1.66 to-0.34) | 27 |
| Healthy Child Behavior With Peers | ||||||
| CONTROL vs LOW | 0.14 | -1.86 | -1.72g | -1.98g | -0.25 (−1.03 to 0.03) | NA |
| CONTROL vs HIGH | 0.26g | -3.34g | -2.53g | -3.39g | -0.86 (−1.76 to-0.27) | 25 |
Abbreviations: CONTROL, weight management education condition; HIGH, 32 sessions of enhanced social facilitation maintenance; LOW, 16 sessions of enhanced social facilitation maintenance; NA, not applicable.
Path A is the effect of treatment on changes in the mediator variable from month 4 to month 12.
Path B is the effect of change in the mediator on change in the weight outcome.
Path C’ is the direct effect of treatment on weight outcome, when accounting for the mediated effect.
Path C is the total effect of treatment on the weight outcome.
Path AB is the product of paths A and B and represents the indirect (mediated) effect
Percentage mediated is the ratio of the indirect effect (path AB) to the total effect (path C) and is shown only for statistically significant indirect effects as determined by 95% CI.
P<.05.
DISCUSSION
This multisite RCT provides substantive information about not only dose response and content of weight-control interventions for children with overweight and obesity, but also mechanisms by which these interventions enhance outcomes. Following FBT, SFM+, a multicomponent weight-control intervention with specialized content, improved child relative weight compared with a highly credible dose-matched weight-management education intervention. Further, the higher dose of SFM+ resulted in the greatest child percentage overweight reductions. These findings are consistent with learning research suggesting that frequent opportunities to receive feedback on desired behaviors aid in acquisition and use of these behaviors.
Mediational analyses show that behavioral and socioenvironmental components of SFM+ yielded the enhanced child weight outcomes. Specifically, continued monitoring and goal setting mediated children’s weight outcomes, consistent with findings from the adult literature.32 Our findings that family support and healthy behaviors with peers mediate children’s treatment outcomes also extend laboratory and observational research findings.33 Family and peers play a crucial role in establishing healthy home and social environments that encourage children to engage in ongoing weight-control behaviors, and their social support should be viewed as a key treatment component.11,12,34 Given that social neglect or rejection of children with overweight and obesity starts in early childhood,33intervening in early to middle childhood through family-based treatment that incorporates social targets may be particularly important, as social (eg, more peer influence) and physiological (eg, puberty) issues could make later intervention more challenging.
Limitations
Limitations include that this trial was conducted in an academic research setting and warrants replication in other settings (eg, primary care, community). Our innovative study design enabled testing of both dose and content of SFM+ and CONTROL. Funding resources prevented including a high-dose CONTROL condition that could have further disentangled dose and content effects, but the lower-dose SFM+ vs CONTROL findings do suggest content effects. Offsetting limitations is the large sample, with diversity across race, ethnicity, and SES.
Conclusions
Our findings provide empirical confirmation of USPSTF recommendations that childhood obesity treatment programs include comprehensive, intensive behavioral interventions (≥26 hours), with higher dose evidencing greater outcomes.4 Enhanced social facilitation maintenance, especially at higher doses, improves weight outcomes, consistent with learning theory.10,35 Enhanced social facilitation maintenance extends opportunities to practice behaviors acquired during FBT and promotes learning new skills (eg, peer engagement) and management of food and activity cues across more contexts of children’s lives to help them engrain healthier eating and activity habits. More than 80% of children in the higher-dose SFM+ achieved clinically significant levels of weight change at 12 months, compared with less than 50% in CONTROL. Given the epidemic of children affected by obesity, to increase treatment access, there is an urgent need to translate effective family-based weight-management interventions, like SFM+, into routine clinical care.36
Supplementary Material
Key Points.
Question: Does treatment designed to strengthen healthy dietary and physical activity habits (enhanced social facilitation maintenance) in children produce better weight loss outcomes than a control condition, and does a higher dose of this treatment provide additional benefits?
Findings: In this randomized clinical trial, 172 parent-child dyads were assigned to 1 of 3 32-week interventions following 16 weeks of family-based behavioral weight loss treatment. The 32-week sessions of enhanced social facilitation maintenance demonstrated better weight outcomes than 16 sessions of enhanced social facilitation maintenance and the control condition.
