Abstract
Pathogenic missense and truncating variants in the GABRG2 gene cause a spectrum of epilepsies, from Dravet syndrome to milder simple febrile seizures. In most cases, pathogenic missense variants in the GABRG2 gene segregate with a febrile seizure phenotype. In this case series, we report a recurrent, de novo missense variant (c.316 G>A; p.A106T) in the GABRG2 gene that was identified in five unrelated individuals. These patients were described to have a more severe phenotype than previously reported for GABRG2 missense variants. Common features include variable early-onset seizures, significant motor and speech delays, intellectual disability, hypotonia, movement disorder, dysmorphic features, and vision/ocular issues. Our report further explores a recurrent pathogenic missense variant within the GABRG2 variant family and broadens the spectrum of associated phenotypes for GABRG2-associated disorders.
Keywords: epilepsy, genetics, seizures, GABRG2, phenotype, missense
Introduction
To date, most reported γ-aminobutyric acid (GABA) type A receptor pathogenic variants associated with idiopathic generalized epilepsies (IGEs) have been found in the γ2 subunit encoded by the GABRG2 gene (Lachance-Touchette et al., 2011; Huang et al., 2014; Kang et al., 2016). GABA type A receptors are pentameric ligand-gated ion channels primarily responsible for mediating fast inhibitory neurotransmission in the mammalian central nervous system (Barnard et al., 1998; Schwartzkroin, 1998). GABA type A receptors hyperpolarize neurons by fluxing chloride ions through a central anion-selective pore that is a pentamer assembled from 19 possible subunits. The most commonly expressed receptor consists of two α subunits, two β subunits, and one γ2 subunit (Sarto-Jackson & Sieghart, 2008).
Pathogenic missense and truncating variants in the GABRG2 gene have been reported as causes of a wide spectrum of epilepsies, from Dravet syndrome and genetic (generalized) epilepsy with febrile seizures plus (GEFS+), to febrile seizures associated with childhood absence epilepsy (CAE) and milder simple febrile seizures (FS) (Macdonald et al., 2010). Additional phenotypes, including learning difficulties and behavioral problems, have been reported in one family with GEFS+ caused by a nonsense variant (p.R136X) in GABRG2 (Johnston et al., 2014). A few individuals from a single family with another GABRG2 nonsense variant (p.Q390X, also referred to as p.Q351X) have been reported to have a clinical diagnosis of Dravet syndrome (Harkin et al., 2002; Hirose, 2006). GABRG2 pathogenic variants are typically inherited in an autosomal dominant manner and may cause different clinical presentations, even within the same family (Wallace et al., 2001; Harkin et al., 2002). Some individuals with GABRG2 pathogenic variants may never develop seizures, indicating incomplete penetrance (Wallace et al., 2001; Hirose, 2006). Only recently, a case series revealed evidence of GABRG2 missense variants being responsible for a more severe epileptic encephalopathy phenotype (Shen et al., 2017). Here we describe a recurrent pathogenic missense variant (c.316 G>A; p.A106T) in the GABRG2 gene in five unrelated patients with severe phenotypes which include significant motor and speech delays, intellectual disability, hypotonia, movement disorder, dysmorphic features, and visual impairment and other ocular issues, in addition to variable early-onset seizures.
Methods
For each proband, the recurrent p.A106T variant in the GABRG2 gene was detected by sequence analysis using one of several Next Generation sequencing platforms in different laboratories. Patient 1 (singleton), and patients 2 and 4 (along with their parents), were tested using typical whole exome sequencing (WES). Patient 1 and parents also had whole genome sequencing (WGS) performed. WES testing involved enriching genomic DNA and capturing targeted exons with Nimblegen reagents using a HGSC custom-designed reagent called VCRome 2.1 (Patient 1) or with the Agilent SureSelect XT Human All Exon V5 Plus kit, per manufacturer’s protocol (Patients 2 and 4). Sequencing was completed on an Illumina HiSeq platform (Patients 1, 2, 4). Data analysis and variant calls were performed using Mercury 1.0, a custom-developed Atlas-SNP and Atlas-indel variant caller (Patient 1), using a proprietary custom-developed bioinformatics pipeline based on the human genome build UCSC hg19 reference sequence (CWES-1.2) (Patient 2), or using the Bench NGS Lab platform (Cartagenia, Leuven, Belgium) and GATK haplotype caller (v2.7–2) (Patient 4).
