SUMMARY
Albinism, the reduction or loss of melanin pigment, is found in many diverse cave-dwelling animals. The mechanisms responsible for loss of melanin pigment are poorly understood. In this study we use a melanogenic substrate assay to determine the position where melanin synthesis is blocked in independently evolved cave planthoppers from Hawaii and Croatia. In this assay, substrates of enzymes responsible for melanin biosynthesis are added to fixed specimens in vitro and their ability to rescue black melanin pigmentation is determined. L-tyrosine, the first substrate in the pathway, did not produce melanin pigment, whereas L-DOPA, the second substrate, restored black pigment. Substrates in combination with enzyme inhibitors were used to test the possibility of additional downstream defects in the pathway.
The results showed that downstream reactions leading from L-DOPA and dopamine to DOPA-melanin and dopaminemelanin, the two types of insect melanin, are functional. It is concluded that albinism is caused by a defect in the first step of the melanin synthesis pathway in cave-adapted planthoppers from widely separated parts of the world. However, Western blots indicated that tyrosine hydroxylase (TH), the only enzyme shown to operate at the first step in insects, is present in Hawaiian cave planthoppers. Thus, an unknown factor(s) operating at this step may be important in the evolution of planthopper albinism. In the cavefish Astyanaxmexicanus, a genetic defect has also been described at the first step of melanin synthesis suggesting convergent evolution of albinism in both cave-adapted insects and teleosts.
INTRODUCTION
Cave-dwelling animals have evolved a suite of regressive phenotypes highlighted by the degeneration of eyes and pigmentation (Porter and Crandall 2003; Culver and Pipan 2009; Juan et al. 2010). Pigmentation normally protects organisms from the harmful effects of UV irradiation, reduces the extent of predation through camouflage and mimicry, and facilitates sexual reproduction by mediating colorful behavioral displays (Protas and Patel 2008). In the darkness of caves, however, these functions are subjected to relaxed selection and pigmentation can disappear without deleterious consequences on fitness. The loss of melanin pigment, which occurs in a wide range of different cave animals (including flatworms, mollusks, crustaceans, insects, and vertebrates), is known as albinism. In addition to cave dwellers, albinism is frequently encountered in diverse animals inhabiting other lightless environments, such as the deep sea, the soil, and in parasites living within the body of their hosts. Albinism is also found in animals living in lighted environments, but it is present at low frequencies in natural populations probably because of deleterious effects on fitness. In all of these situations, the developmental and evolutionary mechanisms underlying albinism are poorly understood.
Melanin is produced in a biochemical pathway whose basic features are conserved across a broad range of different organisms (Riley 1997; Vavricka et al. 2010). This pathway is best characterized in vertebrate melanosomes, the organelles responsible for melanin production (Fig.1A).The first step in the pathway is the conversion of Ltyrosine into L-3, 4-Dihydroxy-L-phenylalanine (L-DOPA), which is subsequently converted through L-DOPAquinone, L-DOPAchrome and a few other intermediates to melanin. The first two steps from L-tyrosine through L-DOPA to LDOPAquinone are catalyzed by the multifunctional enzyme tyrosinaseandtherestbyseveraldifferentenzymes,including the tyrosinase-related proteins and tyrosinase itself.
Fig.1.
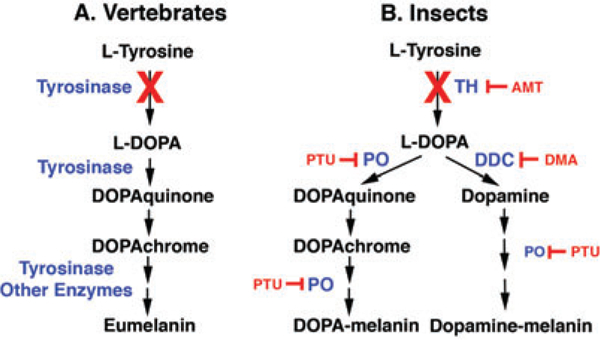
Melaninsynthesispathwayscomparedinvertebratesand insects. (A) The generalized vertebrate melanin synthesis pathway emphasizing the early steps in which tyrosinase successively converts L-tyrosine to L-DOPA and DOPAquinone and subsequent reactions produce eumelanin. (B) A simplified insect melanin synthesis pathway in which TH converts L-tyrosine to L-DOPA and two subsequent branches in which PO is involved in the conversion of L-DOPA to produce DOPA-melanin and DDC is involved in the conversion of L-DOPA to dopamine to produce dopamine-melanin, both through a series of reactions. Enzymes are shown in blue. Enzyme inhibitors are shown in red. Red Xs illustrate the defective steps in the cavefish and planthopper pathways. Abbreviations are explained in the text. Multiple arrows are not representative of the number of steps in the pathways. The consensus insect melanin synthesis pathway (B) is drawn according to True (2003).
