Abstract
OBJECTIVES:
To examine the associations of maternal intake of fruits and vegetables (FVs), considering pesticide residue levels, with fetal growth.
METHODS:
We studied 1777 mothers (1275 white, 502 non-white) and their infants from Project Viva, a prospective pre-birth cohort (1999-2002). We categorized FVs as containing high or low pesticide residues using data from the US Department of Agriculture. We then used a food frequency questionnaire to estimate each participant’s intake of high and low pesticide residue FVs in the first and second trimester. The primary outcomes were small-for-gestational-age (SGA; < 10th percentile in birth-weight-for-gestational-age), large-for-gestational-age (LGA; ≥ 10th percentile in birth-weight-for-gestational-age) and preterm birth (gestational age <37 weeks). We also evaluated whether the associations between high pesticide residue FV intake and birth outcomes were modified by race/ethnicity.
RESULTS:
5.5% of newborns were SGA, 13.7% were LGA, and 7.3% were preterm. Intakes of high or low pesticide residue FVs, regardless of pregnancy trimester, were not associated with risks of SGA, LGA, or preterm birth. In addition, the associations of high pesticide FV intake with SGA and LGA were not modified by race/ethnicity. However, we observed heterogeneity in the relationship between first trimester high pesticide FV intake and risk of preterm birth by race/ethnicity (P value for interaction=0.01), although this relationship did not persist after correction for multiple comparisons (Bonferroni corrected level of significance: P < 2.8×l0−3).
CONCLUSIONS:
There were no clear associations between high or low pesticide FV intake during pregnancy with SGA, LGA or preterm birth.
1. Introduction
Fruits and vegetables (FVs) contain many important nutrients and are considered essential components of a healthy diet (Barbara E Millen et al., 2016). According to the Dietary Guidelines for Americans 2015-2020, consumption of a variety of FVs is recommended throughout the lifespan including during pregnancy (B. E. Millen et al., 2016). Nonetheless, FVs can also serve as a source of exposure to pesticide residues. According to US Department of Agriculture (USDA) Pesticide Data Program, in 2015, 97% of FVs in U.S. markets had detectable pesticide residues, and 56% had detectable levels of three or more individual pesticides (USDA, 2015).
In the US, pesticide tolerances (i.e., amount of pesticide residues that are allowed to remain in or on food) are regulated by the Environmental Protection Agency under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Food Quality Protection Act (FQPA). Although the majority of US foods have residues below tolerance levels (USDA, 2015), it remains unclear whether long-term exposure through diet to low levels of pesticide residues below tolerance levels possess a risk. Pesticide metabolites can be detected in amniotic fluid collected at 15-18 weeks gestation (Bradman et al., 2003). The fetus, due to its rapid growth, immature metabolic pathways, and developing vital organ systems (Berkowitz et al., 2004), may exhibit greater susceptibility to the effects of pesticide residues than adults.
Fetal growth and preterm birth are not only indicators of fetal health but also predictors of long-term health outcomes. A growing number of studies suggest that preterm birth and higher or lower fetal growth are associated with adverse cardiometabolic health later in life (Frankel et al., 1996; Huxley et al., 2000; Irving et al., 2000; Newsome et al., 2003; Rich-Edwards et al., 1997; Rotteveel et al., 2008), although the underlying pathways with later outcomes may be different for preterm birth and fetal growth (Gillman, 2002) . In the literature, a limited number of human studies have examined maternal prenatal urinary concentrations of organophosphate pesticide metabolites in relation to fetal growth and gestational age (Eskenazi et al., 2004; Harley et al., 2011; Naksen et al., 2015; Rauch et al., 2012; Whyatt et al., 2004). However, the results of these studies were mixed, possibly due to differences in timing of exposure (e.g., 1st trimester vs. 2nd trimester vs. 3rd trimester), sources of exposure (residential vs. dietary vs. agricultural exposure), high within-person variability of urinary biomarkers, and different frequencies of paraoxonase 1(PON1) polymorphisms (an enzyme involved in detoxification of organophosphate pesticides (Costa et al., 2013)) across studies. Therefore, the objective of the study was to assess the associations of high and low pesticide residue FV intake during each of the first and second trimesters with fetal growth and preterm birth in a cohort of pregnant women from Eastern Massachusetts. We also examined whether the associations were modified by race/ethnicity, a proxy of frequency in PON1 polymorphism (Chen et al., 2003; Draganov & La Du, 2004).
2. Materials and Methods
2.1. Study population
Project Viva is a prospective pre-birth cohort which recruited women carrying a singleton pregnancy during their initial obstetric care visit at Atrius Harvard Vanguard Medical Associates between 1999 and 2002 in Eastern Massachusetts, USA. Details of the cohort have been described previously (Oken et al., 2015). Briefly, research assistants collected demographic and health history information including race/ethnicity, date of last menstrual period, maternal and paternal height and pre-pregnancy weight, and smoking history via interview and questionnaire. We also provided participants a take-home food frequency questionnaire (FFQ) at the first trimester (~10 weeks of gestation) and second trimester (~26-28 weeks of gestation) study visits. Of 2128 women who delivered a live-bom infant, 1777 completed a first-trimester FFQ and 1666 completed a second-trimester FFQ. Compared to women who completed the first trimester FFQ, women who did not complete it were younger (mean 29.7 vs. 32.2 years), comprised a higher proportion of blacks (38.0% vs. 12.3%), never smokers (73.7% vs. 67.4%), had a higher mean pre-pregnancy BMI (26.3 vs. 24.6 kg/m2) and were less educated (40.4% vs. 69.4% with at least college degree). However, they had similar maternal and paternal height. Institutional review boards of Harvard Pilgrim Health Care, Brigham and Women’s Hospital, and Beth Israel Deaconess Medical Center approved the study protocols and all mothers provided written informed consent.
