Abstract
Background
The reduction of global malaria burden over the past 15 years is much attributed to the expansion of mass distribution campaigns (MDCs) of long-lasting insecticidal nets (LLIN). In Madagascar, two LLIN MDCs were implemented and one district also benefited from a community-based continuous distribution (CB-CD). Malaria incidence dropped but eventually rebounded after a decade.
Methods
Data from a sentinel surveillance network over the 2009–2015 period was analyzed. Alerts were defined as weekly number of malaria cases exceeding the 90th percentile value for three consecutive weeks. Statistical analyses assessed the temporal relationship between LLIN MDCs and (i) number of malaria cases and (ii) malaria alerts detected, and (iii) the effect of a combination of MDCs and a CB-CD in Toamasina District.
Findings
Analyses showed an increase of 13.6 points and 21.4 points in the percentile value of weekly malaria cases during the second and the third year following the MDC of LLINs respectively. The percentage of alert-free sentinel sites was 98.2% during the first year after LLIN MDC, 56.7% during the second year and 31.5% during the third year. The number of weekly malaria cases decreased by 14% during the CB-CD in Toamasina District. In contrast, sites without continuous distribution had a 12% increase of malaria cases.
Interpretation
These findings support the malaria-preventive effectiveness of MDCs in Madagascar but highlight their limited duration when not followed by continuous distribution. The resulting policy implications are crucial to sustain reductions in malaria burden in high transmission settings.
Keywords: Malaria, Madagascar, LLINs effectiveness, Sentinel surveillance
Research in context
Evidence before this study
Triennial mass distribution campaigns (MDCs) of long-lasting insecticidal nets (LLINs) have become an essential component of the global effort to eliminate malaria. Studies have shown the effectiveness of this strategy in several regions of sub-Saharan Africa although there is growing evidence of faster than expected wear-and-tear of bed nets and decline of insecticide concentrations. We searched PubMed for studies using malaria surveillance data as a proxy for vector control effectiveness with the search terms “sentinel”, “long-lasting insecticidal nets” and “malaria”. No study performed a longitudinal analysis of malaria cases and malaria alert from sentinel health center registries to estimate the outcomes of MDC.
Added value of this study
This study analyzed standard malaria surveillance data to assess the effectiveness of two vector control measures, explicitly three yearly mass distribution campaigns (MDCs) of long-lasting insecticidal nets (LLINs) and a community-based continuous distribution of long-lasting insecticidal nets.
Our analysis showed that almost none of the health centers reached an alarming level of malaria case notifications during the first year after MDC. However, alert thresholds were reached in almost half of the health centers during the second year and in almost two thirds during the third year. Interestingly, malaria notifications kept on decreasing when community-based continuous distribution of LLINs was concurrently implemented.
Implications of all the available evidence
The effectiveness of triennial mass distribution campaigns of LLINs seems to fade after twelve months while adding a concomitant community-based continuous distribution of LLINs maintains the reduction of malaria case notifications over the years. This knowledge is essential to consolidate the benefits of costly mass distribution campaigns and to pursue malaria elimination goals in moderate to high transmission areas.
Alt-text: Unlabelled Box
1. Background
The decrease in malaria disease burden over the past 15 years is mainly attributed to the introduction of new control measures, including (i) Artemisinin-based Combination Therapies (ACTs), (ii) Indoor Residual Spraying (IRS) and (iii) Long-Lasting Insecticidal Nets (LLIN) [1]. An unprecedented expansion of Malaria Control Interventions (MCIs), particularly through mass distribution campaigns (MDCs) of LLINs, has led large regions of Africa, including Madagascar, to consider elimination strategies [2]. While these successes confirm the major impact of MCIs, challenges to achieving elimination must not be under-estimated as residual transmission fosters malaria resurgence. MDCs are cost effective in rapidly reaching universal and equitable coverage of LLINs [3], [4], [5], [6], but both coverage and the protective effectiveness of LLINs decline over time and imply the need for regular replacement [7]. LLIN ownership and access decline between campaigns due to net loss, wear and tear, population movements, and births. It is difficult to decide how frequently successive campaigns should be carried out to compensate for the decline in LLIN coverage [8]. The WHO recommends that mass distribution campaigns should be repeated at an interval of no more than three years unless there is reliable evidence that a longer interval could be appropriate [9]. Indeed, gaps in service coverage can contribute to malaria resurgence before the next MDC [10]. In order to maintain uninterrupted universal coverage and sustain public health impact, complementary continuous distribution mechanisms, through antenatal care (ANC) and Expanded Program on Immunization (EPI) services, have been proposed as an integral part of a comprehensive national LLIN strategy to provide a continuous supply of replacement LLINs [11]. However, delivery through ANC and EPI—even if perfectly operated—is insufficient to maintain high levels of universal access, coverage and utilization. Countries and donors thus continue to rely mainly on MDCs [12].