Meaning: This study provides empirical support that higher-dose and specialized treatment content designed to help families maintain weight-control behaviors following family-based behavioral weight loss treatment enhances weight outcomes.
ACKNOWLEDGEMENTS
Funding/Support: This study was funded by the National Institute of Child Health and Human Development (NICHD) (grant R01HD036904 to Dr Wilfley); National Institute of Mental Health (grant K24MH070446 to Dr Wilfley); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant K23DK060476 to Dr Saelens); National Center for Research Resources (NCRR) (grants KL2RR024994 [Dr Stein], UL1RR024992, and UL1RR025014); National Heart, Lung, and Blood Institute (grant T32HL007456 to Dr Kolko); the NIDDK Nutrition Obesity Research Center (grant P30DK056341); National Center for Advancing Translational Sciences (University of Washington Clinical and Translational Science Award) of the National Institutes of Health (grants UL1TR000448 and UL1TR000423); St. Louis Children’s Hospital Foundation (Washington University Pediatric and Adolescent Research Consortium); and institutional support from Washington University School of Medicine and Seattle Children’s Research Institute.
Role of the Sponsors: The NICHD, National Institute of Mental Health, National Heart, Lung, and Blood Institute, NIDDK, NCRR, and National Center for Advancing Translational Sciences were not involved in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. However, the National Institutes of Health Center for Scientific Review study section reviewers’ comments were used to help refine the study design
Financial Disclosures: Dr Stein is a consultant for BAROnova Inc, and Dr Epstein was a consultant for Kurbo during the completion of this work. No other disclosures were reported.
Footnotes
Previous Presentations: Portions of these data were presented at the Academy of Behavioral Medicine Research Annual Meeting; June 26, 2015; Cambridge, Maryland.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Trial Registration: ClinicalTrials.gov: NCT00759746 (https://clinicaltrials.gov/show/NCT00759746)
Contributor Information
Denise E. Wilfley, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO; wilfleyd@wustl.edu..
Brian E. Saelens, Department of Pediatrics, University of Washington and Seattle Children’s Research Institute, Seattle, WA; brian.saelens@seattlechildrens.org..
Richard I. Stein, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO; RStein@wustl.edu..
John R. Best, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO; john.best@ubc.ca..
Rachel P. Kolko, Department of Psychology, Washington University School of Medicine, St. Louis, MO; kolkorp2@upmc.edu..
Kenneth B. Schechtman, Division of Biostatistics, Washington University School of Medicine and St. Louis Children’s Hospital, St. Louis, MO; ken@wubios.wustl.edu..
Michael Wallendorf, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO; mike@wubios.wustl.edu..
R. Robinson Welch, Department of Psychology, Washington University, St. Louis, MO; welchr@wustl.edu..
Michael G. Perri, University of Florida, Department of Clinical & Health Psychology, Gainesville, FL; mperri@phhp.ufl.edu..
Leonard H. Epstein, Department of Pediatrics, University at Buffalo, Buffalo, NY; lhenet@buffalo.edu..
REFERENCES
- 1.Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13(1):6–13. [DOI] [PubMed] [Google Scholar]
- 2.Hampl SE, Carroll CA, Simon SD, Sharma V. Resource utilization and expenditures for overweight and obese children. Arch Pediatr Adolesc Med. 2007;161(1):11–14. [DOI] [PubMed] [Google Scholar]
- 3.Preventive US Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125(2):361–367. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–2444. [DOI] [PubMed] [Google Scholar]
- 5.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125(2):e396–418. [DOI] [PubMed] [Google Scholar]
- 6.Janicke DM, Steele RG, Gayes LA, et al. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. J Pediatr Psychol. 2014;39(8):809–825. [DOI] [PubMed] [Google Scholar]
- 7.Ho M, Garnett SP, Baur L, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130(6):e1647–1671. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell TB, Amaro CM, Steele RG. Pediatric Weight Management Interventions in Primary Care Settings: A Meta-Analysis. Health Psychol 2016. [DOI] [PubMed] [Google Scholar]
- 9.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298(14):1661–1673. [DOI] [PubMed] [Google Scholar]
