Abstract
Perfluorobutanesulfonic acid (PFBS) is used as the replacement of perfluorooctanesulfonic acid (PFOS) since 2000 because of the concern on PFOS’ persistence in the environment and the bioaccumulation in animals. Accumulating evidence has shown the correlation between the exposure to perfluorinated compounds and enhanced adipogenesis. There is no report, however, of the effect of PFBS on adipogenesis. Therefore, the present work aimed to investigate the role of PFBS in adipogenesis using 3T3-L1 adipocytes. PFBS treatment for 6 days extensively promoted the differentiation of 3T3-L1 preadipocytes to adipocytes, resulting in significantly increased triglyceride levels. In particular, the treatments of PFBS at the early adipogenic differentiation period (day 0-2) were positively correlated with increased the triglyceride accumulation on day 6. PFBS treatments significantly increased the protein and mRNA levels of the master transcription factors in adipocyte differentiation; CCAAT/enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor gamma (PPARγ), along with acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), the key proteins in lipogenesis. PFBS significantly activated the phosphorylation of extracellular signal-regulated kinase1/2 (ERK1/2) after 4-hour treatment, and PFBS' effect on triglyceride was abolished by U0126, a specific MAPK/ERK kinase (MEK) inhibitor. In conclusion, PFBS increased the adipogenesis of 3T3-L1 adipocytes, in part, via MEK/ERK-dependent pathway.
Keywords: PFBS, 3T3-L1 preadipocyte, differentiation, PFAS, ERK
Introduction
Per- and polyfluoroalkyl substances (PFASs) are a large group of surface-active compounds. PFASs, such as perfluorooctanesulfonic acid (PFOS), were extensively used as water, oil, and stain repellents in food packaging, non-stick cookware, and textiles, for over 50 years. These ubiquitous environmental contaminants are highly persistent in the environment, and bioaccumulate in living organisms, posing a growing concern for potential adverse effects on human health (Lau et al. 2004, Joensen et al. 2009, Pérez et al. 2013). Accumulating evidence reveals a strong correlation between exposure to PFASs and the increased risks for metabolic syndromes, including obesity (Halldorsson et al. 2012, Maisonet et al. 2012, Braun et al. 2016, Mora et al. 2017, Liu et al. 2018).
As a replacement for PFOS, its four-carbon cognate, perfluorobutanesulfonic acid (PFBS), is widely used due to its shorter biological half-life in humans of ~1 month compared to that of 5 years for PFOS (Olsen et al. 2007, Olsen et al. 2009). PFBS is also generated as the final degradation product from some of the perfluorobutanesulfonyl fluoride-based chemicals (D'eon et al. 2006). After production and usage for over 15 years, PFBS has become one of the major perfluorinated environmental contaminants (Skutlarek et al. 2006, Zhou et al. 2013). PFBS has been detected in human populations, with increasing concentrations seen in humans from 2006 to 2010 (Glynn et al. 2012). Similar to PFOS, PFBS was found to distribute to fat tissue, although the potential adverse effects are largely unknown and understudied (Bogdanska et al. 2014).
Previously, PFASs, particularly PFOS and perfluorooctanoic acid (PFOA), were reported to promote adipogenesis in 3T3-L1 adipocytes with increased expression of adipogenic genes, including CCAAT/enhancer-binding protein alpha (C/EBPα), peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid binding protein 4 (FABP4) and lipoprotein lipase (LPL) (Watkins et al. 2015, Yamamoto et al. 2015). Further, evidence from an animal study revealed that PFOS administration to mice induced adipogenic gene expression and activated nuclear factor erythroid 2-related factor 2 (Nrf2) signaling in epididymal white adipose tissue (Xu et al. 2016). PFASs have also been reported to activate the peroxisome proliferator-activated receptors (PPARs), PPARα and PPARγ, both in hepatocytes and adipocytes, which play key roles in lipid metabolism (Vanden Heuvel et al. 2006, Rosen et al. 2008, Zhang et al. 2014). Moreover, higher plasma concentration of PFASs was associated with the risk of weight gain in human (Liu et al. 2018). The effects of PFBS on adipogenesis, however, have not been investigated. Therefore, we aimed to examine the effects of PFBS exposure on adipogenesis using 3T3-L1 adipocytes.
Materials and Methods
Materials
3T3-L1 preadipocytes were obtained from American Type Culture Collection (Manassas, VA). Fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM), methylisobutylxanthin, human recombinant insulin, dexamethasone, dimethyl sulfoxide (DMSO), and perfluorobutanesulfonic acid (PFBS, 97%) were purchased from Sigma Aldrich Co. (St. Louis, MO). The amounts of triglyceride and protein were quantified using Infinity triglycerides kit and Pierce BCA protein assay kit, respectively, both from Thermo Fisher Scientifics (Middletown, VA). Trizol and High-Capacity cDNA Reverse Transcription Kit were purchased from Life Technologies (Carlsbad, CA). Taqman Universal Master Mix II was obtained from Applied Biosystems (Carlsbad, CA). Radioimmunoprecipitation assay (RIPA) buffer supplemented with 1% protease inhibitor was purchased from Boston Bioproducts Inc. (Ashland, MA). Rabbit anti-CCAAT/enhancer-binding protein alpha (C/ EBPα), anti-peroxisome proliferator-activated receptor gamma (PPAR γ), anti-acetyl-CoA carboxylase (ACC), anti-phosphor-p42/p44 (pERK), and anti-p42/p44 (ERK) were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti-β-actin was from Santa Cruz Biotechnology (Dallas, TX). Goat anti-rabbit and anti-mouse horseradish peroxidase conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA). The Clarity™ Western ECL Substrate Kit was obtained from Bio-Rad Co. (Hercules, CA).