2.2. Dietary Assessment
We assessed diet using a 140-item, self-administered FFQ based on a well-validated FFQ used in other cohorts (Yuan et al., 2017a; Yuan et al., 2017b) and adapted for use among pregnant women (Fawzi et al., 2004). The first-trimester FFQ assessed diet intake since the last menstrual period and was completed by participants at enrollment. The second-trimester FFQ was self-completed at 26-28 weeks of gestation and assessed diet “during the past 3 months.”
We used the FFQ to determine FV intake for each participant. We also used the FFQ to assign each participant to two dietary pattern scores derived by principal component factor analysis to summarize overall food choices (except for FVs) (Lange et al., 2010). The factor scores were standardized to have a mean of 0 and standard deviation of 1, with higher scores indicating higher adherence to Prudent (more poultry, fish, whole grains) or Western (more red meat, processed meat, refined grains, and desserts) dietary patterns.
2.3. Pesticide Residue Assessment
FVs were classified as having high versus low pesticide residues using the Pesticide Residue Burden Score (PRBS), a scoring system used to assess pesticide residue status in FVs. The PRBS has been described in greater details elsewhere (Chiu et al., 2015; Y. H. Chiu et al., 2017). Briefly, the PRBS method couples the information from FFQ and pesticide residue surveillance data from US Department of Agriculture Pesticide Data Program (PDP) (USDA, 2013) and has been validated against urinary pesticide metabolites in two cohorts (Y. H. Chiu et al., 2017; Hu et al., 2016). Specifically, we ranked the 36 FVs included in the FFQ according to each of the three contamination measures from Pesticide Data Program: (1) the percentage of samples with any detectable pesticide residues; (2) the percentage of samples with any pesticide residues above the tolerances; and (3) the percentage of samples with three or more different detectable pesticides. For each contamination measure, we assigned a score of 0 for FVs in the lowest tertile, 1 for FVs in the middle tertile, and 2 for FVs in the upper tertile. The PRBS for each FV was the sum of tertile scores across the three measures on a scale of 0 to 6. We classified FVs with a PRBS ≥4 as having high pesticide residues and those with a PRBS <4 as having low pesticide residues (Supplemental Table S1). We summed the intake of high and low pesticide residue FVs, separately, for each participant. Of note, PDP data from 1999 to 2002 only cover 24 items in the FFQ. In order to maximize the available pesticide residue data for nearly all the FV items assessed in the FFQ, we created the PRBS using the PDP data from 1992 to 2013. Among the 24 items with overlap, the spearman correlation of the PRBS based on PDP data from 1992 to 2013 and that based on PDP data from 1999 to 2002 was 0.92.
2.4. Ascertainment of outcomes
We obtained infant birth weight (gram) and delivery date from hospital medical records. We calculated gestational age at delivery in weeks by subtracting the date of the last menstrual period (LMP) from the date of delivery. For women whose prenatal ultrasound (performed at 16-20 weeks of gestation) estimate differed from the LMP estimate by >10 days (~9%), we calculated gestational age at delivery based on the ultrasound results. We defined preterm birth as birth at <37 completed weeks of gestation. We used sex-specific birth-weight-for-gestational age z-scores (an index as fetal growth) from 1999-2000 US national reference data (Oken et al., 2003). We defined small-for-gestational-age (SGA) and large-for-gestational-age (LGA) as sex-specific birth-weight-for-gestational-age z-score below the 10th percentile and greater than or equal to the 90th percentile, respectively.
2.5. Statistical analysis
We classified women into quintiles of high and low pesticide residue FV intake. We fit multivariable logistic regression models for preterm (versus term) birth, and multinomial logistic regression models for SGA and LGA using appropriate-for-gestational-age as the reference group. Given that race/ethnicity may serve as an indicator for different susceptibility to pesticides (white more susceptible than non-white)(Chen et al., 2003), we specified in advance that we would test for possible effect modification by race/ethnicity. For case in which there was effect modification by race/ethnicity at P-values <0.05, we presented the separate estimates for white and non-white mothers (including Hispanic or Latina, African American, Asian or Pacific Islander, and others).
We selected covariates based on prior knowledge through the use of directed acyclic graphs. All models were adjusted for maternal age (years), pre-pregnancy body mass index (BMI, kg/m2), height (meters), smoking status (smoked during pregnancy, former, or never), education level (with or without college degree), annual household income (≤ $70,000 or >$70,000), married or cohabitating (yes or no), total energy intake (kcal/day), Prudent and Western dietary pattern scores, season of dietary assessment (spring, summer, fall, or winter), and paternal height (meters). As high and low pesticide FV intake may confound each other, we additionally adjusted for low pesticide FV intake in the models of high pesticide FV intake, and vice versa. Because pesticide regulation may change across years, we additionally adjusted for calendar year of delivery in the sensitivity analysis. We conducted tests for trend using the median intake of FV in each quintile as a continuous variable in the regression model.
Approximately 8% of participants had missing data on one (n=140) or two (n=5) covariates. We employed multiple imputations to impute the missing values of covariates using 50 imputed datasets. We combined the estimates of multivariable modeling results using Proc MI ANALYZE. We performed all statistical analyses using SAS v9.4 (SAS Institute, Cary, N.C.). Two-sided P-values <0.05 were considered statistically significant. To account for multiple testing, we also compared the P-values to Bonferroni corrected level of significance (P-value < 2.8×10−3).