To reduce malaria burden in high transmission areas, LLIN use is highly recommended by the National Malaria Control Program in the island of Madagascar. Two MDCs of LLINs were organized in Madagascar at the end of 2009 and 2012. However recent increases in malaria-related cases and deaths [13], reflect the fragility of the gains achieved if control efforts are not sustained [1], [14]. Toamasina II district, an area of high malaria endemicity on the East Coast of Madagascar, uniquely benefited from a combination of LLIN MDCs and a continuous community-based distribution of LLINs from September 2013 to June 2014.
1.1. Objectives
Our hypothesis is that longitudinal data from sentinel surveillance networks can be useful to evaluate the effectiveness of malaria control programs. We performed an analysis using such data [15] to assess (i) the temporal relationship between MDCs of LLINs and malaria cases and malaria alerts detected in Madagascar over the 2009–2015 period, and (ii) the impact of a combination of mass distribution and continuous distribution in Toamasina II district.
2. Material and Methods
2.1. Fever Sentinel Surveillance Network
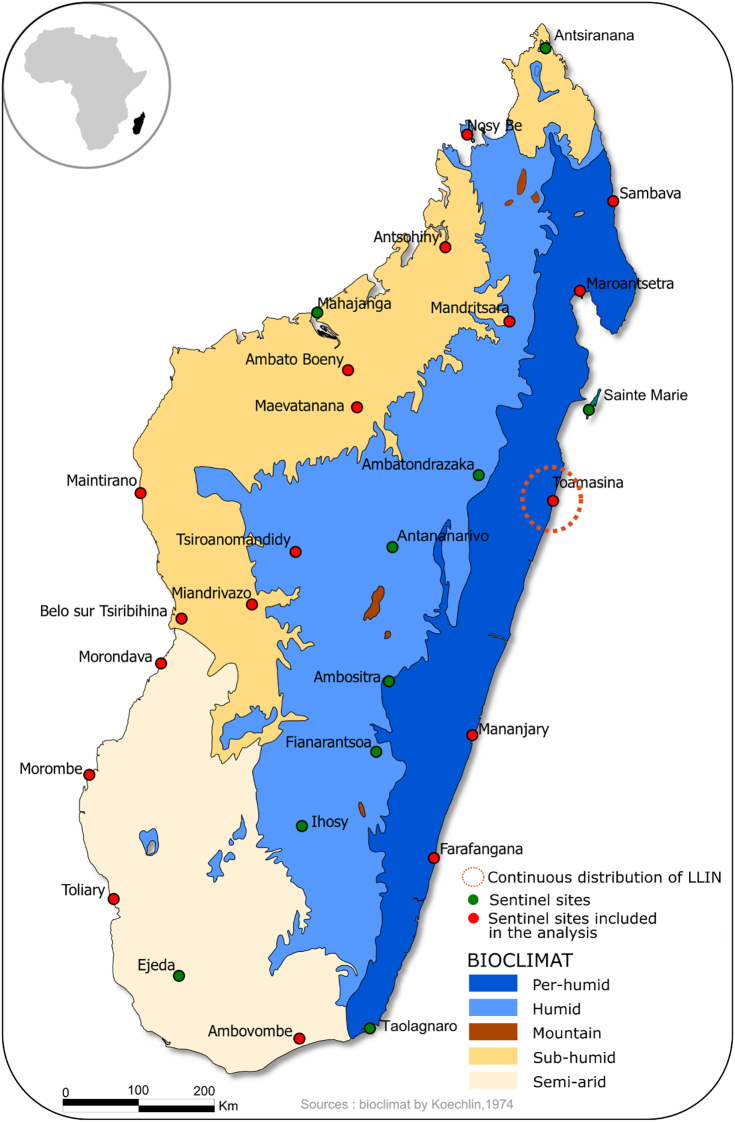
The Institut Pasteur de Madagascar implemented a web-based malaria early warning system [16] using electronically collected data from the Fever Sentinel Surveillance system (FSS) in Madagascar [15]. The FSS is a network of Primary Health Care Centers (PHCC) that expanded from 13 sentinel sites in 2007 to 34 sentinel sites in 2011, out of a total of 1600 health care centers directed by a physician. The FSS includes sites from all the ecosystems of Madagascar for surveillance of fever-associated diseases with all malaria transmission patterns being represented (Fig. 1). In accordance with the national policy, a malaria rapid diagnostic test (RDT) is performed on all febrile cases attending a PHCC in the FSS network. In each sentinel site, data on confirmed malaria cases is aggregated and submitted daily through Short Message Services (SMS). Yearly evaluations of the performance of the FSS showed an average completeness of 95% until 2018, defined as the proportion of SMS received vs. expected from sites. SMS data are also regularly cross-checked with site registries followed by recommendations for improvement. Overall, several publications described the usefulness of the sentinel network in detecting outbreaks [15], [17].
Fig. 1.
Location of the health centers participating in the sentinel surveillance system in Madagascar and surrounding climate.