One of the types of human albinisms is known as oculocutaneous albinism(OCA) because of the absence of melaninin both the eyes and skin. Four types of OCA are known:OCA1 is caused by mutations in the tyrosinase gene, OCA-2 and OCA-4 by mutations in the oca2 and mapt genes, respectively, which function upstream of tyrosinase at the beginning of the pathway, and OCA-3 by mutations in the tyrosinase-related protein-1 gene, which functions downstream of tyrosinase (Oeting and King 1999). The presence of multiple human OCAs implies that the melanin synthesis is vulnerable to change throughout the biosynthetic pathway.
Little is known about what steps of the melanin synthesis pathway have been changed during the evolution of albinism in cave animals with the notable exception of the cavefish Astyanax mexicanus (Jeffery 2006). In this species, the first step in melanin production, the conversion of L-tyrosine into L-DOPA, is affected due to loss-of-function mutations in the oca2 gene (Protas et al. 2006). Therefore, according to the human nomenclature, cavefish would be classified as OCA-2albinos.The precisefunction ofOCA2protein iscurrently unclear but it could possibly control the availability of L-tyrosine for conversion to L-DOPA by tyrosinase. Consistent with its disruption at the first step of the pathway, cavefish melanin pigmentation can be rescued by providing exogenous L-DOPA but not L-tyrosine to specimens in vitro (McCauley et al. 2004). Furthermore, the first step of the pathway has been targeted repeatedly by different oca2 mutations in several independently evolved Astyanax cavefish lineages (McCauley et al. 2004; Protas et al. 2006), suggesting that albinism has evolved multiple times by convergence. The evolution of pigmentation has also been studied in another albino cave animal, the crustacean Asellus aquaticus (Protas et al. 2011). In this species, albinism is inherited as a recessive trait caused either by mutation in a single gene locus or in two different gene loci.
The details of the insect melanin synthesis pathway are not as well resolved as in vertebrates and differ from them in several fundamental aspects (Fig. 1B; True 2003). First, tyrosine hydroxylase (TH), rather than tyrosinase, is responsible for the conversion of L-tyrosine to L-DOPA. Second, phenol oxidase (PO) catalyzes the conversion of LDOPA to DOPAquinone and some of the downstream reactions in the pathway. Third, there are two types of insect melanins: L-DOPA-melanin and dopamine-melanin, which are produced by separate branches of the pathway beginning at the level of L-DOPA. In one branch, L-DOPA is converted to L-DOPAquinone by PO, and downstream reactions produce DOPA-melanin. In the other branch, LDOPA is converted to dopamine by DOPAdecarboxylase (DDC), and subsequent reactions also involving PO activity produce dopamine-melanin. DOPA-melanin and dopaminemelanin are secreted into the hemolymph or deposited into the cuticle by epidermal cells, where they are responsible for dark pigmentation. In addition to pigmentation, melanin and the enzymes participating in its production have been implicated in cell-mediated immunity (Marmaras and Lampropoulou2009)andhardeningofthecuticleduringmolting (Sugumaran 1988; Hopkins and Kramer 1992; Gorman and Arakane 2010).
Similar to some vertebrates, insects have become adapted to life in caves, resulting in the reduction or loss of eyes, reduced wings, and albinism in many species (Barr1968). However, the mechanisms underlying albinism in cave-adapted insects are unknown. In order to shed light on these mechanisms and examine their possible convergence, we have studied melanin synthesis in two independently evolved cixiid planthopper species from lava tubes in Hawaii (Howarth 1972; Fennah 1973) and limestone caves in Croatia. Surfacedwelling cixiid planthoppers spend their immature stages as juveniles associated with plant roots in the soil. After the final molt, adults emerge, migrate to the surface, and feed on the aerial portions of the host plant(s). Mature adults of caveadapted Hawaiian planthoppers and possibly the Croatian species as well have undergone an adaptive shift in which they remain associated with roots in caves rather than migrate to the surface (Howarth 1987). The present study describes the convergent evolution of albinism in Hawaiian and Croatian cave planthoppers and compares its underlying causes with those in a distantly related organism, Astyanax cavefish.