3. Results
The baseline characteristics of the 1777 mother-child pairs are shown in Table 1. Most participants were white (72%), followed by black (12%), Hispanic or Latina (7%) and Asian (6%) (Table 1). Of 1777 infants, 5.5% were SGA, 13.7% were LGA, and 7.3% were preterm. Infants born to the 502 non-white mothers were more likely to be preterm (11.4% vs. 5.7%) or SGA (9.2% vs. 4.1%), and less likely LGA (9.4% vs. 15.4%) than infants born to white mothers. Among 1543 mothers with both first- and second-trimester FFQs, intakes of FVs in the first and second trimester were positively correlated (rspearman = 0.69 for high pesticide FVs, and rspearman = 0.60 for low pesticide FVs). High and low pesticide residue FV intakes were also positively correlated to each other (rspearman = 0.54 for the first trimester and 0.54 for the second trimester). On average, consumption of high pesticide residue FVs was similar between white and nonwhite mothers (mean(SD): 2.4 (1.3) vs. 2.3 (1.7) servings/day for white vs. non-white), while consumption of low pesticide residues FVs was lower for white mothers compared to non-white mothers (mean(SD): 3.0 (1.5) vs. 3.4 (2.1) servings/day for white vs. non-white).
Table 1.
Characteristics of 1777 pregnant women and their infants in Project Viva
| Total (n=1777) | White (n=1275) | Non-white (n=502) | |
|---|---|---|---|
| Mean (SD) | |||
| Maternal characteristics | |||
| First trimester | |||
| High pesticide FV intake, servings/d | 2.3 (1.4) | 2.4 (1.3) | 2.3 (1.7) |
| Low pesticide FV intake, servings/d | 3.1 (1.7) | 3.0 (1.5) | 3.4 (2.1) |
| Age, years | 32.2 (4.9) | 32.9 (4.3) | 30.5 (5.9) |
| Gestational age at enrollment, wks | 10.4 (2.5) | 10.4 (2.4) | 10.6 (2.8) |
| Pre-pregnancy BMI, kg/m2 | 24.6 (5.3) | 24.2 (4.9) | 25.6 (5.9) |
| Total energy intake, kcal/day | 2060 (673) | 2058 (592) | 2066 (844) |
| Prudent dietary pattern | −0.0 (1.0) | 0.2 (0.9) | −0.5 (1.0) |
| Western dietary pattern | −0.0 (1.0) | −0.1 (0.9) | 0.1 (1.2) |
| N (%) | |||
| Race/ethnicity | |||
| . Non-Hispanic black | 219 (12.3) | ||
| . Hispanic or Latino | 115 (6.5) | ||
| . Asian | 103 (5.8) | ||
| . Non-Hispanic White | 1275 (71.8) | ||
| . Other | 65 (3.7) | ||
| Married or cohabitating | |||
| . No | 119 (6.7) | 35 (2.7) | 84 (16.7) |
| . Yes | 1658 (93.3) | 1240 (97.3) | 418 (83.3) |
| College graduate or higher | |||
| . No | 544 (30.6) | 300 (23.5) | 244 (48.6) |
| . Yes | 1233 (69.4) | 975 (76.5) | 258 (51.4) |
| Annual household income >$70,000/y | |||
| . No | 671 (37.7) | 377 (29.6) | 294 (58.5) |
| . Yes | 1106 (62.3) | 898 (70.4) | 208 (41.5) |
| Nulliparous | |||
| . No | 904 (50.9) | 632 (49.6) | 272 (54.2) |
| . Yes | 873 (49.1) | 643 (50.4) | 230 (45.8) |
| Smoking status | |||
| . Never | 1198 (67.4) | 813 (63.8) | 385 (76.7) |
| . Former | 383 (21.6) | 331 (25.9) | 53 (10.5) |
| . During pregnancy | 195 (11.0) | 131 (10.3) | 64 (12.8) |
| Height | |||
| . <1.6 m | 311 (17.5) | 188 (14.7) | 123 (24.5) |
| . 1.6 - <1.7 m | 947 (53.3) | 671 (52.6) | 276 (55.0) |
| . ≥1.7 m | 519 (29.2) | 416 (32.6) | 103 (20.5) |
| Paternal height | |||
| . <1.7 m | 162 (9.1) | 68 (5.4) | 94 (18.7) |
| . 1.7 -<1.8 m | 719 (40.5) | 512 (40.2) | 207 (41.3) |
| . ≥1.8 m | 895 (50.4) | 695 (54.5) | 201 (40.0) |
| Child characteristics | |||
| Sex | |||
| . Male | 893 (50.3) | 639 (50.1) | 254 (50.6) |
| . Female | 884 (49.7) | 636 (49.9) | 248 (49.4) |
| Preterm (<37 weeks) | |||
| . No | 1647 (92.7) | 1202 (94.3) | 445 (88.6) |
| . Yes | 130 (7.3) | 73 (5.7) | 57 (11.4) |
| Birth weight for gestational age and sex | |||
| . Small for gestational age (<10th %tile) | 98 (5.5) | 52 (4.1) | 46 (9.2) |
| . Average for gestational age (10th - <90th %tile) | 1436 (80.8) | 1027 (80.5) | 409 (81.5) |
| . Large for gestational age (≥90th %tile) | 243 (13.7) | 196 (15.4) | 47 (9.4) |
| Season of birth | |||
| . Spring | 475 (26.7) | 128 (25.5) | 276 (21.7) |
| . Summer | 483 (27.2) | 126 (25.1) | 347 (27.2) |
| . Fall | 394 (22.2) | 118 (23.5) | 357 (28.0) |
| . Winter | 425 (23.9) | 130 (25.9) | 295 (23.1) |
| Mean (SD) | |||
| Birth weight for gestational age z-score | 0.19 (0.95) | 0.28 (0.94) | −0.03 (0.96) |
| Gestational age at birth, weeks | 39.5 (1.9) | 39.6 (1.7) | 39.0 (2.3) |
First trimester intake of high or low pesticide residue FVs was not associated with risks of SGA, LGA, or preterm birth in both unadjusted or adjusted models (Table 2). In addition, the associations of first trimester high pesticide FV intake with SGA and with LGA were not modified by race/ethnicity (P-values for interaction =0.17 and 0.63, respectively). However, there was heterogeneity in the relationship of first trimester high pesticide FV intake with risk of preterm birth by race/ethnicity (P-value for interaction=0.01) (Figure 1). Specifically, among white mothers, the adjusted odds ratios (ORs) of delivering a preterm infant were 1.14 (95% confidence interval (CI), 0.45 to 2.89), 1.47 (95%CI, 0.59 to 3.65), 2.03 (95%CI, 0.81 to 5.08) and 1.70 (95%CI, 0.62 to 4.67) for women in increasing quintiles of high pesticide residue FV intake as compared to the lowest quintile (P, trend=0.25). In contrast, among non-white mothers, the corresponding ORs of delivering a preterm infant were 1.63 (95%CI, 0.74 to 3.60), 0.76 (95%CI, 0.29 to 1.97), 1.18 (95%CI, 0.41 to 3.40) and 0.64 (95%CI, 0.18 to 2.27), respectively (P, trend=0.42).