Different methods have been proposed by WHO [18] to define malaria alert thresholds in resource-constrained environments [19], [20], [21] such as i) the weekly mean number of malaria cases + 2 standard deviations (SD) and ii) the cumulative sum (C-SUM). These methods enable software packages to define a baseline of normal incidence for a specific area at specific times. However, these methods are limited by the need for historical data over at least five years excluding epidemic years [19]. To overcome this constraint, the FSS alert system uses an alternative, less restrictive, method in which the alert threshold is reached when the weekly number of malaria cases is exceeding the 90th percentile value for three consecutive weeks. Percentile values are calculated for each sentinel site using all the surveillance data since the inclusion of the site in the sentinel network. Thus, time series of weekly numbers of malaria cases were transformed into percentile values. This percentile method is not season-dependent and was used successfully to detect malaria outbreaks in Madagascar [16].
Sentinel sites from the Central Highlands of Madagascar, where malaria transmission is low and therefore not targeted for MDCs, were excluded from our analyses. Therefore, 18 of the 34 sentinel sites were included in this observational study using time trends. All RDT confirmed malaria cases attending the selected OPDs since 2007 were eligible for analysis in this study.
2.2. Malaria control intervention
In Madagascar, vector control relies on LLIN distributions and IRS campaigns. The assessment of the latter was not considered in the present study since IRS is mostly focalized in the Central Highlands and Fringe transmission patterns of Madagascar, i.e. in areas of low transmission of malaria [22]. IRS was not carried out in sentinel site areas included in the study.
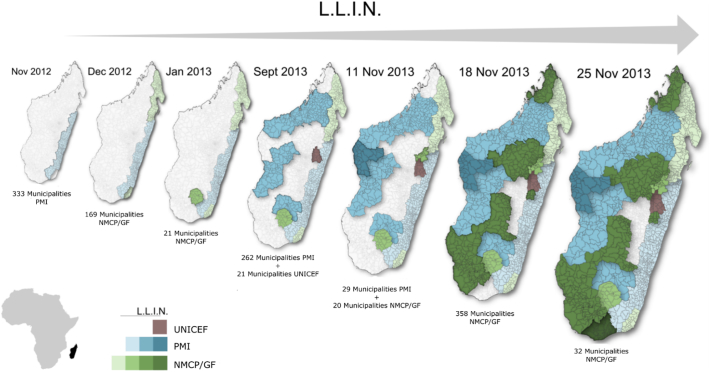
The seasonal variation of malaria transmission in Madagascar is well known, with higher malaria prevalence rates in the rainy season from November to May. MDCs are therefore planned before the high transmission season to maximize LLIN utilization and expected impact. Two MDCs were held in Madagascar from 2009 to 2013. The first took place from November 2009 to January 2010. The second was conducted in two stages, in November 2012 on the Eastern coast (in areas represented by five of the sentinel sites included in the study), and in October 2013 (15 sites in the rest of the country) (Fig. 2).
Fig. 2.
Mass LLIN campaign distribution held in 2012–2013 in Madagascar. UNICEF: United Nations Children's Fund, PMI: President's Malaria Initiative, NMCP/GF: National Malaria Control Program.
Madagascar has an extensive network of community agents, who provide health education, prevention services, and limited medical care to members of their communities. A community-based distribution system was piloted in the District of Toamasina II on the East Coast of Madagascar from September 2013 to June 2014. The pilot distribution was based on a ‘push–pull’ system, with delivery (push) of LLINs to a nearby distribution point, and demand (pull) from households. Local religious leaders provided LLINs to households presenting coupons obtained from community agents in their village. Households were eligible for coupon issuance if there were uncovered sleeping spaces or if the household included newly married couples, recent immigrants, pregnant women, or children of vaccination age [23]. This scheme was conceived to provide families with new nets as needed and to maintain at least 90% of households with at least one LLIN [23].
Time–space data on MCIs were provided by the National Malaria Control Program and President's Malaria Initiative (PMI). Data on MDC were available at district level on a weekly basis and encoded as a binary variable: weekly absence or presence of distribution. As illustrated on Fig. 2, MDCs are not always concurrent.
MDCs unfolded over several weeks in each area. During the study period, 18 sites benefited from the two MDCs described above, including one (Toamasina) that benefited from an additional continuous distribution of LLINs from September 2013 to June 2014 [24].
2.3. Statistical Analysis
In this study the unit of analysis is sentinel sites, not individuals. Reporting of malaria cases from sentinel sites are aggregated, with all sites sending their surveillance data at least on a weekly basis. Hence missing data are not addressed in this statistical analysis. For each sentinel site, weekly malaria cases were converted into percentiles ranks. This score indicates, for each site, the percentage of weeks with lower malaria case notifications since the beginning of the surveillance. Hence, a site ranked at the 90th percentile on a given week had only 10% of its total weeks with more malaria cases.