Patients 2, 3, and 5 were tested by Next Generation sequencing panels with exon-level array CGH for epilepsy-related genes. Coding regions and splice junctions of the genes on the epilepsy panels were either sequenced simultaneously by massive parallel sequencing (Patients 2 and 3) or enriched using a proprietary targeted capture system (Patient 5) and sequenced on an Illumina Next Generation sequencing platform, with sequence fill-ins and variant confirmations performed by conventional dideoxy sequencing. Concurrent deletion/duplication testing was performed for most genes in the panels by array CGH using a custom-developed exon-level oligo array. Probe sequences and locations were based on human genome build GRCh37/UCSC hg19. Confirmation of the p.A106T variant was completed by traditional Sanger sequencing for all patients, the parental samples provided for Patients 1, 2, 3, and 4, and for the maternal sample provided for Patient 5. Paternal testing was not completed for Patient 5. The potential pathogenicity of the variant was evaluated using criteria from the American College of Medical Genetics (Richards et al., 2015).
Results
The p.A106T variant in the GABRG2 gene was first identified in Patient 2 via an epilepsy panel. At that time, the variant was classified as a variant of unknown significance as missense pathogenic variants had been reported in association with febrile seizures, which Patient 2 did not have. Patient 2 then had whole exome sequencing performed, and the p.A106T variant was observed again. Subsequently, this variant was also identified in two patients via whole exome sequencing (Patients 1 and 4), and in two patients via epilepsy panels (Patients 3 and 5). Paternal testing was not completed in Patient 5, but the other four cases are confirmed (paternity and maternity confirmed) or assumed (paternity and/or maternity not confirmed) de novo. Based on this new evidence, the p.A106T variant was reclassified as pathogenic in 2016.
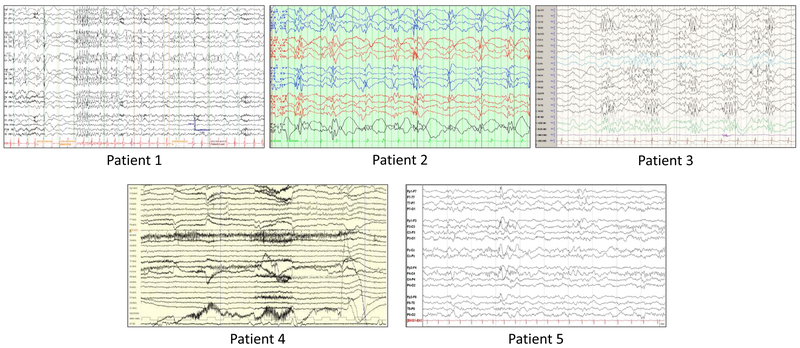
Genetic test information, demographics, and clinical features for the five patients were collected and compared (Table 1). One patient had a family history of seizures whereby a first cousin was reported with teenage-onset epilepsy. All five patients experienced early seizures and/or staring spells; age of onset ranged from the first day of life (generalized convulsions) to four months old. The types of seizures were highly variable; the majority (4/5) of the patients had generalized tonic-clonic seizures and 5/5 patients had intractable seizures. Patient 4 was successfully treated with pyridoxine/vitamin B6 and folinic acid for almost 12 years until she experienced a new seizure episode, starting with a tonic-clonic seizure that coincided with menarche. On a regimen of high dose vitamin B, folinic acid, medroxyprogesterone injections, and oxcarbazepine, she was seizure free for a period of time, but when the vitamin B dose was lowered the seizures recurred. Focal, myoclonic, and neonatal seizures were also observed in some of the patients. Figure 1 includes representative EEG tracings for all five patients.