Table 2.
Associations of high and low pesticide fruit and vegetable intake during first trimester with SGA, LGA, and preterm birth in 1777 mother-infant pairs participating in Project Viva.
| Quintiles of high pesticide FV intake | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P, trend1 | |
| Range of intake, serving/d | 0.1-1.2 | 1.2-1.8 | 1.8-2.4 | 2.4-3.3 | 3.3-13.2 | |
| SGA | ||||||
| Case/N | 24/365 | 20/347 | 23/364 | 12/347 | 19/354 | |
| Unadjusted OR | 1.00 (reference) | 0.91 (0.49, 1.70) | 1.01 (0.54, 1.90) | 0.54 (0.25, 1.16) | 0.82 (0.40, 1.68) | 0.36 |
| Adjusted OR2 | 1.00 (reference) | 0.98 (0.51, 1.88) | 1.20 (0.62, 2.33) | 0.72 (0.32, 1.60) | 1.27 (0.57, 2.86) | 0.74 |
| LGA | ||||||
| Case/N | 45/365 | 47/347 | 52/364 | 51/347 | 48/354 | |
| Unadjusted OR | 1.00 (reference) | 1.05 (0.67, 1.64) | 1.11 (0.70, 1.75) | 1.09 (0.68, 1.75) | 1.08 (0.66, 1.77) | 0.72 |
| Adjusted OR2 | 1.00 (reference) | 1.16 (0.73, 1.86) | 1.11 (0.69, 1.80) | 1.24 (0.75, 2.04) | 1.14 (0.65, 2.01) | 0.63 |
| Preterm | ||||||
| Case/N | 26/365 | 27/347 | 24/364 | 28/347 | 25/354 | |
| Unadjusted OR | 1.00 (reference) | 1.17 (0.66, 2.06) | 0.99 (0.54, 1.82) | 1.26 (0.69, 2.30) | 1.03 (0.54, 1.98) | 0.96 |
| Adjusted OR2 | 1.00 (reference) | 1.14 (0.64, 2.04) | 0.99 (0.53, 1.85) | 1.28 (0.67, 2.41) | 1.01 (0.49, 2.10) | 0.96 |
| Quintiles of low pesticide FV intake | ||||||
| Range of intake, serving/d | 0.3-1.7 | 1.7-2.5 | 2.5-3.2 | 3.2-4.3 | 4.3-13.4 | |
| SGA | ||||||
| Case/N | 24/351 | 19/366 | 17/354 | 18/352 | 20/354 | |
| Unadjusted OR | 1.00 (reference) | 0.79 (0.42, 1.49) | 0.78 (0.40, 1.53) | 0.90 (0.45, 1.79) | 0.95 (0.46, 1.93) | 0.95 |
| Adjusted OR2 | 1.00 (reference) | 0.89 (0.46, 1.70) | 0.93 (0.46, 1.85) | 1.12 (0.54, 2.31) | 1.23 (0.56, 2.70) | 0.50 |
| LGA | ||||||
| Case/N | 43/351 | 47/366 | 51/354 | 60/352 | 42/354 | |
| Unadjusted OR | 1.00 (reference) | 1.02 (0.65, 1.60) | 1.14 (0.72, 1.81) | 1.39 (0.88, 2.21) | 0.91 (0.55, 1.52) | 0.99 |
| Adjusted OR2 | 1.00 (reference) | 0.88 (0.55, 1.41) | 1.03 (0.64, 1.66) | 1.24 (0.76, 2.03) | 0.81 (0.46, 1.43) | 0.78 |
| Preterm | ||||||
| Case/N | 29/351 | 26/366 | 25/354 | 21/352 | 29/354 | |
| Unadjusted OR | 1.00 (reference) | 0.83 (0.48, 1.46) | 0.81 (0.45, 1.46) | 0.68 (0.36, 1.27) | 0.97 (0.52, 1.81) | 0.93 |
| Adjusted OR2 | 1.00 (reference) | 0.84 (0.48, 1.49) | 0.80 (0.44, 1.46) | 0.67 (0.35, 1.29) | 0.88 (0.44, 1.75) | 0.69 |
Abbreviations: FV, fruit and vegetable; LGA, large-for-gestational-age; N, number of participants; SGA, small-for-gestational-age; OR, odds ratio.
P, trend was calculated using a trend test with median intake in each quintile as a continuous variable in the model.
The models were adjusted for maternal age, pre-pregnancy BMI, smoked during pregnancy, marital/ cohabitating status, parity, household income, education, dietary patterns, total energy intake, height and paternal height, season of diet assessment, and low pesticide fruit and vegetable intake.
Figure 1.