The outcome in this analysis is a “malaria alert” (or failure in the survival analysis), defined as three consecutive weeks above the 90th percentile of malaria cases. The main exposure is the time in weeks after a LLIN distribution. Sentinel sites were considered as a population and a Kaplan Meier survival curve described the delay between a LLIN distribution and a censorship or a failure. Right-censoring occurred at the beginning of a new MDC, a new malaria control policy (i.e., continuous LLIN distribution) or at the end of follow-up. The beginning of a time series defined a priori was seven weeks after the beginning of the MDCs of LLIN to account (i) for the delay between beginning of LLIN distribution and achieving effective coverage around sentinel sites, and (ii) for the delay between malaria infection and diagnosis. Data management was conducted using Stata 13 (Stata Corp., College Station, USA) and the graphical output was performed using R 3.3.2 (R Development Core Team).
The temporal relationship between MDCs of LLINs and percentile values of the number of malaria cases was estimated using a linear regression model. The period following MDC was divided into three phases: (i) > 7 weeks and ≤ 52 weeks (reference), (ii) > 52 weeks and ≤ 104 weeks, (iii) > 104 weeks after the beginning of a MDC. This variable defining the period post-MDC was our main exposure. Similarly, the risk of malaria alert over the three post-distribution phases following campaign as defined above, was estimated using a logistic regression model (alert vs no alert). Since the number of cases had been previously transformed into percentiles by site, the structure of the sample was taken into account and there was no need to use mixed effect or generalized estimating equation models to further account for non-independence of observations.
Finally, we assessed the effect of the combination of a MDC followed by a community-based continuous distribution intervention in Toamasina II district. Using linear regression model, the percentile values of malaria cases of Toamasina sentinel site were compared with those of four other sites, also located on the eastern coast of Madagascar and sharing the same malaria transmission pattern. All five sites benefited from MDCs in November 2009 and in November and December 2012 (Fig. 1). Beginning 9 months (36 weeks) after the December 2012 MDC the community-based distribution pilot ran for an additional 9 months, from September 2013 to June 2014 in Toamasina. This period of 18 months (72 weeks) following MDC was divided into two phases: (i) > 7 weeks and ≤ 36 week, and (ii) > 36 weeks and ≤ 72 weeks after the beginning of the MDC, covering respectively, the period after the MDC and before the beginning of the continuous distribution and the period covering the continuous distribution. Due to the low number of alerts during the period, no logit model was performed.
All models were controlled for available potential confounders, namely site-specific weekly rainfall from the National Oceanographic and Atmospheric Administration's [25], lagged for 8 weeks [16]; malaria seasons were empirically categorized as low (June to October), moderate (November to March), and high (April to May). GLM models were developed using R 3.3.2 (R Development Core Team (2005). R: A language and environment for statistical computing, reference index version 3.3.2 R Foundation for Statistical Computing, Vienna, Austria.).
3. Results
From November 9, 2009 to June 22, 2015, the 18 sentinel sites reported a total of 4221 weekly reports of malaria cases. Four of the five malaria transmission patterns were represented: 11 sentinel sites were located in the western transmission pattern, 5 in the eastern, 1 in the southern and 1 in the fringes. These patterns cover, respectively, 21.0, 27.5, 13.7 and 5.9% of the Malagasy population (Fig. 1).
In all sites, at least two LLIN MDC were implemented. Overall, 1790, 1520 and 911 weekly reports of malaria cases were received ≤ 52 weeks, > 52 and ≤ 104, and > 104 weeks after the beginning of a MDC, respectively. Among these 4221 reports, 217 reported totals above the malaria alert threshold for the specific site: 1.8% (4/217) within 52 weeks following a MDC, 53.1% (116/217) from 52 to 104 weeks and 44.7% (97/217) after 104 weeks (Fig. 3).
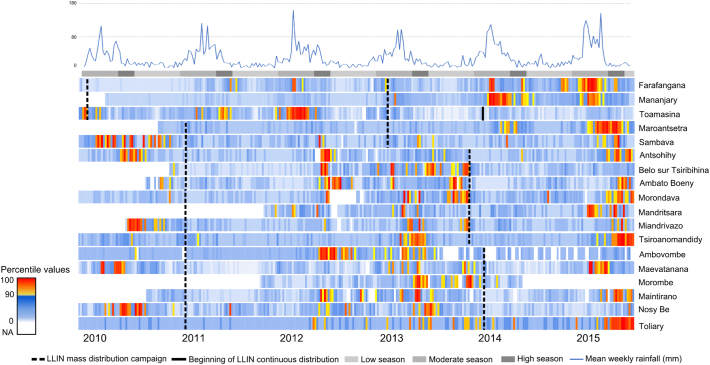
Fig. 3.
Percentile value of malaria cases vs LLIN.