Table 1:
Summary of Patient Phenotypes
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | ||
|---|---|---|---|---|---|---|
| Gender | Female | Male | Male | Female | Female | |
| Age | 6 years | 10 years | 5 years | 13 years | 7 months | |
| Age of seizure onset | 4 months, 2 yearsa |
3 months | 6 weeks | Day 1, 7 monthsb |
Day 2 | |
| Family history of epilepsy | No | No | Yesc | No | No | |
| Testing platform | Singleton Whole Exome Sequencing and Trio Whole Genome Sequencing | Epilepsy Panel, Trio Whole Exome Sequencing | Epilepsy Panel | Trio Whole Exome Sequencing | Epilepsy Panel | |
| Inheritance | confirmed de novo | confirmed de novo | assumed de novo | confirmed de novo | not completed | |
| CLINICAL INFORMATION | Frequency | |||||
| Seizures (5/5) | ||||||
| Epileptic encephalopathy | + | + | + | 3/5 | ||
| Focal seizures | + | + | + | + | 4/5 | |
| Generalized tonic-clonic seizures | + | + | + | + | 4/5 | |
| Neonatal seizures (general convulsions) | + | + | 2/5 | |||
| Myoclonic seizures | + | + | 2/5 | |||
| Intractable seizures | + | + | + | + | + | 5/5 |
| Physical/Cognitive Development (5/5) | ||||||
| Intellectual disability | + | + | + | + | N/A | 4/4d |
| Gross motor delay (non-ambulatory) | + | + | + | + | + | 5/5 |
| Fine motor delay | + | + | + | + | + | 5/5 |
| Speech delay (non-verbal) | + | + | + | + | N/A | 4/4d |
| Developmental regression | + | 1/5 | ||||
| Movement (4/5) | ||||||
| Cerebral palsy | + | 1/5 | ||||
| Dysmetria | + | + | 2/5 | |||
| Hyperkinetic movements | + | 1/5 | ||||
| Ataxia/movement disorder | + | + | 2/5 | |||
| Constant stereotypic, jerky movements | + | 1/5 | ||||
| Visual Impairment (4/5) | ||||||
| Cortical blindness/visual impairment | + | + | + | + | 4/5 | |
| Ocular (4/5) | ||||||
| Esotropia | + (bilateral) | + (unilateral) | 2/5 | |||
| Nystagmus | + | + | + (resolved) | + | 4/5 | |
| Dysmorphic Features (4/5) | ||||||
| Dysmorphic facial features (mild) | + | + | 2/5 | |||
| Downslanting palpebral fissures | + | + | 2/5 | |||
| Ptosis | + | 1/5 | ||||
| Bilateral inverted nipples | + | 1/5 | ||||
| Gastrointestinal (3/5) | ||||||
| Dysphagia | + | + | 2/5 | |||
| Gastrointestinal reflux | + | 1/5 | ||||
| Constipation | + | + | 2/5 | |||
| Gastrostomy tube placement | + | + | 2/5 | |||
| Other Clinical Features (5/5) | ||||||
| Hypotonia | + | + | + | + | + | 5/5 |
| Failure to thrive | + | 1/5 | ||||
onset of staring spells and delays at age 4 months; onset of generalized seizures at age 2 years
onset of generalized convulsions at day 1 of life; onset of myoclonic seizures at age 7 months
paternal first-cousin (female) with teenage-onset epilepsy
total count did not include Patient 5, who was too young to have exhibited the sign/symptom
yo = years old
Figure 1: Representative EEGs.
Patient 1 - Seizure with head drop and behavioral arrest at 5 years old, generalized spikes followed by relative voltage attenuation, fast generalized spike-wave complexes.
Patient 2 - Runs of spike and slow wave at 1 Hz. EEG obtained at 10 years old.
Patient 3 - Sleep epileptiform discharges at 6 years old. High amplitude spike and polyspike discharges with prominence in the temporal-occipital regions bilaterally (T5-O1, T6-O2).
Patient 4 - Bilateral peak waves in the frontal areas. EEG obtained at 12 years old.