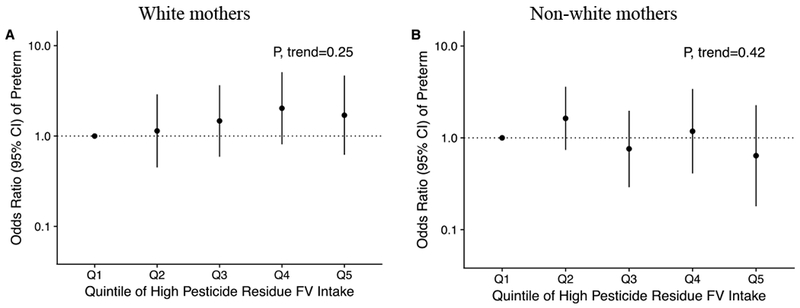
Associations of high pesticide residue fruit and vegetable intake during the first trimester with preterm birth in infants of 1275 white mothers and 502 non-white mothers participating in Project Viva. The models were adjusted for maternal age, pre-pregnancy BMI, smoked during pregnancy, marital/cohabitating status, parity, household income, education, dietary patterns, total energy intake, season of diet assessment, height and paternal height, and first trimester low pesticide fruit and vegetable intake. P-value for interaction=0.01. Quintile 1 was used as the reference group. Abbreviations: FV, fruit and vegetable; OR, odd ratios.
We also examined risks of SGA, LGA, and preterm birth according to high and low pesticide residue FV intake during the second trimester (Table 3). Overall, second trimester intake of high or low pesticide FV intake was not associated with SGA, LGA or preterm birth in unadjusted or adjusted models. In addition, there was no evidence of heterogeneity in the relationship between second trimester high pesticide FV intake and birth outcomes by race/ethnicity (P-values for interaction all >0.20). The results remained unchanged when additionally adjusted for calendar year of delivery (data not shown).
Table 3.
Associations of high and low pesticide fruit and vegetable intake during second trimester with SGA, LGA, and preterm birth in 1666 mother-infant pairs participating in Project Viva.
| Quintiles of high pesticide FV intake | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P, trend1 | |
| Range of intake, serving/d | 0.1-1.2 | 1.2-1.8 | 1.9-2.5 | 2.5-3.4 | 3.4-13.5 | |
| SGA | ||||||
| Case/N | 17/331 | 21/329 | 21/338 | 13/333 | 21/335 | |
| Unadjusted OR | 1.00 (reference) | 1.25 (0.64, 2.45) | 1.15 (0.57, 2.32) | 0.68 (0.31, 1.51) | 1.04 (0.49, 2.20) | 0.70 |
| Adjusted OR2 | 1.00 (reference) | 1.29 (0.65, 2.59) | 1.29 (0.62, 2.68) | 0.73 (0.32, 1.68) | 1.11 (0.48, 2.54) | 0.80 |
| LGA | ||||||
| Case/N | 40/331 | 44/329 | 46/338 | 51/333 | 43/335 | |
| Unadjusted OR | 1.00 (reference) | 1.04 (0.65, 1.66) | 0.98 (0.61, 1.58) | 1.06 (0.65, 1.71) | 0.90 (0.54, 1.50) | 0.80 |
| Adjusted OR | 1.00 (reference) | 0.96 (0.59, 1.57) | 0.85 (0.51, 1.42) | 0.92 (0.55, 1.54) | 0.78 (0.44, 1.39) | 0.50 |
| Preterm | ||||||
| Case/N | 25/331 | 21/329 | 19/338 | 19/333 | 19/335 | |
| Unadjusted OR | 1.00 (reference) | 0.89 (0.48, 1.64) | 0.79 (0.41, 1.52) | 0.75 (0.38, 1.48) | 0.65 (0.32, 1.33) | 0.17 |
| Adjusted OR2 | 1.00 (reference) | 0.82 (0.44, 1.56) | 0.77 (0.39, 1.54) | 0.70 (0.34, 1.45) | 0.60 (0.27, 1.34) | 0.19 |
| Quintiles of low pesticide FV intake | ||||||
| Range of intake, serving/d | 0.1-1.8 | 1.8-2.5 | 2.5-3.3 | 3.3-4.4 | 4.4-13.5 | |
| SGA | ||||||
| Case/N | 19/342 | 15/323 | 16/331 | 22/336 | 21/334 | |
| Unadjusted OR | 1.00 (reference) | 0.85 (0.42, 1.74) | 1 (0.49, 2.04) | 1.36 (0.68, 2.72) | 1.29 (0.62, 2.70) | 0.30 |
| Adjusted OR2 | 1.00 (reference) | 0.83 (0.41, 1.72) | 1.05 (0.50, 2.18) | 1.51 (0.73, 3.11) | 1.53 (0.69, 3.41) | 0.14 |
| LGA | ||||||
| Case/N | 33/342 | 39/323 | 59/331 | 49/336 | 44/334 | |
| Unadjusted OR | 1.00 (reference) | 1.27 (0.77, 2.11) | 2.04 (1.26, 3.29) | 1.66 (1.00, 2.75) | 1.50 (0.88, 2.56) | 0.19 |
| Adjusted OR2 | 1.00 (reference) | 1.20 (0.71, 2.02) | 1.82 (1.10, 3.00) | 1.27 (0.74, 2.19) | 0.96 (0.53, 1.76) | 0.61 |
| Preterm | ||||||
| Case/N | 28/342 | 16/323 | 14/331 | 18/336 | 27/334 | |
| Unadjusted OR | 1.00 (reference) | 0.62 (0.33, 1.19) | 0.55 (0.28, 1.10) | 0.74 (0.38, 1.44) | 1.22 (0.64, 2.33) | 0.28 |
| Adjusted OR2 | 1.00 (reference) | 0.59 (0.30, 1.13) | 0.53 (0.26, 1.06) | 0.68 (0.34, 1.37) | 1.13 (0.55, 2.32) | 0.44 |
Abbreviations: FV, fruit and vegetable; LGA, large-for-gestational-age; N, number of participants; SGA, small-for-gestational-age; OR, odd ratios.
P, trend was calculated using a trend test with median intake in each quintile as a continuous variable in the model.
The models were adjusted for maternal age, pre-pregnancy BMI, smoked during pregnancy, marital/ cohabitating status, parity, household income, education, dietary patterns, total energy intake, height and paternal height, season of diet assessment, and low pesticide fruit and vegetable intake.