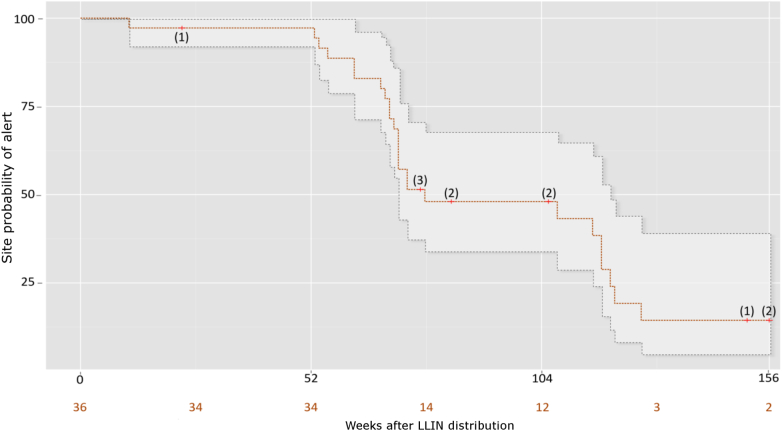
The survival analysis (Fig. 4) showed that within the first year following LLINs' MDC, only one out of the 36 sentinel sites reached the malaria alert threshold at least once, with 98.2% of sites remaining alert free (95% CI [94.8–100]). Between 52 and 104 weeks after MDC, the percentage of sites remaining alert free dropped to 56.7% (95% CI [44.8–71.7]), and between 104 and 156 weeks post MDC it further dropped to 31.5% (CI 95% [20.9–47.5]). Eleven sentinel sites were right-censored during follow-up: one sentinel site (Toamasina) at the beginning of the continuous distribution program in the district, 8 sentinel sites due to a new MDC, and 2 sentinel sites were censored after reaching the maximum follow-up period of 156 weeks (three years). Eleven sentinel sites were right-censored during the same follow-up period: one sentinel site (Toamasina) at the start of the continuous distribution program in the area, 8 sentinel sites due to a new MDC, and 2 sentinel sites were censored after reaching the maximum follow-up period of 156 weeks (three years).
Fig. 4.
Kaplan Meier of site week probability of alert. Censored observations are denoted by red crosses and numbered in brackets. The number of sites remaining at risk at different points is numbered in orange. The 95-confidence interval is denoted by dots.
The mean of percentile values of weekly malaria cases over the 18 sites was 50.2% (95% CI [49.3–51.2], p-value < 0.001) during the first year following mass campaign, 64.3% (95% CI [63.1–65.2]) during the second year and 71.9% (CI 95% [70.5–73.3]) in the third year following mass campaign. Linear regression including data from all 18 sentinel sites showed an increase of 13.6 points (95%CI [12.1–15.1], p-value < 0.001) and 21.4 points (95%CI [19.6–23.1], p-value < 0.001) in the percentile value of weekly malaria cases during the second and the third year following the MDC of LLINs respectively (Table 1).
Table 1.
Estimation of the impact of mass distribution campaign of long-lasting insecticidal nets by Linear Regression Model of percentile values of number of malaria cases for each week of the period at each of the 18 sentinel sites. Rainfall: site specific estimated rainfall in millimeters lagged for 8 weeks; CI: confidence interval.
| Coefficient (β) | 95% CI | t value | p-Value | |
|---|---|---|---|---|
| Intercept | 47.00 | 46.14 to 48.61 | 75.41 | < 0.001 |
| ≤ 52 weeks after MDC | Ref. | – | – | – |
| > 52 and ≤ 104 weeks after MDC | 13.57 | 12.08 to 15.07 | 17.75 | < 0.001 |
| > 104 weeks after MDC | 21.38 | 19.64 to 23.09 | 24.03 | < 0.001 |
| Low season | Ref. | – | – | – |
| Moderate season | 3.01 | 1.50 to 4.52 | 3.92 | < 0.001 |
| High season | 9.67 | 8.14 to 11.78 | 10.07 | < 0.001 |
| Rainfall (mm) | 0.003 | − 0.002 to 0.009 | 1.15 | 0.251 |
The logistic regression model, using data from all 18 sentinel sites also showed that the probability of a malaria alert at surveillance sites increased dramatically during the second year (odds ratio (OR): 36.8 [95% CI: 15.4–120.4]) and was further increased during the third year (OR 53.8 [22.4–176.4]) after adjusting for rainfall and malaria seasonality (Table 2). The association between numbers of alerts and season (OR: 2.8 [95% CI: 2.0–4.1]) was much weaker than the associations between numbers of alerts and time since LLIN distribution.
Table 2.
Estimation of the impact of mass distribution campaign of long-lasting insecticidal nets by logistic regression of the occurrence of alerts for each week of the period and each sentinel site. Rainfall: site specific estimated rainfall in millimeters lagged for 8 weeks; OR: Odds ratio; CI: confidence interval.
| Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|
| Intercept | < 0.001 | ||
| ≤ 52 weeks after MDC | Ref. | – | – |
| > 52 and ≤ 104 weeks after MDC | 36.84 | 15.43 to 120.37 | < 0.001 |
| > 104 weeks after MDC | 53.83 | 22.42 to 176.42 | < 0.001 |
| Low season | Ref. | – | – |
| Moderate season | 1.01 | 0.70 to 1.46 | 0.95 |
| High season | 2.83 | 1.98 to 4.08 | < 0.001 |
| Rainfall (mm) | 1.00 | 0.99 to 1.00 | 0.63 |
The linear regression model, restricted to the 5 sentinel sites located on the eastern coast of Madagascar showed after 36 weeks and beyond of the MDC a sustained 14% decrease (95% CI [7.7–20.6], p < 0.001) of malaria cases, as compared to the previous period, in Toamasina, district that benefited from additional continuous distribution of LLINs. In other sites without continuous LLIN distribution (Farafangana, Mananjary, Maroantsetra and Sambava), a 12% increase (95% CI [8.1–16.2], p < 0.001) of malaria cases was observed over the same period (Table 3).