- 10.Wilfley DE, Van Buren DJ, Theim KR, et al. The use of biosimulation in the design of a novel multilevel weight loss maintenance program for overweight children. Obesity (Silver Spring). 2010;18 Suppl 1:S91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvy SJ, de la Haye K, Bowker JC, Hermans RC. Influence of peers and friends on children’s and adolescents’ eating and activity behaviors. Physiol Behav. 2012;106(3):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amini M, Djazayery A, Majdzadeh R, Taghdisi MH, Sadrzadeh-Yeganeh H, Eslami-Amirabadi M. Children with Obesity Prioritize Social Support against Stigma: A Qualitative Study for Development of an Obesity Prevention Intervention. Int J Prev Med. 2014;5(8):960–968. [PMC free article] [PubMed] [Google Scholar]
- 13.Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull. 2006;132(3):354–380. [DOI] [PubMed] [Google Scholar]
- 14.Karpicke JD, Roediger HLI. Repeated retrieval during learning is the key to long-term retention. J Mem Lang. 2007;57:151–162. [Google Scholar]
- 15.Perri MG, Shapiro RM, Ludwig WW, Twentyman CT, McAdoo WG. Maintenance strategies for the treatment of obesity: an evaluation of relapse prevention training and posttreatment contact by mail and telephone. J Consult Clin Psychol. 1984;52(3):404–413. [DOI] [PubMed] [Google Scholar]
- 16.Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol. 2000;19(1 Suppl):57–63. [DOI] [PubMed] [Google Scholar]
- 17.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 19.Epstein L, Paluch R, Roemmich J, Beecher M. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilfley DE, Kass AE, Kolko RP. Counseling and behavior change in pediatric obesity. Pediatr Clin North Am. 2011;58(6):1403–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkley JE, Salvy SJ, Roemmich JN. The effect of simulated ostracism on physical activity behavior in children. Pediatrics. 2012;129(3):e659–666. [DOI] [PubMed] [Google Scholar]
- 22.Storch EA, Milsom VA, Debraganza N, Lewin AB, Geffken GR, Silverstein JH. Peer victimization, psychosocial adjustment, and physical activity in overweight and at-risk-for-overweight youth. J Pediatr Psychol. 2007;32(1):80–89. [DOI] [PubMed] [Google Scholar]
- 23.Hayden-Wade HA, Stein RI, Ghaderi A, Saelens BE, Zabinski MF, Wilfley DE. Prevalence, characteristics, and correlates of teasing experiences among overweight children vs. non-overweight peers. Obes Res. 2005;13(8):1381–1392. [DOI] [PubMed] [Google Scholar]
- 24.Faith MS, Leone MA, Ayers TS, Heo M, Pietrobelli A. Weight criticism during physical activity, coping skills, and reported phyiscal activity in children. Pediatrics. 2002;110(2 Pt 1):e23. [DOI] [PubMed] [Google Scholar]
- 25.Gray WN, Janicke DM, Ingerski LM, Silverstein JH. The impact of peer victimization, parent distress and child depression on barrier formation and physical activity in overweight youth. J Dev Behav Pediatr. 2008;29(1):26–33. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. [PubMed] [Google Scholar]
- 27.Barratt W The Barratt Simplified Measure of Social Status (BSMSS). http://socialclassoncampus.blogspot.com/2012/06/barratt-simplified-measure-of-social.html. Published June 14, 2012. Accessed August 28, 2017
- 28.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. Am J Hum Biol. 2007;19(4):487–494. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer HC, Frank E. Evaluation of comparative treatment trials: assessing clinical benefits and risks for patients, rather than statistical effects on measures. JAMA. 2010;304(6):683–684. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. [DOI] [PubMed] [Google Scholar]
- 31.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- 32.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring). 2007;15(12):3091–3096. [DOI] [PubMed] [Google Scholar]
- 33.Perri MG, Limacher MC, von Castel-Roberts K, et al. Comparative effectiveness of three doses of weight-loss counseling: two-year findings from the rural LITE trial. Obesity (Silver Spring). 2014;22(11):2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulik N, Valle CG, Tate DF. Friend and Family Support for Weight Loss in Adolescent Females. Child Obes. 2016;12(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutelle KN, Bouton ME. Implications of learning theory for developing programs to decrease overeating. Appetite. 2015;93:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melnyk BM, Grossman DC, Chou R, et al. USPSTF perspective on evidence-based preventive recommendations for children. Pediatrics. 2012;130(2):e399–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.