Cell culture
3T3-L1 preadipocytes were maintained in DMEM supplemented with 10% (v/v) FBS. Two days after confluence (day 0), preadipocytes were induced for differentiation with DMEM containing 10% FBS and a mixture of methylisobutylxanthin (0.5 mM), dexamethasone (1 mM), and insulin (1 mg/mL), as described previously (Sun, Qi et al. 2016). At day 2, the medium was switched to DMEM with 10% FBS and insulin only. From day 4, insulin was removed from the medium and cells maintained in DMEM with 10% FBS,changing medium every two days. During differentiation, cells were treated with PFBS at increasing concentrations or with vehicle (dimethylsulfoxide, DMSO). All treatments included DMSO at a final concentration of 0.1%.
Measurement of cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based assay (Gerlier and Thomasset 1986). 3T3-L1 preadipocytes were seeded in a 96-well plate at a density of 5 × 103 cells per well. 2 days after confluence, cells were exposed to PFBS for 6 days. The cells were then treated with 5 mg/ml MTT at 37 °C for 4 h. After incubation, MTT-formazan was dissolved in DMSO and the absorbance at 540 nm was determined using a SpectraMax i3 microplate reader (Molecular Devices, Sunnyvale, CA).
Triglyceride quantification
Triglyceride (TG) levels were determined using a commercial kit (Infinity™ Triglycerides Reagent; Thermo Scientific). Briefly, at day 6 cells were washed twice with phosphate-buffered saline (PBS) and harvested by scraping the cells from the culture plate in PBS containing 1% Triton-X. Cell homogenates were obtained by sonication and TG concentrations were determined uisng the commercial kit according to the manufacturer’s instructions. Protein concentrations were measured using Pierce BCA protein assay kit (Thermo Scientific) and used for normalization of samples.
Real-time PCR (q-PCR) analysis
Total RNA was isolated from cells using TRIzol Reagent (Life technologies, Carlsbad, CA) according to manufacturer’s instruction. Conversion of total RNA to single stranded cDNA was performed using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA). Gene expression assays for CCAAT/enhancer-binding protein a (C/ EBPα, Mm00514283_s1), peroxisome proliferator-activated receptor gamma (PPARγ, Mm00440940_m1), acetyl-CoA carboxylase (ACC, Mm01304257_m1), fatty acid synthase (FAS, Mm00662319_m1), and fatty acid binding protein 4 (FABP4, Mm00445878_m1) were performed with Taqman Universal Master Mix II (Applied Biosystems, Carlsbad, CA) on a StepOne Plus real time PCR instrument (Applied Biosystems, Carlsbad, CA). The results of target gene expression levels were normalized to the expression level of the18S rRNA gene(Hs99999901_s1) using the 2-ΔΔCt method (Livak and Schmittgen 2001).
Immunoblotting
Cells were lysed with RIPA buffer supplemented with Protease & Phosphatase Inhibitor Cocktail (100X, Thermo Scientific, Rockford, IL). Proteins in the cell lysates were separated by electrophoresis using a 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE). Electrophoresed proteins were transferred to an Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking with 5% skim milk for two hours, the membrane was incubated overnight under 4 °C with primary antibodies at appropriate dilutions. Horseradish peroxidase conjugated goat anti-rabbit or anti-mouse IgG was used as the secondary antibody. Specific proteins were were detected using an image Station 4000 MM (Carestream Health, New Haven, CT) using the Clarity™ Western ECL Substrate Kit (Bio-Rad Co., Hercules, CA). GAPDH or β-actin was used as an internal control. Image and results were quantified with Image J software (NIH).
Cell counting
After exposure to PFBS (0, 10, 50, 100 μM for 48 hours, cells were trypsinized and collected by centrifugation at 1000g for 5 minutes. Cell suspension was then subjected to cell counting with hemocytometer (Fisher Scientific, Horsham, PA).
Data Analysis
Data in figure 1-6 were analyzed with one-way ANOVA using the Statistical Analysis System (SAS Institute, Cary, NC). Data in figure 7 were analyzed with two-way ANOVA. All data are expressed as the mean ± standard error of the mean (S.E.). Multiple comparisons among groups were performed using Tukey’s test. P values less than 0.05 are reported as statistically significant. N is defined as the number of replicates in each experiment.
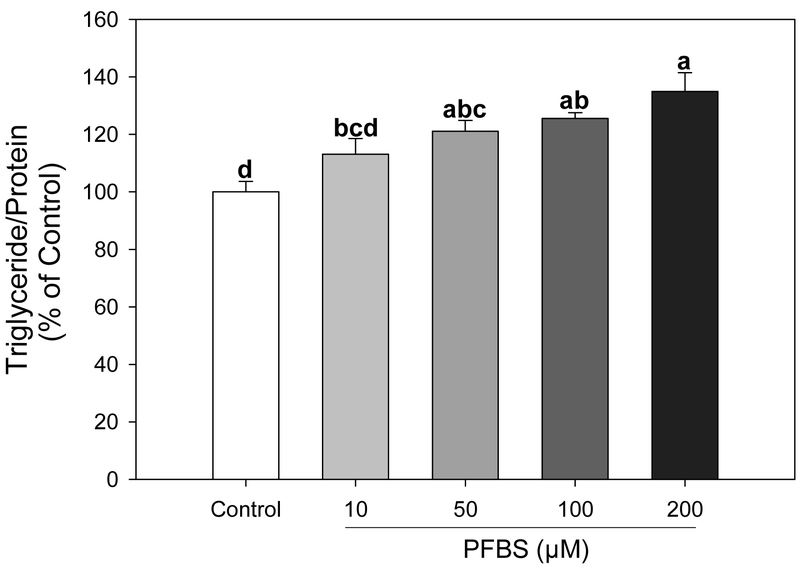
Figure 1.