Table 3.
Estimation of the impact of continuous LLIN distribution by Linear Regression Model of percentile values of number of malaria cases for each week of the period and each of the 5 sentinel sites located on the East Coast of Madagascar. Rainfall: site specific estimated rainfall in millimeters lagged for 8 weeks; CI: confidence interval.
| Coefficient (β) | 95% CI | t value | p-Value | |
|---|---|---|---|---|
| Intercept | 41.24 | 37.45 to 45.02 | 21.34 | < 0.001 |
| > 7 weeks and ≤ 36 weeks after MDC and no continuous LLIN distribution | Ref. | – | – | – |
| > 36 weeks after MDC and no continuous LLIN distribution | 12.16 | 8.09 to 16.22 | 5.86 | < 0.001 |
| > 36 weeks after MDC and continuous LLIN distribution | − 14.15 | − 20.64 to − 7.66 | − 4.28 | < 0.001 |
| Low season | Ref. | – | – | – |
| Moderate season | 20.96 | 16.50 to 25.41 | 9.22 | < 0.001 |
| High season | 17.35 | 12.06 to 22.63 | 6.44 | < 0.001 |
| Rainfall (mm) | − 0.009 | − 0.009 to 0.07 | 1.51 | 0.13 |
4. Discussion
The study results support the malaria-preventive impact of LLIN MDCs in Madagascar; MDCs were almost all followed by drop in malaria alerts across the sentinel surveillance system. However, the duration of this protection seems to be limited to one malaria season if not reinforced with a continuous LLIN distribution. In our analysis, LLINs appear to provide adequate protection at population level only for the first transmission season following the mass distribution. Rebounds in malaria alerts were almost always observed between the three-yearly MDCs of LLINs [10]. Mass campaigns are a cost-effective way to rapidly achieve high and equitable coverage, but experience showed that coverage gaps emerge almost immediately post-campaign through net deterioration, loss of nets, and population growth [11]. Moreover, several studies have shown that variability in net decay appears to be substantial and the average ‘lifespan’ could be considerably less than 3 years [10], [26]. This study suggests that these declines in coverage and effectiveness translate into a reduced impact on the number of malaria cases. So, health policymakers should give attention to both LLIN quality [27] and distribution plans optimizing coverage to maximize impact on morbidity and, likely, on mortality.
Our results revealed that within the first year following a MDC of LLINs in Madagascar only one out of the 18 sites reached an alert threshold, which occurred only 8 weeks following the distribution or one week if considering an expected lag of seven weeks after the campaign to observe its effect. We began the time series seven weeks after the beginning of the LLIN distribution to account for the delay (i) between beginning of distribution and effective coverage of LLINs in studied areas, (ii) and between infection and diagnosis. No alerts were recorded during the seven-week period after LLIN distribution and therefore possible alerts in this early phase were not artificially masked in the Kaplan–Meier curve. Since the MDCs took place just before the high transmission season, all but one site were alert-free during the first malaria season following a MDC.
The odds of a malaria alert increased more than 30-fold during the second year and 50-fold during the third year following MDCs; the percentage of malaria alert free sites dropped to 56.7% at the end of year 2, and further decreased to 31.5% at the end of year 3. The Kaplan Meier curve (Fig. 4) shows steep yearly steps reflecting the seasonality and synchronicity of LLIN MDCs over the sentinel sites (Fig. 3). In summary, sentinel sites are very well protected for one high transmission season but the duration of the impact of MDCs measured by survival analysis and linear regression analysis dropped abruptly after one year. The persistence of the current vector control strategy, mostly based on triennial MDCs of LLINs without continuous distribution, could lead to malaria resurgence well before the three-yearly replacement of the LLINs [10]. This poor outcome had been suspected in a recent malaria outbreak on the southeastern coast of Madagascar [28]. The malaria outbreaks observed in 2014 in the eastern coast of Madagascar (except Toamasina) and in 2015 in the whole sentinel network occurred a year after their respective LLIN MDCs. A failure in the effectiveness of LLINs after only one year could explain this premature upsurge. However, it should be noted that the unexpected resurgence of malaria across Madagascar in 2015 quickly led to a nationwide ACT shortage, but this could not be controlled in this analysis. Shortages in ACTs resulted from the unanticipated increase of clinical cases, which then probably contributed to the epidemic by increasing the parasite reservoir in untreated clinical cases. ACT stock-outs have also been reported during the malaria outbreak on the south-eastern coast of Madagascar in 2012 [28].