Patient 5 - Stage II sleep with symmetrical sleep spindles at 4 months old. Excess beta range activity and frequent bifrontal, synchronous spike and wave discharges.
All patients have hypotonia and motor delays (including being non-ambulatory). With the exception of Patient 5, all patients exhibit intellectual disability, speech delays (including being non-verbal), and have at least one dysmorphic feature indicated. Four of the five patients have visual impairment or ocular issues, and four of the five patients have a movement disorder.
Patient 1
Patient 1 is a six-year-old female who was hypotonic from birth. Concerns for developmental delay and staring spells were raised at four to five months of age. By two years of age, she began having generalized tonic and atonic seizures, clinically characterized by head drop, and possibly myoclonic astatic seizures. Her seizures have been largely refractory to treatment; surgical treatment is now being considered. A twenty-four hour electroencephalogram (EEG) at age five years was abnormal, with one 15 second seizure and two brief (one to two second) seizures of generalized onset associated with reported head drop. There were near continuous multifocal spikes in sleep and absence of normal awake and sleep features, consistent with seizure of generalized mechanism of onset and a diffuse encephalopathy of non-specific etiology. She was noted to have a unilateral multicystic dysplastic kidney prenatally and has a history of contralateral vesicoureteral reflux, now resolved. Her mild dysmorphic features include prominent forehead and nasal bridge, short columella, slightly deep-set eyes, and a wide mouth. She has bilateral fifth finger clinodacytly, small fifth fingernails and toenails, slightly broad great toes, and prominent finger and toe pads. Brain magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) at 27 months of age were normal, with cavum septum pellucidum et vergae, mild prominence of perivascular spaces noted. Studies on cerebrospinal fluid (CSF) at age four years were essentially normal: monoamine neurotransmitter metabolites, neopterin, tetrahydrobiopterin, 5-methyltetrahydrofolate, succinyladenosine, 2-hydroxybutyric acid, and sialic acid. Metabolic work-up and extensive genetic testing were non-diagnostic.
Patient 2 (also Patient 2 in Shen et al., 2017)
Patient 2 is a 10-year-old male who first presented with seizures at three months of age. He subsequently developed intractable partial complex seizures. Some seizure episodes lasted for 10 to 60 seconds and involved head turning and stiffening of both arms. These occurred about every two weeks. Other episodes, occurring two to three times per week, consisted of the patient putting his head down, turning to the right, and staring blankly for about 30 seconds. In some instances he would drop his head and start laughing. He presented with gastrointestinal issues in the first year of life, including gastroesophageal reflux disease, dysphagia, and constipation. This prompted placement of a gastrostomy-tube at 15 months old. MRI of the brain was normal at two years of age, but a second MRI performed at age eight years revealed progressive volume loss in the frontal lobes and frontal horns of the lateral ventricles. A video EEG was performed at age three-and-a-half years and was abnormal due to multifocal sharp waves and diffuse excessive beta. Additionally, brain MRS was normal at age four years, and CSF monoamine neurotransmitter metabolite levels were normal at age two-and-a-half years.
Patient 3
Patient 3 is a five-year-old male who had episodes that were suspicious for seizures around six weeks of age, then began having convulsions at three months old. Phenobarbital treatment seemed effective for a period of time, but then he started experiencing generalized tonic-clonic seizures. These seizures lasted for 30 to 60 seconds and involved arching of the back, full-body twitching, non-purposeful extremity movement, and upward eye deviation. His parents believed that fevers, fatigue, and overstimulation triggered the seizures. They have reported episodes as he is falling asleep which entailed opening of his eyes, back arching, full-body stiffening, and arm extension for about five seconds before returning back to sleep. He experienced developmental regression when he initially had seizures, but his development has remained static since then. MRI of the brain was normal around seven-and-a-half months of age, but brain MRS revealed elevated lactate levels. An EEG performed at one-and-a-half years of age revealed right frontotemporal discharges with background slowing. Previous CSF monoamine neurotransmitter metabolic testing was normal. CSF folate was not measured.