4. Discussion
We evaluated associations of high and low pesticide residue FV intake during pregnancy with risks of SGA, LGA, and preterm delivery in a pre-birth cohort in Massachusetts. Overall, intake of high or low pesticide residue FVs, regardless of timing during the first or second trimesters, was not associated with birth outcomes (i.e. SGA, LGA, and preterm birth). While we found some heterogeneity in the relationship of first trimester high pesticide FV intake with risk of preterm birth by race/ethnicity (a non-significant positive trend for white mothers and a non-obvious trend for non-white mothers), the relationship was not robust to correction for multiple comparisons.
FVs are known to benefit health in general (Wang et al., 2014), and they may improve birth outcomes as well. Micronutrients in FVs may protect against restricted growth by improving placental function or reducing risks of hypertension (Ahn et al., 2007; Appel et al., 1997; Bergen et al., 2012; Fall et al., 2009). Epidemiologic studies suggest that a diet rich in FVs was associated with lower risks of SGA (Knudsen et al., 2008; Thompson et al., 2010), higher infant birth weight (Chia et al., 2016; Murphy et al., 2014; Ramon et al., 2009), although some did not find an association with birth outcomes (Coelho Nde et al., 2015). Because FVs can serve as a vehicle of exposure to pesticide residues as well, any potential overall benefit of FV consumption on birth outcomes might be diminished by harmful pesticides that these foods contain (Chiu et al., 2015; Chiu et al., 2016; Chiu et al., 2018). To the best of our knowledge, no previous studies have considered pesticide residue status when examining the associations of maternal FV intake with fetal growth. In spite of the prevalent use of pesticides in modern conventional agriculture (USDA, 2015) and evidence of reduction in pesticide exposure with an organic diet (Bradman et al., 2015; Lu et al., 2006; Oates et al., 2014), little attention has been paid to potential health effects from ingestions of pesticide residues via FVs.
While no studies have assessed the associations of organic food consumption or dietary pesticide exposure with fetal growth, a few have shown that consumption of organically produced food during pregnancy was associated with a lower risk of preeclampsia (Torjusen et al., 2014) and hypospadias (Brantsaeter et al., 2016), and the possible explanation was that consuming organic food may reduce exposure to pesticides. In the literature, there is also much interest regarding the effect of prenatal pesticide exposure on fetal growth. Although the results have been inconsistent, some studies have noted that these associations could be modified by PON1 polymorphisms. For example, in a recent pooled analysis (total sample size~1100) of 4 cohorts (CHAMACOS, HOME, Columbia, and Mount Sinai birth cohorts), Harley et al. showed that overall, there were no associations between maternal urinary total organophosphate pesticide metabolites and birth weight; however, in black women and in infants born with less susceptible PON1 genotype (PON1192RR), prenatal urinary dimethyl organophosphate pesticide concentrations were inversely associated with birth weight and birth length (Harley et al., 2016). On the other hand, a pilot study of 52 mothers and children in Thailand found that higher maternal dialkylphosphate urinary concentrations (collected multiple times during pregnancy) were associated with lower birth weight and shorter gestational age among newborns of mothers with low PON1 activity, and no associations were found for newborns of mothers with high maternal PON1 activity (Naksen et al., 2015). These disparate results between these studies may be attributed to diverse racial/ethnic composition, socio-economic status, differences in timing, routes, levels, and classes of pesticide exposure across studies.
In the present study, we focused on pesticide exposure through the intake of FVs during first and second trimesters in a cohort of women with relatively higher socioeconomic status in Eastern Massachusetts. Overall, there was no clear evidence for an association between high or low pesticide residue FV intake during pregnancy and adverse birth outcomes such as SGA, LGA, and preterm birth. In addition, although there was heterogeneity in the association between high pesticide residue FV intake during the first trimester and preterm birth by race/ethnicity, the significance of the association did not withstand correction for multiple testing. These findings may reflect the absence of an effect or result from potential inherent limitations of the study. For example, while we previously have shown that the PRBS predicts urinary pesticide metabolites and allows adequate characterization of pesticide exposure through diet (Y.-H. Chiu et al., 2017; Hu et al., 2016), exposure misclassification is still possible due to the measurement error in the FFQ as well as poor time-integration between the FFQ (1999-2002) and pesticide assessment (1992-2013) despite the fact that the PRBS is relatively constant across years (as reflected by the high correlations of the PRBS using PDP data from 1992 to 2013 versus that from 1999-2002). Second, PRBS captured overall pesticide exposure instead of targeting a certain pesticide. Thus, we may fail to identify the specific pesticide(s) that are associated with fetal growth and preterm birth. Third, the relationship between pesticide residue exposure and birth outcomes are likely to be modified by PON1 polymorphisms (Harley et al., 2016). While we have tested the interaction by white mothers versus non-white mothers, race/ethnicity may be considered a crude proxy of pesticide susceptibility (Chen et al., 2003; Draganov & La Du, 2004). Because we did not measure PON1 polymorphism, there may be some misclassification of susceptibility and subsequent bias. Also, our participants were recruited at their initial obstetric visit at the median gestational age of 9.9 weeks— at a time beyond the most common window of pregnancy loss (before 9 weeks) (Mumford et al., 2016). Since pesticide exposure may affect pregnancy loss (Arbuckle et al., 2001; Bell et al., 2001; Chiu et al., 2018), survival bias may arise if there were unmeasured common causes for pregnancy loss and birth outcomes. Furthermore, despite our adjustment for a large number of potential confounders, there may be residual bias from residual and unmeasured confounding (e.g. residential use of pesticides). Lastly, fetal size and gestational age, used as markers of fetal growth in this study, are only a snapshot of growth trajectory (Gillman, 2002). Future studies should further evaluate the effect of pesticide exposure on intrauterine growth, postnatal growth, and child development.
Our study also has some strengths, including a prospective study design, a relatively large sample size, and detailed covariate information on many maternal factors that have previously been associated with fetal growth. In addition, the availability of a full-length FFQ during the first trimester and second trimester of pregnancy allowed evaluation of the most susceptible prenatal time period in which an exposure may have a feto- or embyrotoxic effect (Rozman & Klaassen, 2007; Sadler, 2011).