PFBS promotes triglyceride accumulation in 3T3-L1 cells. After treatment for 6 days, adipocytes were harvested and measured for triglyceride content that were normalized by protein. Numbers are mean ± S.E. (n=4). Means with different letters are significantly different at P< 0.05.
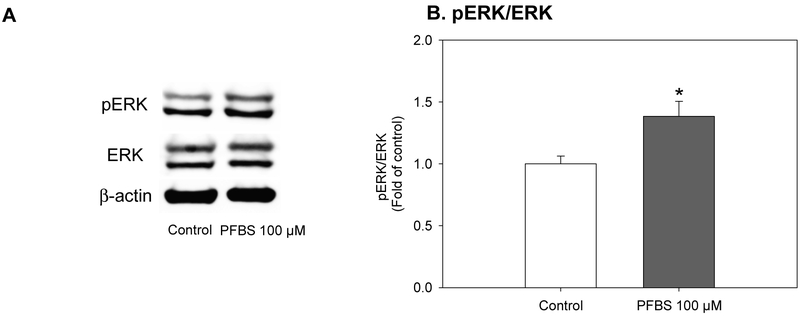
Figure 6.
Activation of ERK pathway by PFBS. Upon induction of differentiation, preadipocytes were treated by PFBS (100 μM) for 4 hours. Then, phosphorylation of ERK was determined by immunoblotting. A. Representative results; B. Ratio of phospho-ERK to ERK. Numbers are mean ± S.E. (n=3). * indicates a significant difference against the control (P< 0.05).
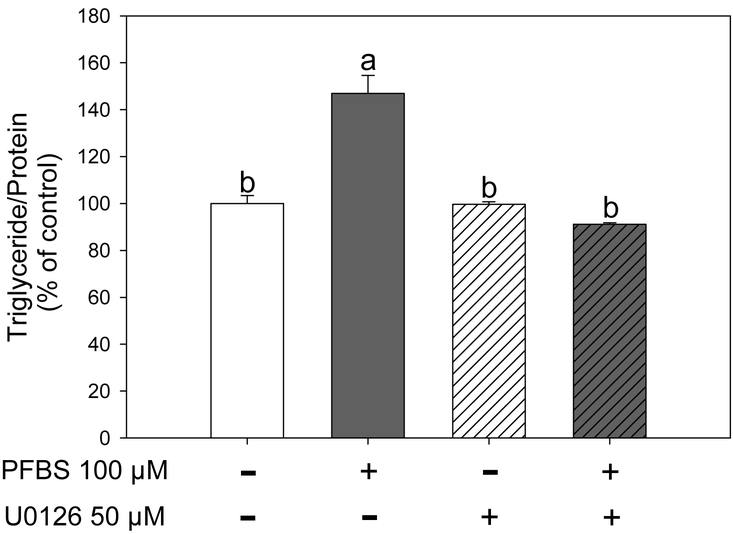
Figure 7.
Activation of ERK with PFBS was abolished by MEK1/2 specific inhibitor U0126. During differentiation, the cells were treated with PFBS (100 μM) and U0126 (50 μM). On day 6, triglyceride contents were measured. Numbers are mean ± S.E. (n=4). Means with different letters are significantly different at P< 0.05.
Results
PFBS had no effect on cell viability at concentrations up to 200 μM after a 6-day exposure. (Supplementary Figure S1). PFBS treatment during adipogenic differentiation significantly promoted lipid accumulation in a dose dependent manner compared with the DMSO control (Figure 1). Cells treated with 200 μM PFBS showed a 35% increase in triglyceride content compared to the control (P<0.0001). Treatments of 50 μM and 100 μM PFBS also significantly elevated TG levels above the control (21% and 25% increases, respectively). From these results, it was decided to use PFBS concentrations up to 100 μM for the following experiments.