Patient 4
Patient 4 is a 13-year-old female who presented with generalized convulsions and hypotonia on her first day of life. She was born at full term, but delivery was induced due to decreased fetal movement and oligohydramnios. She began having generalized convulsions which were not well characterized. The convulsions were controlled with phenobarbital and the patient was discharged after one week. She was readmitted at age seven months due to persistent myoclonic seizures. The myoclonic seizures did not respond to phenobarbital. The patient was treated with pyridoxine (vitamin B6) and folinic acid, given extremely low GABA levels in her cerebrospinal fluid. This treatment was effective for years, but a seizure was observed again at age 12 years and four months. She is now also treated with oxcarbazepine and medroxyprogesterone injections, as hormonal influences are suspected. The seizures appear to be related to her menarche. After the vitamin B6 dose was lowered due to fear of toxicity, the patient started experiencing seizures again. At age 13, she has complex partial seizures four to five times a month with smile, drooling, and head turn. She experienced abnormal eye movements with delayed visual maturity during the first two years of life, and electroretinography at age six years showed signs of a retinopathy. Brain MRS has not been performed for this patient, but MRI of the brain was normal at ages two, six, and 12 years. Repeat cerebrospinal fluid studies revealed normal GABA levels. An electromyogram (EMG) was inconclusive. An EEG at 12 years old showed diffuse beta activity with short clusters of left frontolateral sharp waves, but no epileptiform discharges. Additionally, an EEG at 12 years old revealed bilateral peak waves in the frontal areas.
Patient 5
Patient 5 is a seven-month-old female who presented on day two of life with neonatal seizures. Pregnancy and delivery were unremarkable, with an initial Apgar score of five. The baby was floppy and cyanotic requiring vigorous stimulation, suctioning, and continuous positive airway pressure by mask. Apgar score at five minutes was eight and the baby was slow to feed. Head computed tomography (CT), sepsis evaluation, transaminases, and lactate were normal. On the second day of life, the patient developed apnea episodes felt to be seizures. MRI showed two tiny periventricular cysts but was otherwise normal. The seizures resolved upon treatment with levetiracetam. An attempt was made to taper the medications but bilateral upper extremity jerking episodes occurred and levetiracetam was resumed. At four months of age, episodes of stiffening with behavioral arrest occurred. Urine organic acids, Fragile X, karyotype, and microarray were normal. Examination and video EEG monitoring were unremarkable. Seizures recurred at seven months of age; video EEG monitoring captured focal onset electrographic seizures with little clinical accompaniment, episodes of behavioral arrest and eye deviation associated with a focal ictal pattern, and episodes of sudden arousal from sleep with eye deviation associated only with diffuse slowing. The EEG background showed excessive beta activity not attributable to sedative medications. Oxcarbazepine was administered in addition to levetiracetam and seizures resolved. The physical examination over time became more concerning with lack of a social smile and poor visual interaction.
Discussion
To date, pathogenic variants in the GABRG2 gene, including 11 truncating variants (four nonsense, four frame-shifts, two splice-sites, and one large deletion) and 16 missense variants, have been reported in a subset of families and individuals with a variety of phenotypes ranging from epileptic encephalopathies (including Dravet syndrome) to genetic (generalized) epilepsy with febrile seizures plus (GEFS+), to febrile seizures associated with childhood absence epilepsy (CAE) and milder simple febrile seizures (FS) (Baulac et al., 2001; Wallace et al., 2001; Harkin et al., 2002; Kananura et al., 2002; Audenaert et al., 2006; Hirose, 2006; Sun et al., 2008; Macdonald et al., 2010; Shi et al., 2010; Cantarín-Extremera et al., 2011; Lachance-Touchette et al., 2011; Balan et al., 2013; Carvill et al., 2013; Tian et al., 2013; Johnston et al., 2014; Boillot et al., 2015; Reinthaler et al., 2015; Shen et al., 2017). In these studies, the majority of pathogenic variants in GABRG2 segregated predominantly with a FS phenotype. Additional genetic or epigenetic modifiers have been proposed to determine the associated variable epilepsy component (Sun et al., 2008). However, until now the link between FS and GABRG2 defects has been poorly understood.