5. Conclusions
In this study, we found that intake of high pesticide residue FVs, regardless of trimester of pregnancy, was not associated with SGA, LGA, or preterm birth. We also found no associations between low pesticide FV intake during pregnancy and these outcomes. Given the paucity of data on this topic and the inherent limitations acknowledged earlier, further understanding of prenatal dietary pesticide exposure on birth and later childhood outcomes could result from confirmation using repeated measures of exposure biomarkers at the relevant time window (e.g, preconception periods, early, and late pregnancy), incorporating genotyping data, and collecting detailed covariate information (e.g., organic food consumption frequency, occupation, and residential pesticide exposure history) in future studies.
Supplementary Material
Highlights:
Little is known about whether intake of pesticide residues from fruits and vegetables during pregnancy may have adverse health effects on offspring.
Neither intake of high or low pesticide residue fruits and vegetables during pregnancy was associated with preterm birth or fetal growth.
More studies are needed to further investigate the impact of prenatal pesticide exposure on birth and later childhood outcomes.
Acknowledgements
We thank the participants and staff of Project Viva. This work was supported by grants from the National Institutes of Health (grant R01 HD 034568, K24HD069408, UG3OD023286, P30DK046200, and K23ES024803).
Abbreviations:
- BMI—
body mass index
- CI—
confidence interval
- EPA—
Environmental Protection Agency
- FFQ—
food frequency questionnaire
- FV—
fruit and vegetable
- SGA—
small for gestational age
- LGA—
large for gestational age
- PON1—
paraoxonase 1
- PRBS—
Pesticide Residue Burden Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interests
None of the authors declares any actual or potential competing financial interests. The findings and conclusions in this report are those of authors and do not necessarily represent the official position of the National Institutes of Health.
Declarations of interests: None. The views expressed in this article do not necessarily represent the views of the US Government, the Department of Health and Human Services or the National Institutes of Health.
References
- Ahn YM, Kim YJ, Park H, Park B, & Lee H (2007). Prenatal vitamin C status is associated with placental apoptosis in normal-term human pregnancies. Placenta, 28(1), 31–38. doi: 10.1016/j.placenta.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, … Karanja N (1997). A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med, 336(16), 1117–1124. doi: 10.1056/nejm199704173361601 [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Lin Z, & Mery LS (2001). An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ Health Perspect, 109(8), 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EM, Hertz-Picciotto I, & Beaumont JJ (2001). A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology, 12(2), 148–156. [DOI] [PubMed] [Google Scholar]
- Bergen NE, Jaddoe VW, Timmermans S, Hofman A, Lindemans J, Russcher H, … Steegers EA (2012). Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG, 119(6), 739–751. doi: 10.1111/j.1471-0528.2012.03321.x [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, … Wolff MS (2004). In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect, 112(3), 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, & Eskenazi B (2003). Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect, 777(14), 1779–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Quiros-Alcala L, Castorina R, Schall RA, Camacho J, Holland NT, … Eskenazi B (2015). Effect of Organic Diet Intervention on Pesticide Exposures in Young Children Living in Low-Income Urban and Agricultural Communities. Environ Health Perspect, 723(10), 1086–1093. doi: 10.1289/ehp.1408660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsaeter AL, Torjusen H, Meltzer HM, Papadopoulou E, Hoppin JA, Alexander J, … Haugen M (2016). Organic Food Consumption during Pregnancy and Hypospadias and Cryptorchidism at Birth: The Norwegian Mother and Child Cohort Study (MoBa). Environ Health Perspect, 724(3), 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kumar M, Chan W, Berkowitz G, & Wetmur JG (2003). Increased influence of genetic variation on PON1 activity in neonates. Environ Health Perspect, 777(11), 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia AR, de Seymour JV, Colega M, Chen LW, Chan YH, Aris IM, … Chong MF (2016). A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr, 704(5), 1416–1423. doi: 10.3945/ajcn.116.133892 [DOI] [PubMed] [Google Scholar]
- Chiu Y-H, Williams PL, Minguez-Alarcon L, Gillman MW, Sun Q, Ospina M, … Chavarro JE (2017). Comparison of questionnaire-based estimation of pesticide residue intake from fruits and vegetables with urinary concentrations of pesticide metabolites Accepted in the Journal of Exposure Science and Environmental Epidemiology in July 2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, … Chavarro JE (2015). Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod, 30(6), 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Levine H, … Chavarro JE (2016). Intake of Fruits and Vegetables with Low-to-Moderate Pesticide Residues Is Positively Associated with Semen-Quality Parameters among Young Healthy Men. J Nutr, 746(5), 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Williams PL, Gillman MW, Gaskins AJ, Minguez-Alarcon L, Souter I, … Chavarro JE (2018). Association Between Pesticide Residue Intake From Consumption of Fruits and Vegetables and Pregnancy Outcomes Among Women Undergoing Infertility Treatment With Assisted Reproductive Technology. JAMA Intern Med, 778(1), 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Williams PL, Minguez-Alarcon L, Gillman M, Sun Q, Ospina M, … Chavarro JE (2017). Comparison of questionnaire-based estimation of pesticide residue intake from fruits and vegetables with urinary concentrations of pesticide biomarkers. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho Nde L, Cunha DB, Esteves AP, Lacerda EM, & Theme Filha MM (2015). Dietary patterns in pregnancy and birth weight. Rev Saude Publica, 49, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Cole TB, Marsillach J, & Furlong CE (2013). Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology, 307, 115–122. doi: 10.1016/j.tox.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganov DI, & La Du BN (2004). Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol, 369(1), 78–88. doi: 10.1007/s00210-003-0833-1 [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, … Holland NT (2004). Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect, 112(10), 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall CH, Fisher DJ, Osmond C, & Margetts BM (2009). Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull, 30(4 Suppl), S533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, & Gillman MW (2004). Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol, 14(10), 754–762. doi: 10.1016/j.annepidem.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Frankel S, Elwood P, Sweetnam P, Yarnell J, & Smith GD (1996). Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet, 348(9040), 1478–1480. doi: 10.1016/s0140-6736(96)03482-4 [DOI] [PubMed] [Google Scholar]