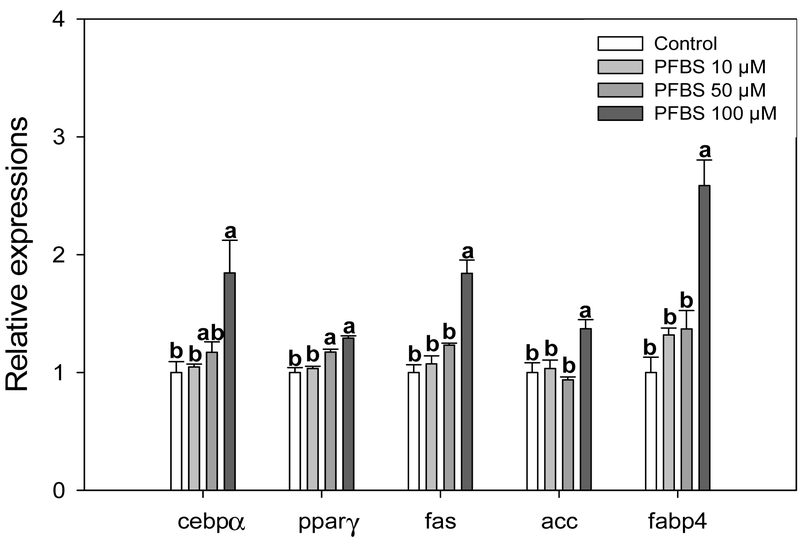
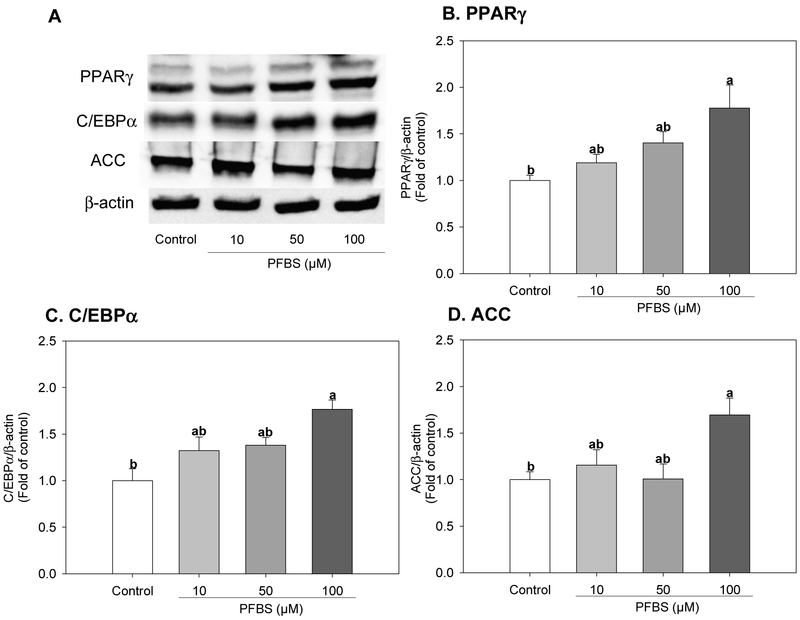
Next, we assessed whether PFBS potentiated adipogenesis through the upregulation of genes known to be involved in adipogenic differentiation and/or lipid metabolism. Treatment with 100 μM PFBS for 6 days significantly increased the mRNA levels of genes encoding two master regulators of adipogenic differentiation; CCAAT/enhancer-binding protein alpha (C/EBPα, 84%, P=0.0047) and peroxisome-proliferator activated receptor gamma (PPARγ, 29%, P<0.0001), as well as their target gene fatty acid binding protein 4 (FABP4, 159%, P<0.0001) (Figure 2) when compared with the control. The mRNA expression level of key regulatory enzymes involved in de novo lipogenesis; fatty acid synthase (FAS, 84%, P<0.0001) and acetyl-CoA carboxylase (ACC, 37%, P=0.0049), were also significantly increased by 100 μM PFBS treatments compared with the control (Figure 2). Likewise, 100 μM PFBS treatments also significantly increased the protein expression levels of PPARγ and C/EBPα compared with the control by 76% (P=0.006) and 77% (P=0.0017), respectively (Figure 3B and C). In a similar manner, the protein expression of ACC was also increased by PFBS (69%, P=0.0021, Figure 3D) compared to the control.
Figure 2.
Effects of PFBS on adipogenic gene expression. Cells were induced to differentiation for 6 days with or without PFBS (10, 50, 100 μM). mRNA levels of indicated genes were quantified by real-time PCR and the ddCt was determined using 18S RNA as a housekeeping gene. C/EBPα, CAATT element binding protein alpha; PPARγ, Peroxisome proliferator-activated receptor gamma; FAS, Fatty acid synthase; ACC, Acetyl-CoA carboxylase; and FABP4, Fatty acid binding protein 4. Numbers are mean ± S.E. (n=3). Means with different letters are significantly different at P< 0.05.
Figure 3.
Effects of PFBS on protein levels of molecular mediators of adipogenesis. Cells were induced to differentiation for 6 days with or without PFBS (10, 50, 100 μM). Protein levels of indicated genes were quantified by Western blot. A. Representative results; B. PPARγ, Peroxisome proliferator-activated receptor gamma; C. C/EBPα, CAATT element binding protein alpha; D. ACC, Acetyl-CoA carboxylase. Numbers represent mean ± S.E. (n=3). Means with different letters were significantly different at P< 0.05.
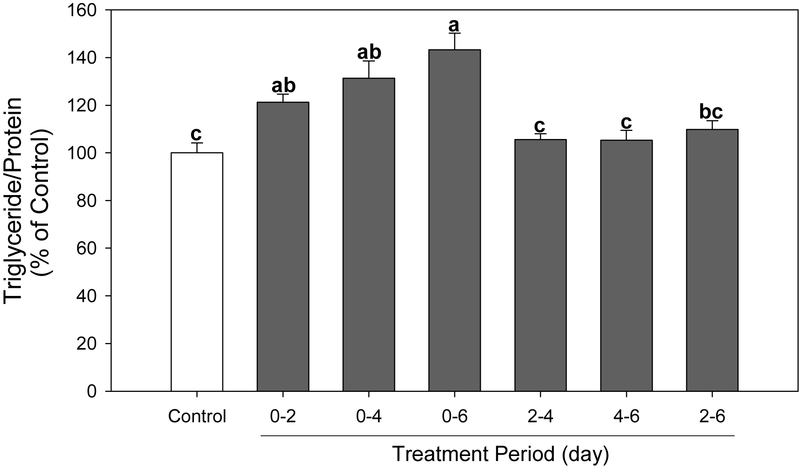
To determine the specific adipogenic developmental stage targeted by PFBS, we next examined the effect of PFBS on lipid accumulation at different exposure intervals. A significant increase in lipid accumulation was observed in cells treated with PFBS at day 0-2 (P=0.0078), day 0-4 (P=0.0020) and day 0-6 (P<0.0001), while treatment after day 2 showed no effect compared to the control (Figure 4). It is apparent that longer treatment of PFBS potentiated fat accumulation (from day 0 to 2-6 days). These finding suggested that PFBS induced adipogenesis, particularly targeting events in early adipogenesis.