Here we describe a recurrent pathogenic missense variant (c.316 G>A; p.A106T) in exon 3 of the GABRG2 gene (RefSeq#: NM_000816.3) in five unrelated patients. The A106T variant was not observed in any publicly available variant databases, indicating it is not a common benign variant in these populations. This variant was reported as a de novo pathogenic variant in four of the five patients tested by an epilepsy gene panel and/or whole exome or whole genome sequencing (the fifth patient has not had paternal testing completed). While the seizure types are highly variable, only one of the five patients reported a possible history of FS (Patient 3), whereas FS is the most common type of seizure in most GABRG2 positive cases. All five patients in this case series show complex phenotypes. Note that the fifth patient is much younger (seven months old) than the other cases, so some of the complex phenotypic features seen in the other four patients may not yet be apparent in Patient 5. The most frequent phenotypes in this case series include significant gross motor delay (non-ambulatory), fine motor delay, speech delay (non-verbal), intellectual disability, and hypotonia (Table 1). Furthermore, variable movement disorder, dysmorphic features, and visual impairment and ocular issues have also been seen in most of these patients (Table 1). While these features are highly variable, they have not been recognized in other GABRG2-associated cases published prior to 2016, but are consistent with a recent report of five pathogenic/likely pathogenic missense variants in GABRG2 (Shen et al., 2017).
The p.A106T variant was located in the β1-β2 inner loop of the N-terminal extracellular topological domain of the GABRG2 protein that contributes to the subunit interface at the junction of the transmembrane domain. It is highly possible that this de novo variant may decrease the GABA potency by disrupting this structural domain important for GABA type A receptor function. A recent in vitro functional study from Shen et.al demonstrated that not only did the p.A106T variant decrease macroscopic GABA-evoked currents in p.A106T transfected HEK293T cells by 30%, but it also reduced surface levels of mutant γ2L subunits by 26% compared to co-expressed wild-type γ2L subunits. These results all suggest that the p.A106T variant reduces biogenesis of GABA type A receptors (Shen et al., 2017).
Conclusions
It is evident that the position and severity of the pathogenic variants in GABRG2 determine the spectrum of associated phenotypes, but genotype-phenotype correlations are still poorly understood at this time. Further studies are needed to explain the phenotypic variations caused by different pathogenic variants in this gene. The phenotypes identified in four of our five patients with the de novo p.A106T variant include variable early-onset seizures, intellectual disability, motor and speech delays, hypotonia, movement disorder, dysmorphic features, and/or visual impairment/ocular issues. Recent electrophysiological in vitro studies demonstrated that the p.A106T variant resulted in a significant reduction in current amplitude. Additionally, there was impairment of the ³2 subunit surface expression. The authors concluded that the p.A106T variant has a major effect on GABAA receptor function and alters kinetic properties (Shen et al., 2017). The p.A106T variant is de novo in all patients for whom both maternal and paternal testing was completed, which is also consistent with a more severe outcome for this variant. This observation stands in contrast to a majority of other reported GABRG2 pathogenic variants with milder outcomes, which are inherited. Further in vitro and in vivo studies are needed to explore the molecular mechanism behind the complex and unique phenotypes associated with the p.A106T variant and other missense variants causing a severe early-onset encephalopathy phenotype. Our report further explores a recurrent pathogenic missense variant within the fast growing GABRG2 variant family and broadens the spectrum of phenotypes associated with variants in the GABRG2 gene.