MATERIALS AND METHODS
Animals
Late instars and adults of the Hawaiian cixiid planthopper Oliarus polyphemus were collected by aspiration from Metro siderus polymorpha roots and lava tube walls in Kaumana Cave near Hilo, HI, USA. Animals were maintained in humidified plastic chambers containing roots as food until being assayed. Fourth and fifth in stars of an undescribed Croatian cixiid species were collected from roots and under rocks in Male ponte jama and Špilja kod Nerezinog dola on the island of Mljet, Croatia and were maintained in humidified chambers containing coniferous needles prior to the assay. Immature developmental stages of both cave species were classified according to Sforza et al. (1999). Surface-dwelling planthoppers were collected in central Dalmatia, Croatia, Drosophila melanogaster (Oregon R strain) was a gift of Dr. Leslie Pick, University of Maryland, and A. mexicanus was obtained from the Jeffery Laboratory, University of Maryland.
Melanogenic substrate assays
Melanogenic substrate assays were adapted from those previously developed for the cavefish A. mexicanus (McCauley et al. 2004) and several different insect species (Jones and Sinclair 1958; Nijhout 1980; Hiruma et al. 1985; Walter et al. 1996; Fatahashi and Fugiwara 2005). Assays were either conducted in the field immediately after animal collection or after living or fixed specimens were brought to the laboratory. Animals were fixed in 5% formalin dissolved in phosphate buffered saline (PBS) for 1 hr at room temperature. The fixative was removed by five washes in PBS before adding the substrates. The specimens were subsequently immersed in various substrates or combinations of substrates and inhibitors: 0.1% L-tyrosine, 0.1%L-DOPA,0.1%dopaminehydrochloride,amixtureof0.1% L-DOPA or 0.1% dopamine and 0.1% phenylthiourea (PTU), or 1 mM alpha-methyl-DL-tyrosine (AMT) or 1 mM 3-(3,4-Dihydroxyphenyl)-2-methyl-L-alanine sesquihydrate (DOPA methyl-L-alanine). The substrates and substrate-inhibitor mixtures were dissolved in phosphate buffer (pH 7.4). All substrates and inhibitors were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Assays containing dopamine were conducted in the absence of light. Control specimens were immersed in buffered water (pH 7.4) instead of substrates. The assays were terminated after pigment deposition occurred (2–5 days) by washing specimens in PBS and postfixation in 5% formalin or 4% paraformaldehyde. Some specimens were embedded in polyester wax and serial sectioned at 10 μm.
Protein extraction and Western blots
Protein extracts were prepared by homogenizing animals in RIPA buffer containing a protease inhibitor cocktail (SigmaAldrich). The homogenates were cleared by centrifugation, and the supernatants were stored at −20°C. Protein extracts were subjected to electrophoresis through 10% SDS-polyacrylamide (SDS/PAGE) gels containing prestained protein markers and transferred to PVDF filters (Bio-Rad Laboratories, Hercules, CA) by standard methods (Harlow and Lane 1988). The filters were incubated with 5% nonfat dry milk in TBST buffer (50 mM Tris-HCl, pH 7.6, 100 mM NaCl, 0.1% Tween-20) to block nonspecific binding, then washed three times for 5 min in TBST, incubated overnight in a 1:100 dilution of mouse TH monoclonal antibody (clone LNC1, Millipore, Billerica, MA, USA) in TBST at 4°C, and finally incubated for 1 hr at room temperature in a 1:25,000 dilution of HRP-conjugated anti-mouse IgG secondary antibody. After three washes for 5 min with TBST, the signals were visualized with Chemiluminescence Luminol (Santa Cruz Biotechnology, Santa Cruz, CA, USA) used according to the supplier’s specifications. Images of X-ray films were taken with a digital camera.