The analysis from the sentinel site of Toamasina suggests that a combination of MDCs followed by a community-based continuous distribution of LLINs succeeded in maintaining a low number of malaria cases for several years. The weekly number of cases in Toamasina was 14% below the reference period (after MDC and before continuous distribution) while malaria cases in sentinel sites located on the same malaria transmission pattern without continuous distribution were 12% above the reference period. Community-based continuous distribution, although challenging to implement (training, ongoing supervision, strong logistics and supply chains) [23], appeared to have preserved the benefits of previous MDCs with a positive public health impact.
There are some limitations in the current study that should be noted and nuanced. While we provide evidence that LLIN MDC prevented malaria alerts for one year, control sites would be needed to fully demonstrate that this increase was caused by a transient impact of MDC. However, all coastal areas of Madagascar with moderate to high transmission were receiving triennial MDC of LLINs and controls would be ethically unacceptable. Additional malaria control activities concomitant to MDCs could be an alternative explanation to the short-lived effectiveness, although no such event was reported. Our analysis was based solely on surveillance data and encourages the use of this readily available source to assess the performance of innovative malaria control programs. However, this approach comes with intrinsic limitations such as the use of cost-effectiveness data or the lack of randomized controls to compare intervention effectiveness.
MDCs are considered here as a uniform intervention although different brands (likely with varying bio-efficacy) were distributed. Routine malaria surveillance does not include “ITN use and access data” and we could therefore not make any inferences in this regard. However, results from recent Malaria Indicator Surveys show that Madagascar ranks among the highest rates in ITN use among those with access to a bednet; suggesting this is not a significant factor [29]. Moreover, other factors such as biomedical perception of malaria could affect the effectiveness of MDC of LLINs and could be unevenly distributed in Madagascar [30].
Only Toamasina benefited from a continuous net distribution which might not be representative of the situation in other areas in the country. This intervention was added on top of the triennial MDCs with hopes of improving malaria control. However neighboring sentinel sites had similar malaria endemicity to Toamasina until the LLIN continuous distribution started. Perhaps Toamasina underwent special meteorological conditions or a reinforced access to antimalarials preventing a malaria rebound. Nevertheless, the positive outcomes of this novel strategy were detected using surveillance data and are worth sharing.
Despite its limitations, the sentinel surveillance system in Madagascar provided a real-time evaluation of the impact of malaria control interventions at both national and regional levels. Information on the duration of LLIN impact at population level will further help malaria control programs to define the optimal timing of subsequent net distribution campaigns. As electronic-Health technologies develop, surveillance data is increasingly available and similar analyses could be conducted at low cost in many countries. A scale-up of e-Health solutions, as currently occurring in Madagascar, opens the doors to more solid designs such as Cluster Randomized Control Trials. The use of routine sentinel surveillance to monitor and assess effectiveness of MCIs over time could easily guide malaria elimination strategies.
Authors' Contributions
PP initiated the project. FG drafted the manuscript. PP, FG, TK and YM contributed to the data analysis, interpretation and writing. MR, VH, CR and JH contributed to data interpretation and writing. LR, MR, LR and RR contributed the data collection and preparation of the data. All authors reviewed and approved the final manuscript.
Funding
This research was supported by ASPR within the US DHHS (Assistant Secretary for Preparedness and Response with the US Department of Health and Human Services, grant No. 5 IDSEP140020-03-00) and by the USAID (grant No. AID-687-G-13-00003).
Declaration of Interests
No conflicts of interest.
Ethics Approval and Consent to Participate
Not applicable.
Acknowledgments
Acknowledgments
We thank administration authorities and health authorities from the Ministry of Health, the National Malaria Control Program and the President's Malaria Initiative. We especially thank all the sentinel surveillance team of the Institut Pasteur de Madagascar. We thank Bienvenue Rahoilijaona and Reziky Mangahasimbola for data base management.
Disclaimers
“The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.”
Consent for Publication
All authors approved the manuscript's submission for publication.