Acknowledgements
We wish to acknowledge the collaborations with our patients and their families, who consented to share their stories in an effort to add to the published information on epilepsy genes.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, and Sunyaev SR (2010). A method and server for predicting damaging missense mutations. Nat Methods, April 7(4), 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert D, Schwartz E, Claeys KG, Claes L, Deprez L, Suls A, Van Dyck T, Lagae L, Van Broeckhoven C, Macdonald RL and De Jonghe P (2006). A novel GABRG2 mutation associated with febrile seizures. Neurology, 67(4), 687–690. [DOI] [PubMed] [Google Scholar]
- Balan S, Sathyan S, Radha SK, Joseph V, Radhakrishnan K and Banerjee M (2013). GABRG2, rs211037 is associated with epilepsy susceptibility, but not with antiepileptic drug resistance and febrile seizures. Pharmacogenetics and Genomics, 23(11), 605–610. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN and Langer SZ (1998). International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacological Reviews, 50(2), 291–314. [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R and LeGuern E (2001). First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nature Genetics, 28(1), 46–48. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Song L, Zhang H and Macdonald RL (2002). Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. The Journal of Neuroscience, 22(13), 5321–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillot M, Morin-Brureau M, Picard F, Weckhuysen S, Lambrecq V, Minetti C, Striano P, Zara F, Iacomino M, Ishida S and An-Gourfinkel I (2015). Novel GABRG2 mutations cause familial febrile seizures. Neurology Genetics, 1(4), e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarin-Extremera V, Garcia-Penas JJ, Gutiérrez-Solana LG, Garcia-Fernandez M, Ruiz-Falcó ML, Duat-Rodríguez A and Lopez-Marin L (2011). Clinical, electroencephalographic and genomic characteristics of patients with epilepsy with febrile seizures plus. Revista de Neurologia, 52(7), 404–411. [PubMed] [Google Scholar]
- Carvill GL, Heavin SB, Yendle SC, McMahon JM, O’Roak BJ, Cook J, Khan A, Dorschner MO, Weaver M, Calvert S and Malone S (2013). Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nature Genetics, 45(7), 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, Del Angel G, Rivas MA, Hanna M and McKenna A (2011). A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics, 43(5), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J and Macdonald RL (2006). δ subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of α4β2δ GABAA receptors. The Journal of Neuroscience, 26(5), 1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MJ, Song L, Arain F and Macdonald RL (2004). The juvenile myoclonic epilepsy GABAA receptor α1 subunit mutation A322D produces asymmetrical, subunit position-dependent reduction of heterozygous receptor currents and α1 subunit protein expression. The Journal of Neuroscience, 24(24), 5570–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF and Scheffer IE (2002). Truncation of the GABA A-receptor γ2 subunit in a family with generalized epilepsy with febrile seizures plus. The American Journal of Human Genetics, 70(2), 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S (2006). A new paradigm of channelopathy in epilepsy syndromes: intracellular trafficking abnormality of channel molecules. Epilepsy Research, 70, 206–217. [DOI] [PubMed] [Google Scholar]
- Huang X, Hernandez CC, Hu N and Macdonald RL (2014). Three epilepsy-associated GABRG2 missense mutations at the γ+/β− interface disrupt GABA A receptor assembly and trafficking by similar mechanisms but to different extents. Neurobiology of Disease, 68, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AJ, Kang JQ, Shen W, Pickrell WO, Cushion TD, Davies JS, Baer K, Mullins JG, Hammond CL, Chung SK and Thomas RH, 2014. A novel GABRG2 mutation, p. R136*, in a family with GEFS+ and extended phenotypes. Neurobiology of Disease, 64, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananura C, Haug K, Sander T, Runge U, Gu W, Hallmann K, Rebstock J, Heils A and Steinlein OK (2002). A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Archives of Neurology, 59(7), 1137–1141. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Shen W and Macdonald RL (2009). The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. The Journal of Neuroscience, 29(9), 2845–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W and Macdonald RL (2013). Trafficking‐deficient mutant GABRG2 subunit amount may modify epilepsy phenotype. Annals of Neurology, 74(4), 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Zhou C, Xu D and Macdonald RL (2015). The human epilepsy mutation GABRG2 (Q390X) causes chronic subunit accumulation and neurodegeneration. Nature Neuroscience, 18(7), 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ and Macdonald RL (2016). Molecular pathogenic basis for GABRG2 mutations associated with a spectrum of epilepsy syndromes, from generalized absence epilepsy to Dravet syndrome. JAMA neurology, 73(8), 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC (2009). Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc, 4, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lachance-Touchette P, Brown P, Meloche C, Kinirons P, Lapointe L, Lacasse H, Lortie A, Carmant L, Bedford F, Bowie D and Cossette P (2011). Novel α1 and γ2 GABAA receptor subunit mutations in families with idiopathic generalized epilepsy. European Journal of Neuroscience, 34(2), 237–249. [DOI] [PubMed] [Google Scholar]
- Li H and Durbin R (2009). Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics, 25(14), 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G and Durbin R (2009). The sequence alignment/map format and SAMtools. Bioinformatics, 25(16), 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H (2012). Exploring single-sample SNP and INDEL calling with whole-genome de novo assembly. Bioinformatics, 28(14), 1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ and Feng HJ (2006). GABA A Receptor Mutations Associated with Generalized Epilepsies 1. Advances in Pharmacology, 54, 147–169. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ and Gallagher MJ (2010). Mutations in GABAA receptor subunits associated with genetic epilepsies. The Journal of Physiology, 588(11), 1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinthaler EM, Dejanovic B, Lal D, Semtner M, Merkler Y, Reinhold A, Pittrich DA, Hotzy C, Feucht M, Steinböck H and Gruber‐Sedlmayr U (2015). Rare variants in γ‐aminobutyric acid type A receptor genes in rolandic epilepsy and related syndromes. Annals of Neurology, 77(6), 972–986. [DOI] [PubMed] [Google Scholar]
- Retterer K, Scuffins J, Schmidt D, Lewis R, Pineda-Alvarez D, Stafford A, Schmidt L, Warren S, Gibellini F, Kondakova A and Blair A (2014). Assessing copy number from exome sequencing and exome array CGH based on CNV spectrum in a large clinical cohort. Genetics in Medicine, 17(8), 623–629. [DOI] [PubMed] [Google Scholar]
- Richards CS, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, and Rehm HL; on behalf of the ACMG Laboratory Quality Assurance Committee. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17, 405–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde M, Lyon E, Ward BE, Spector E; Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee. (2008). ACMG Recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genetics in Medicine, 10, 294–300. [DOI] [PubMed] [Google Scholar]
- Sarto-Jackson I and Sieghart W (2008). Assembly of GABAA receptors (Review). Molecular Membrane Biology, 25(4), 302–310. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA (1998). GABA synapses enter the molecular big time. Nature Medicine, 4(10), 1115. [DOI] [PubMed] [Google Scholar]
- Shen D, Hernandez CC, Shen W, Hu N, Poduri A, Shiedley B, Rotenberg A, Datta AN, Leiz S, Patzer S, Boor R, Ramsey K, Goldberg E, Helbig I, Ortiz-Gonzalez XR, Lemke JR, Marsh ED, and Macdonald RL (2017). De novo GABRG2 mutations associated with epileptic encephalopathies. Brain, 140(1), 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Huang MC, Ishii A, Yoshida S, Okada M, Morita K, Nagafuji H, Yasumoto S, Kaneko S, Kojima T and Hirose S (2010). Mutational analysis of GABRG2 in a Japanese cohort with childhood epilepsies. Journal of Human Genetics, 55(6), 375–378. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhang Y, Liang J, Liu X, Ma X, Wu H, Xu K, Qin J, Qi Y and Wu X (2008). SCN1A, SCN1B, and GABRG2 gene mutation analysis in Chinese families with generalized epilepsy with febrile seizures plus. Journal of Human Genetics, 53(8), 769–774. [DOI] [PubMed] [Google Scholar]
- Tian M, Mei D, Freri E, Hernandez CC, Granata T, Shen W, Macdonald RL and Guerrini R (2013). Impaired surface αβγ GABA A receptor expression in familial epilepsy due to a GABRG2 frameshift mutation. Neurobiology of Disease, 50, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE and Berkovic SF (2001). Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nature Genetics, 28(1), 49–52. [DOI] [PubMed] [Google Scholar]