Figure 4.
Effects of PFBS on triglyceride accumulation with different treatment periods. Cells were exposed to PFBS (100 μM) on indicated time period and harvested at day 6. Numbers are mean ± S.E. (n=4). Means with different letters are significantly different at P<0.05.
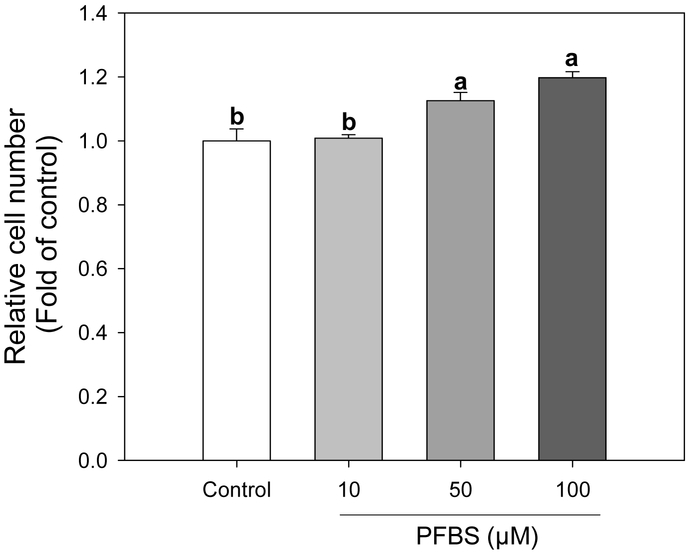
Upon exposure to adipogenic inducers, such as methylisobutylxanthin, dexamethasone, and insulin, growth-arrested preadipocytes re-enter the cell cycle and undergo two rounds of cell division in the subsequent 2 days, a process called mitotic clonal expansion (MCE). MCE is a prerequisite for the expression of adipogenic genes that produce the terminal differentiated phenotype. As observed in Figure 5, the effect of PFBS targets the early stage of differentiation in 3T3-L1 cells when MCE is triggered. Therefore, we next examined whether MCE was affected by PFBS. Treatments with PFBS (50 & 100 μM) for 48 h significantly increased the relative cell number compared to the control by 13% (P=0.0076) and 20% (P=0.0005) compared to the control, respectively, which implied the activation of MCE by PFBS treatment (Figure 5).
Figure 5.
Activation of mitotic clonal expansion in preadipocytes by PFBS. Upon induction of differentiation, preadipocytes were treated by PFBS (10, 50 and 100 μM) for 48 hours. Then, cell numbers were determined by hemocytometer. Numbers are mean ± S.E. (n=6). Means with different letters are significantly different at P< 0.05.
The activation of ERK is one of the crucial steps in the early stages of adipogenesis. The inhibition of ERK phosphorylation during the early adipogenesis has been shown to significantly inhibit fat accumulation in the later stages (Prusty et al. 2002). Also, ERK activation was reported to be essential to MCE. Therefore, we next determine whether the effect of PFBS was dependent on the activation of ERK. The treatment of PFBS (100 μM) for 4 hours significantly increased the ratio of pERK and ERK by 38% compared to the control (Figure 6, P=0.047).
To further confirm if the enhanced adipogenesis by PFBS is mediated by ERK activation, we next co-treated the cells with PFBS (100 μM) and U0126 (50 μM), a specific MEK/ERK pathway blocker, for 6 days. PFBS significantly increased fat accumulation consistent with the results shown in Fig. 1 (P<0.0001), while U0126 treatment alone did not change the fat content. However, the increased fat accumulation induced by PFBS was abolished with co-treatment of U0126 (Figure 7). These results suggested that PFBS increased fat accumulation, in part, via a MEK/ERK-dependent manner.
Discussion
In the current study, we demonstrated that PFBS exposure contributes to increased adipogenesis of 3T3-L1 adipocytes. To our knowledge, this is the first report on the correlation between PFBS exposure and adipogenesis. Further, we found that the effect of PFBS on adipogenesis occurs primarily during early stage of adipogenesis, and mitotic clonal expansion (MCE) is induced by PFBS treatment during this developmental interval. The current results also suggest that PFBS influence adipogenesis, in part, via the up regulation of the MEK/ERK-dependent pathway, which affects MCE in the early stages of adipogenesis that is a crucial step f adipogenesis and the activation of this step has been reported to induce adipogenesis (Tang et al. 2003).