RESULTS
Albinism in cave-adapted planthoppers
Surface-dwelling planthoppers are darkly pigmented due to melanin presence throughout their bodies, including in the eyesandwings(Fig.2 AandB).Theextentofpigmentregression in the Hawaiian and Croatian planthopper species was determined by microscopy. All of the developmental stages tested, late in stars and adults of the Hawaiian species and late in stars of the Croatian species, lacked black pigmentation externally, although they showed yellow-tan pigmentation on the dorsal cuticle of the head, thorax, abdomen, and vestigial wings (when present) (Fig. 2B inset, C–H). Serial sectioning showed that there was also no internal melanin pigmentation in either species (data not shown). From this, we conclude that the Hawaiian and Croatian cave planthopper species are albinos lacking any black pigmentation.
Fig. 2.
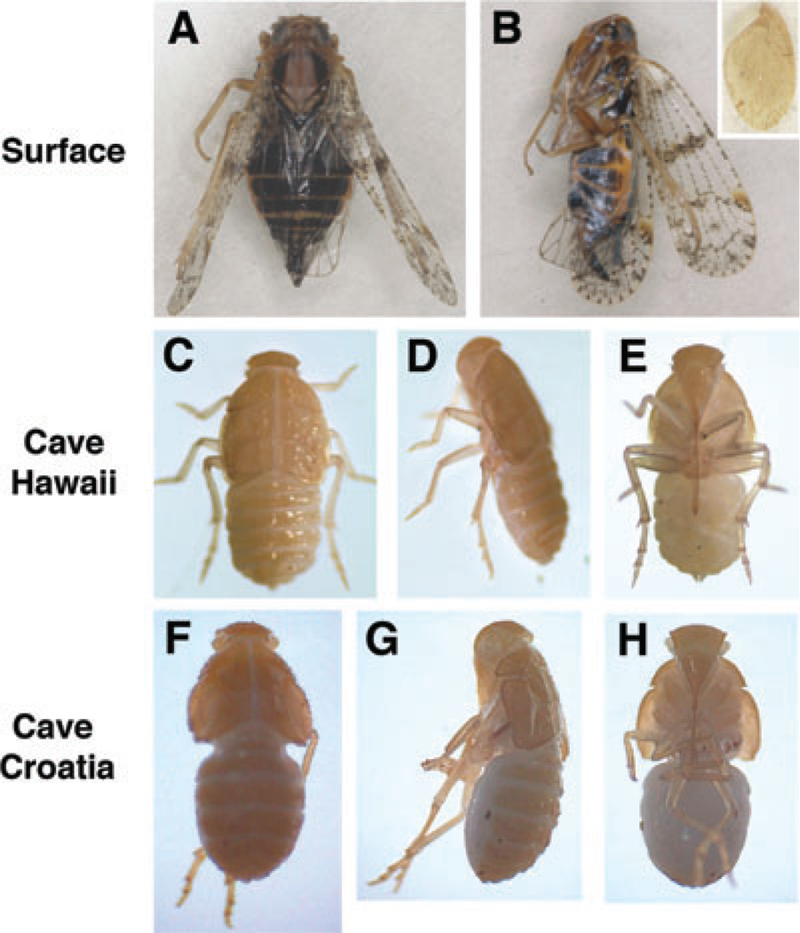
Pigmentation and albinism in planthoppers. (A, B). A surface-dwelling cixiid planthopper viewed from the dorsal (A) and lateral (B) sides showing melanin pigmentation in the body and wings. Inset in (B) shows the vestigial wing of an albino Hawaiian cave planthopper at the same magnification as the surface planthopper. (C–H). Albino fifth instar Hawaiian (C–E) and Croatian (F–H) cave planthoppers viewed from the dorsal (C, F), lateral (D, G), and ventral (E, H) sides.
L-DOPA produces melanin pigmentation in albino planthoppers
The melanogenic substrate assay was used to determine the position of the defect(s) in melanin synthesis leading to albinism in the Hawaiian and Croatian cave planthoppers. In this assay, different substrates were added to lightly fixed and permeabilized specimens, and the appearance of black pigmentation was determined by microscopy. When late instars and adult planthoppers were incubated in L-DOPA black pigment was formed throughout the body, including the dorsal cuticle, abdomen, legs, the shafts, and surrounding areas of sensory bristles and hairs on the body and wings (when present), and the antennal bases in the head (Fig. 3A–C; Table 1). Controls exposed to the same conditions without L-DOPA did not form black pigment (Table 1). Additional controls were done to determine whether black pigment deposition was due to an enzymatic reaction. First, fixed animals were heated to 65°C prior to the assay to denature proteins and destroy enzyme activity. High temperature abolished the ability of L-DOPA to rescue pigmentation (Table 1). Second, specimens were incubated with a mixture of L-DOPA and the PO inhibitor PTU. The accumulation of black pigment was eliminated or substantially reduced by PTU (Fig. 3D–F; Table 1). In contrast, incubation of specimenswithL-DOPAandtheTHinhibitorADTdidnotaffect black pigment formation (Table 1), consistent with TH function at the step before L-DOPA in the pathway. These results support the conclusion that pigment synthesis was due to an enzymatic reaction.