Availability of Data and Materials
Data are available from the Ministry of Health and from the Institut Pasteur de Madagascar.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Geneva: WHO . Vol 365. 2014. Global Malaria Programme WHO: World Malaria Report 2014. [Google Scholar]
- 2.Ministère de la Santé Publique de Madagascar . 2012. Plan Strategique De Lutte Contre Le Paludisme Madagascar. [Google Scholar]
- 3.Bhatt S., Weiss D.J., Cameron E. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015 doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesteman T., Randrianarivelojosia M., Raharimanga V., Randrianasolo L., Piola P., Rogier C. Effectiveness of malaria control interventions in Madagascar: a nationwide case-control survey. Malar J. 2016 doi: 10.1186/s12936-016-1132-x. Accepted:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2 doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Gerstl S., Dunkley S., Mukhtar A. Long-lasting insecticide-treated net usage in eastern Sierra Leone - the success of free distribution. Tropical Med Int Health. 2010;15(4):480–488. doi: 10.1111/j.1365-3156.2010.02478.x. [DOI] [PubMed] [Google Scholar]
- 7.Statement C., Systems C.D., Treated I. 2011. RBM Vector Control Working Group (VCWG) Continuous Distribution Workstream; pp. 1–2. [Google Scholar]
- 8.Dhiman S., Veer V. Culminating anti-malaria efforts at long lasting insecticidal net? J Infect Public Health. 2014;7(6):457–464. doi: 10.1016/j.jiph.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 9.WHO Global Malaria Programme WHO recommendations for achieving universal coverage with long-lasting insecticidal nets in malaria control. WHO. 2014;2013(September 2013):2013–2015. [Google Scholar]
- 10.Gnanguenon V., Azondekon R., Oke-Agbo F., Beach R., Akogbeto M. Durability assessment results suggest a serviceable life of two, rather than three, years for the current long-lasting insecticidal (mosquito) net (LLIN) intervention in Benin. BMC Infect Dis. 2014;14(1):69. doi: 10.1186/1471-2334-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Global Malaria Programme . 2013. Report to MPAC September 2013, Geneva. 2013. Methods for achieving universal coverage with long-lasting insecticidal nets in malaria control; pp. 1–27. (September) [Google Scholar]
- 12.Theiss-Nyland K., Lynch M., Lines J. Assessing the availability of LLINs for continuous distribution through routine antenatal care and the expanded Programme on immunizations in sub-Saharan Africa. Malar J. 2016;15(1):255. doi: 10.1186/s12936-016-1309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howes R.E., Mioramalala S.A., Ramiranirina B. Contemporary epidemiological overview of malaria in Madagascar: operational utility of reported routine case data for malaria control planning. Malar J. 2016;15(1):502. doi: 10.1186/s12936-016-1556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J.M., Smith D.L., Cotter C. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11(1):122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randrianasolo L., Raoelina Y., Ratsitorahina M. Sentinel surveillance system for early outbreak detection in Madagascar. BMC Public Health. 2010 doi: 10.1186/1471-2458-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girond Florian, Randrianasolo Laurence, Randriamampionona Lea. Analysing trends and forecasting malaria epidemics in Madagascar using a sentinel surveillance network: a Web-based application. Malar J. 2017:1–11. doi: 10.1186/s12936-017-1728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randriamampionona L., Piola P., Heraud J. Lessons from the evaluation of the influenza sentinel surveillance system in Madagascar, 2009–2014. Bull World Health Organ. 2017:375–381. doi: 10.2471/BLT.16.171280. (February) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . 2006. Systems for the early detection of malaria epidemics in Africa. An analysis of current practices and future priorities; pp. 1–100.http://www.who.int/malaria/publications/atoz/9789241594882/en/ [Google Scholar]
- 19.Geneva WHO . Third Meeting of the Technical Support Network. 2002. Prevention and control of malaria epidemics. 10–11 December 2001. [Google Scholar]
- 20.World Health Organization . Third meeting of the technical support network. 2002. Prevention and control of malaria epidemics. [Google Scholar]
- 21.World Health Organization . 2004. Field guide for malaria epidemic assessment and reporting. [Google Scholar]
- 22.Ministère de la Santé Publique Programme National de Lutte contre le Paludisme . Vol. 2011. Ministère de La Santé Publique; Antananarivo, Madagascar: 2011. Revue Du Programme Paludisme À Madagascar. [Google Scholar]
- 23.De Beyl C.Z., Kilian A., Brown A. Evaluation of community - based continuous distribution of long - lasting insecticide - treated nets in Toamasina II District, Madagascar. Malar J. 2017:1–14. doi: 10.1186/s12936-017-1985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenker H. 2015. NetWorks end of project report 2015. [Google Scholar]
- 25.National Oceanic and Atmospheric Administration's. http://iridl.ldeo.columbia.edu/SOURCES/.NOAA/.NCEP/.CPC/.FEWS/.Africa/.DAILY/.RFEv2/.est_prcp/.
- 26.World Health Organization . 2011. Guidelines for monitoring the durability of long-lasting insecticidal mosquito nets under operational conditions; p. 1. [Google Scholar]
- 27.Randriamaherijaona Sanjiarizaha, Raharinjatov Jacky. Raymond Beach SB. Durability monitoring of Long-Lasting Insecticidal (mosquito) Nets (LLINs) in Madagascar: 1-physical integrity and insecticidal activity. Manuscr Submitt. 2017:1–11. doi: 10.1186/s13071-017-2419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesteman T., Rafalimanantsoa S.A., Razafimandimby H. Multiple causes of an unexpected malaria outbreak in a high-transmission area in Madagascar. Malar J. 2016;15(1):57. doi: 10.1186/s12936-016-1113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenker H., Ricotta E., Olapeju B. Baltimore, MD. PMI | VectorWorks project, Johns Hopkins Center for communication programs. May 2018. Insecticide-treated nets (ITN) access and use report. [Google Scholar]
- 30.Mattern C., Pourette D., Raboanary E. Tazomoka is not a problem. Local perspectives on malaria, fever case management and bed net use in Madagascar. PLoS One. 2016;11(3):1–14. doi: 10.1371/journal.pone.0151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Ministry of Health and from the Institut Pasteur de Madagascar.