ERK1/2 are activated by phosphorylation of threonine and tyrosine residues by the dual specificity kinase MAPK/ERK kinase 1/2 (MEK1/2), which is essential to the induction of MCE and the subsequent adipogenesis (Qiu et al. 2001, Bost et al. 2005). The activation of this pathway during early adipogenesis promotes adipogenesis by activating the transcription factors to initiate the expression of PPARγ and C/EBPα. Conversely, the inhibition of MEK/ERK pathway by U0126 has been suggested to inhibit adipogenesis (Prusty et al. 2002). Our results suggest that the effect of PFBS on increase adipogenesis is in part dependent on the activation of the MEK/ERK pathway. Thus, PFBS appears to initially activate the MEK/ERK pathway, which further induces an accelerated MCE, showing increase in cell number after 48-hour treatment. Other PFASs, including PFOS, have been reported to increase cell number of 3T3- L1 cells (Watkins et al. 2015), and PFOS activates ERK in multiple cell lines, including HAPI microglial cells and cerebellar granule cells (Lee et al. 2013, Wang et al. 2015), which are consistent to the current results. Similar effects were likewise observed following treatments with PFOA or perfluorohexanesulfonic acid (PFHxS) (Upham et al. 2009, Lee et al. 2014). Therefore, we speculate that other PFASs might share common mechanism for adipogenesis as PFBS. However, this is the first to report on the role of PFBS in adipogenesis.
It is not clear how PFBS activates ERK in the current study, however, previously it was reported that PFOS and PFOA elevate cytoplasmic calcium levels by releasing calcium ions from intracellular calcium stores (Liu et al. 2011), where elevated calcium levels can subsequently activate ERK through calmodulin kinase I (CaMKI) (Schmitt et al. 2004, Chuderland and Seger 2008). Alternatively, elevated calcium levels can activate phospholipase C and protein kinase C, leading to ERK activation (Huang 1989, Orton et al. 2005). Further studies are needed to determine if PFBS activates ERK via modulating calcium levels similar to that of PFOS and PFOA.
PFBS is generally believed to be less toxic than PFOS due to its shorter biological halflife of a month in comparison to 5.4 years for PFOS (Olsen et al. 2007, Olsen et al. 2009). In our study, PFOS treatment had no effect on adipogenesis (Supplementary Figure S2). This is consistent with a previous study reporting no effect of PFOS on adipocyte differentiation, while perfluorooctanoic acid (POFA) potentiated adipocyte differentiation in 3T3-L1 adipocytes (Yamamoto et al. 2015). Others reported that PFOS was less potent than perfluorohexane sulfonate (PFHxS), a short-chain cognate of PFOS, in adipogenesis (Watkins et al. 2015, Xu et al. 2016). Even though PFBS is more potent in adipogenesis than PFOS, tissue levels of PFBS are relatively low compared to other PFASs: 5-40 fold lower than that of PFOS (Bogdanska et al. 2014). However, it is reported that PFBS and PFOS have similar tissue distribution (Bogdanska et al. 2014) and the concentration of PFBS in humans currently is rising (Glynn et al. 2012). Thus, more attention should be paid to the potential effects of long-term exposure of PFBS as well as interactions with other PFASs including PFOS on adipogenesis.
A few studies have examined the concentration of PFBS in humans or animals and have led to a 7 μg/L (23 nM) health risk limit for ground water established in Minnesota, and the EPA has set a subchronic reference dose of 0.2 mg/kg/d and chronic reference dose of 0.02 mg/kg/d (EPA 2014). In a 90-day sub-chronic oral administration study on rats, the no-observed-adverse-effect level (NOAEL) for PFBS was set at 60 mg/kg/d for males and 600 mg/kg/d for females (Lieder et al. 2009). In a pharmacokinetic study following the exposure to 16 mg/kg/d of 35S-labeled PFBS, 35S labeled PFBS was found to distribute to epididymal fat and blood at 5 μmol/kg and 19 μmol/kg (~19 μM), respectively (Bogdanska et al. 2014). Serum PFBS levels of factory workers have been found to be approximately 23 ng/ml (77 nM) (Fu et al. 2015). Given this limited data set, the doses used in the current study may not be achievable. Nevertheless, the effects of obesogens can be aggravated by dietary fat intake as previous studies have shown the interaction between obesogens treatment and dietary fat (Xiao et al. 2017, Sun, Xiao et al. 2016, Sun et al. 2017, Xiao et al. 2018).
To summarize, our current study reports the effects of PFBS, the replacement compound for PFOS, on adipogenesis. These results are significant in elucidating a potential link between the risk of developing obesity and the exposure to the replacement of PFOS, which is a known obesogens. However, the current results are limited to an in vitro model using relatively high concentrations of PFBS. Further studies in animals, as well as epidemiology studies, are needed to further confirm our in vitro results.
Supplementary Material
Highlights.
-
-
Perfluorobutanesulfonic acid (PFBS) promoted the adipogenesis in 3T3-L1 cells.
-
-
PFBS targets early adipogenesis in 3T3-L1 cells.
-
-
Effects of PFBS on adipogenesis is in part via MAPK/ERK-mediated mechanism.