- Gillman MW (2002). Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol, 31(2), 294–299. [PubMed] [Google Scholar]
- Harley KG, Engel SM, Vedar MG, Eskenazi B, Whyatt RM, Lanphear BP, … Wolff MS (2016). Prenatal Exposure to Organophosphorous Pesticides and Fetal Growth: Pooled Results from Four Longitudinal Birth Cohort Studies. Environ Health Perspect, 124(7), 1084–1092. doi: 10.1289/ehp.1409362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Huen K, Aguilar Schall R, Holland NT, Bradman A, Barr DB, & Eskenazi B (2011). Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican-American women. PLoS One, 6(8), e23923. doi: 10.1371/journal.pone.0023923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chiu Y-H, Hauser R, Chavarro J, & Sun Q (2016). Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: A validation study. Environ Int, 92, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley RR, Shiell AW, & Law CM (2000). The role of size at birth and postnatal catchup growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens, 18(7), 815–831. [DOI] [PubMed] [Google Scholar]
- Irving RJ, Belton NR, Elton RA, & Walker BR (2000). Adult cardiovascular risk factors in premature babies. Lancet, 355(9221), 2135–2136. doi: 10.1016/s0140-6736(00)02384-9 [DOI] [PubMed] [Google Scholar]
- Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, & Olsen SF (2008). Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr, 62(4), 463–470. doi: 10.1038/sj.ejcn.1602745 [DOI] [PubMed] [Google Scholar]
- Lange NE, Rifas-Shiman SL, Camargo CA Jr., Gold DR, Gillman MW, & Litonjua AA (2010). Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J Allergy Clin Immunol, 126(2), 250–255, 255.e251–254. doi: 10.1016/j.jaci.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, & Bravo R (2006). Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect, 114(2), 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, … Neuhouser ML (2016). The 2015 Dietary Guidelines Advisory Committee scientific report: development and major conclusions. Advances in Nutrition: An International Review Journal, 7(3), 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, … Lichtenstein AH (2016). The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv Nutr, 7(3), 438–444. doi: 10.3945/an.116.012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Silver RM, Sjaarda LA, Wactawski-Wende J, Townsend JM, Lynch AM, … Schisterman EF (2016). Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod, 57(3), 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Stettler N, Smith KM, & Reiss R (2014). Associations of consumption of fruits and vegetables during pregnancy with infant birth weight or small for gestational age births: a systematic review of the literature. Int J Womens Health, 6, 899–912. doi: 10.2147/ijwh.s67130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavomyutikam P, Srinual N, … Barr DB (2015). Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort, Thailand. Environ Res, 142, 288–296. doi: 10.1016/j.envres.2015.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, & Law CM (2003). Is birth weight related to later glucose and insulin metabolism?—A systematic review. Diabet Med, 20(5), 339–348. [DOI] [PubMed] [Google Scholar]
- Oates L, Cohen M, Braun L, Schembri A, & Taskova R (2014). Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res, 132, 105–111. doi: 10.1016/j.envres.2014.03.021 [DOI] [PubMed] [Google Scholar]
- Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, … Gillman MW (2015). Cohort profile: project viva. Int J Epidemiol, 44(1), 37–48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, & Gillman MW (2003). A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr, 3, 6. doi: 10.1186/1471-2431-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon R, Ballester F, Iniguez C, Rebagliato M, Murcia M, Esplugues A, … Vioque J (2009). Vegetable but not fruit intake during pregnancy is associated with newborn anthropometric measures. J Nutr, 139(3), 561–567. doi: 10.3945/jn.108.095596 [DOI] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano AM, … Lanphear BP (2012). Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ Health Perspect, 120(1), 1055–1060. doi: 10.1289/ehp.1104615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, … Hennekens CH (1997). Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Bmj, 375(7105), 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotteveel J, van Weissenbruch MM, Twisk JW, & Delemarre-Van de Waal HA (2008). Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely bom young adults. Pediatrics, 122(2), 313–321. doi: 10.1542/peds.2007-2012 [DOI] [PubMed] [Google Scholar]
- Rozman KK, & Klaassen CD (2007). Casarett and Doull’s toxicology: the basic science of poisons. p421–423: McGraw-Hill, New York. [Google Scholar]
- Sadler TW (2011). Lanman’s medical embryology. Lippincott Williams & Wilkins. [Google Scholar]
- Thompson JM, Wall C, Becroft DM, Robinson E, Wild CJ, & Mitchell EA (2010). Maternal dietary patterns in pregnancy and the association with small-for-gestational-age infants. Br J Nutr, 103(11), 1665–1673. doi: 10.1017/s0007114509993606 [DOI] [PubMed] [Google Scholar]
- Torjusen H, Brantsaeter AL, Haugen M, Alexander J, Bakketeig LS, Lieblein G, … Meltzer HM (2014). Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian Mother and Child Cohort Study. BMJ Open, 4(9), e006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. (2013). US Department of Agriculture pesticide data program (PDP), annual summary. URL: http://www.ams.usda.gov/AMSv1.0/PDP USDA, Agricultural Marketing Service. [Google Scholar]
- USDA. (2015). Pesticide Data Program (PDP), annual sumary. URL: https://www.ams.usda.gov/datasets/pdp Agricultural Marketing Service [Google Scholar]
- Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, & Hu FB (2014). Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Bmj, 349, g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, … Perera FP (2004). Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect, 112(10), 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, … Willett WC (2017a). Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol doi: 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, … Willett WC (2017b). Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol, 185(7), 570–584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