Acknowledgement
This project was in part supported by NIH R01ES028201. We are thankful for discussions with Dr. Karilyn Sant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogdanska J, Sundström M, Bergström U, Borg D, Abedi-Valugerdi M, Bergman Ï, DePierre J and Nobel S (2014). "Tissue Distribution of 35S-Labelled Perfluorobutanesulfonic Acid in Adult Mice Following Dietary Exposure for 1–5 Days." Chemosphere 98: 28–36. [DOI] [PubMed] [Google Scholar]
- Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, Dani C, Hofman P, Pagès G and Pouysségur J (2005). "The Extracellular Signal–Regulated Kinase Isoform ERK1 Is Specifically Required for in Vitro and in Vivo Adipogenesis." Diabetes 54(2): 402–411. [DOI] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K and Lanphear BP (2016). "Prenatal Perfluoroalkyl Substance Exposure and Child Adiposity at 8 Years of Age: The Home Study." Obesity 24(1): 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuderland D, and Seger R (2008). "Calcium Regulates ERK Signaling by Modulating Its Protein-Protein Interactions." Communicative & Integrative Biology 1(1): 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'eon JC, Hurley MD, Wallington TJ and Mabury SA (2006). "Atmospheric Chemistry of N-Methyl Perfluorobutane Sulfonamidoethanol, C4F9SO2N (CH3) CH2CH2OH: Kinetics and Mechanism of Reaction with OH." Environmental Science & Technology 40(6): 1862–1868. [DOI] [PubMed] [Google Scholar]
- EPA (2014). "Provisional Peer-Reviewed Toxicity Values for Perfluorobutane Sulfonate (Casrn 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (Casrn 29420-49-3)." From https://cfpub.epa.gov/ncea/pprtv/documents/PotassiumPerfluorobutaneSulfonate.pdf. [PubMed]
- Fu J, Gao Y, Wang T, Liang Y, Zhang A, Wang Y and Jiang G (2015). "Elevated Levels of Perfluoroalkyl Acids in Family Members of Occupationally Exposed Workers: The Importance of Dust Transfer." Scientific Reports 5: 9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlier D and Thomasset N (1986). "Use of MTT Colorimetric Assay to Measure Cell Activation." Journal of Immunological Methods 94(1-2): 57–63. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S and Darnerud PO (2012). "Perfluorinated Alkyl Acids in Blood Serum from Primiparous Women in Sweden: Serial Sampling During Pregnancy and Nursing, and Temporal Trends 1996–2010." Environmental Science & Technology 46(16): 9071–9079. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, Henriksen TB and Olsen SF (2012). "Prenatal Exposure to Perfluorooctanoate and Risk of Overweight at 20 Years of Age: A Prospective Cohort Study." Environmental Health Perspectives 120(5): 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K-P (1989). "The Mechanism of Protein Kinase C Activation." Trends in Neurosciences 12(11): 425–432. [DOI] [PubMed] [Google Scholar]
- Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebæk NE and Jørgensen N (2009). "Do Perfluoroalkyl Compounds Impair Human Semen Quality?" Environmental Health Perspectives 117(6): 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Butenhoff JL and Rogers JM (2004). "The Developmental Toxicity of Perfluoroalkyl Acids and Their Derivatives." Toxicology and Applied Pharmacology 198(2): 231–241. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Choi S-Y and Yang J-H (2014). "PFHxS Induces Apoptosis of Neuronal Cells via ERK1/2-Mediated Pathway." Chemosphere 94: 121–127. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee H-G and Yang J-H (2013). "Perfluorooctane Sulfonate-Induced Apoptosis of Cerebellar Granule Cells Is Mediated by ERK 1/2 Pathway." Chemosphere 90(5): 1597–1602. [DOI] [PubMed] [Google Scholar]
- Lieder PH, Chang S-C, York RG and Butenhoff JL (2009). "Toxicological Evaluation of Potassium Perfluorobutanesulfonate in a 90-Day Oral Gavage Study with Sprague-Dawley Rats." Toxicology 255(1-2): 45–52. [DOI] [PubMed] [Google Scholar]
- Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L, Qi L, Bray GA, DeJonge L and Coull B (2018). "Perfluoroalkyl Substances and Changes in Body Weight and Resting Metabolic Rate in Response to Weight-Loss Diets: A Prospective Study." PLoS Medicine 15(2): e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jin Y, Liu W, Wang F and Hao S (2011). "Possible Mechanism of Perfluorooctane Sulfonate and Perfluorooctanoate on the Release of Calcium Ion from Calcium Stores in Primary Cultures of Rat Hippocampal Neurons." Toxicology in Vitro 25(7): 1294–1301. [DOI] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD (2001). "Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2– Δδct Method." Methods 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM and Marcus M (2012). "Maternal Concentrations of Polyfluoroalkyl Compounds During Pregnancy and Fetal and Postnatal Growth in British Girls." Environmental Health Perspectives 120(10): 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, Ye X and Sagiv SK (2017). "Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood." Environmental Health Perspectives 125(3): 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL and Zobel LR (2007). "Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers." Environmental Health Perspectives 115(9): 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Chang S-C, Noker PE, Gorman GS, Ehresman DJ, Lieder PH and Butenhoff JL (2009). "A Comparison of the Pharmacokinetics of Perfluorobutanesulfonate (PFBS) in Rats, Monkeys, and Humans." Toxicology 256(1-2): 65–74. [DOI] [PubMed] [Google Scholar]
- Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR and Kolch W (2005). "Computational Modelling of the Receptor-Tyrosine-Kinase-Activated MAPK Pathway." Biochemical Journal 392(2): 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, BarcelÓ D and Farré M (2013). "Accumulation of Perfluoroalkyl Substances in Human Tissues." Environment International 59: 354–362. [DOI] [PubMed] [Google Scholar]
- Prusty D, Park B-H, Davis KE and Farmer SR (2002). "Activation of MEK/ERK Signaling Promotes Adipogenesis by Enhancing Peroxisome Proliferator-Activated Receptor γ (PPARγ) and C/EBPα Gene Expression During the Differentiation of 3t3-L1 Preadipocytes." Journal of Biological Chemistry 277(48): 46226–46232. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Wei Y, Chen N, Jiang M, Wu J and Liao K (2001). "DNA Synthesis and Mitotic Clonal Expansion Is Not a Required Step for 3T3-L1 Preadipocyte Differentiation into Adipocytes." Journal of Biological Chemistry 276(15): 11988–11995. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C and Corton JC (2008). "Toxicogenomic Dissection of the Perfluorooctanoic Acid Transcript Profile in Mouse Liver: Evidence for the Involvement of Nuclear Receptors Pparα and Car." Toxicological Sciences 103(1): 46–56. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Wayman GA, Nozaki N and Soderling TR (2004). "Calcium Activation of ERK Mediated by Calmodulin Kinase I." Journal of Biological Chemistry 279(23): 24064–24072. [DOI] [PubMed] [Google Scholar]
- Skutlarek D, Exner M and Farber H (2006). "Perfluorinated Surfactants in Surface and Drinking Waters." Environmental Science and Pollution Research International 13(5): 299. [DOI] [PubMed] [Google Scholar]
- Sun Q, Qi W, Xiao X, Yang S-H, Kim D, Yoon KS, Clark JM and Park Y (2017). "Imidacloprid Promotes High Fat Diet-Induced Adiposity in Female C57BL/6J Mice and Enhances Adipogenesis in 3T3-L1 Adipocytes Via the AMPKα-Mediated Pathway." Journal of Agricultural and Food Chemistry 65(31): 6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Qi W, Yang JJ, Yoon KS, Clark JM and Park Y (2016). "Fipronil Promotes Adipogenesis Via Ampkα-Mediated Pathway in 3T3-L1 Adipocytes." Food and Chemical Toxicology 92: 217–223. [DOI] [PubMed] [Google Scholar]
- Sun Q, Xiao X, Kim Y, Kim D, Yoon KS, Clark JM and Park Y (2016). "Imidacloprid Promotes High Fat Diet-Induced Adiposity and Insulin Resistance in Male C57BL/6J Mice." Journal of Agricultural and Food Chemistry 64(49): 9293–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q-Q, Otto TC and Lane MD (2003). "Mitotic Clonal Expansion: A Synchronous Process Required for Adipogenesis." Proceedings of the National Academy of Sciences 100(1): 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Park J-S, Babica P, Sovadinova I, Rummel AM, Trosko JE, Hirose A, Hasegawa R, Kanno J and Sai K (2009). "Structure-Activity-Dependent Regulation of Cell Communication by Perfluorinated Fatty Acids Using in Vivo and in Vitro Model Systems." Environmental Health Perspectives 117(4): 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR and Gillies PJ (2006). "Differential Activation of Nuclear Receptors by Perfluorinated Fatty Acid Analogs and Natural Fatty Acids: A Comparison of Human, Mouse, and Rat Peroxisome Proliferator-Activated Receptor-α, -β, and-γ, Liver X Receptor- β, and Retinoid X Receptor-α." Toxicological Sciences 92(2): 476–489. [DOI] [PubMed] [Google Scholar]
- Wang C, Nie X, Zhang Y, Li T, Mao J, Liu X, Gu Y, Shi J, Xiao J and Wan C (2015). "Reactive Oxygen Species Mediate Nitric Oxide Production through ERK/JNK MAPK Signaling in Hapi Microglia after PFOS Exposure." Toxicology and Applied Pharmacology 288(2): 143–151. [DOI] [PubMed] [Google Scholar]
- Watkins AM, Wood CR, Lin MT and Abbott BD (2015). "The Effects of Perfluorinated Chemicals on Adipocyte Differentiation in Vitro." Molecular and Cellular Endocrinology 400: 90–101. [DOI] [PubMed] [Google Scholar]
- Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, Park Y, 2017. Permethrin alters glucose metabolism in conjunction with high fat diet by potentiating insulin resistance and decreases voluntary activities in female C57BL/6J mice. Food Chem. Toxicol 108, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Sun Q, Kim Y, Yang S-H, Qi W, Kim D, Yoon KS, Clark JM and Park Y (2018). "Exposure to Permethrin Promotes High Fat Diet-Induced Weight Gain and Insulin Resistance in Male C57BL/6J Mice." Food and Chemical Toxicology 111: 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shimpi P, Armstrong L, Salter D and Slitt AL (2016). "PFOS Induces Adipogenesis and Glucose Uptake in Association with Activation of NRF2 Signaling Pathway." Toxicology and Applied Pharmacology 290: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Yamane T, Oishi Y and Kobayashi-Hattori K (2015). "Perfluorooctanoic Acid Binds to Peroxisome Proliferator-Activated Receptor γ and Promotes Adipocyte Differentiation in 3T3-L1 Adipocytes." Bioscience, Biotechnology, and Biochemistry 79(4): 636–639. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren X-M, Wan B and Guo L-H (2014). "Structure-Dependent Binding and Activation of Perfluorinated Compounds on Human Peroxisome Proliferator-Activated Receptor γ." Toxicology and Applied Pharmacology 279(3): 275–283. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liang Y, Shi Y, Xu L and Cai Y (2013). "Occurrence and Transport of Perfluoroalkyl Acids (PFAAs), Including Short-Chain Pfaas in Tangxun Lake, China." Environmental Science & Technology 47(16): 9249–9257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.