Fig.3.

MelanogenicsubstrateassaysincaveplanthoppersafterincubationinL-DOPA,L-DOPAandPTU,L-tyrosine,ordopamine. (A–C). Adult Hawaiian cave planthopper (A, B) and fifth instar Croatian (C) cave planthopper showing production of black pigment after incubation in L-DOPA. (D–F). Adult Hawaiian cave planthopper (D, E) and fifth instar Croatian cave planthopper (F) lacking black pigment after incubation in L-DOPA and the PO inhibitor PTU. (G–I). Adult Hawaiian cave planthopper (G, H) and fifth instar Croatian cave planthopper (I) showing no black pigment deposition after incubation in L-tyrosine. (J–L). Adult Hawaiian cave planthopper (J, K) and fifth instar Croatian (L) cave planthopper showing production of black pigment after incubation in dopamine. (B, E, H, K). Wings of Hawaiian cave planthoppers dissected from the bodies after completion of the assays.
Table 1.
The effects of substrates, various treatments, and enzyme inhibitors on rescue of black pigmentation in Hawaiian and Croatian cave planthoppers
| Pigment deposition (+ or −/N) |
||||||
|---|---|---|---|---|---|---|
| Treatments | ||||||
| Substrate | Pre- | Co- | Post- | Enzyme affected | Hawaiian | Croatian |
| None | — | — | — | — | (−/2) | (−/3) |
| L-tyrosine | — | — | — | — | (−/6) | (−/5) |
| L-tyrosine | — | — | L-DOPA | — | (+/2) | (+/2) |
| L-DOPA | — | — | — | — | (+/8) | (+/8) |
| L-DOPA | 65°C | — | — | all | (−/2) | (−/2) |
| L-DOPA | — | PTU | — | PO | (−/3) | (−/3) |
| L-DOPA | — | AMT | — | TH | (+/2) | (+/1) |
| L-DOPA | — | DMA | — | DDC | (+/1) | (+/3) |
| Dopamine | — | — | — | — | (+/4) | (+/5) |
| Dopamine | — | PTU | — | PO | (−/3) | (−/3) |
N: number of specimens. Other abbreviations are explained in the text.
Based on the ability of L-DOPA to produce black pigmentation, we conclude that PO and all of the downstream enzymes are present and potentially functional in at least one of the two pathways responsible for melanin production (see below). In addition, these data suggest that the defect in melanin synthesis is upstream of L-DOPA in albino planthoppers of both species, possibly at the step in which L-tyrosine is converted to L-DOPA.
L-tyrosine does not produce melanin pigmentation in albino planthoppers
Hawaiian and Croatian planthoppers were incubated in Ltyrosine to determine whether the melanin synthesis path way is disrupted at the step between L-tyrosine and L-DOPA. The rationale for conducting these experiments was as follows. Considering the demonstration that L-DOPA incubation produces melanin pigmentation (see above), if Ltyrosine incubation produced melanin production, then the defect would be located upstream of L-tyrosine, where as if Ltyrosine failed to rescue pigmentation, then the defect would be located between L-tyrosine and L-DOPA. All of the developmental stages we tested in both planthopper species failed to produce black pigment during L-tyrosine incubation (Fig.3G–I; Table 1). When the same specimens were subsequently incubated in L-DOPA black pigment was formed (Table 1), showing that they were able to produce pigment when incubated with an appropriate substrate. These results indicate that the Hawaiian and Croatian planthopper species are unable to use L-tyrosine to make black pigment. Therefore, it is concluded that melanin synthesis is probably blocked at the step between L-tyrosine and L-DOPA in cave planthoppers.
Downstream steps in the melanin synthesis pathway are not affected in albino planthoppers
The insect melanin synthesis pathway branches in two directions from the point of L-DOPA production: one branch leads to L-DOPA-melanin and the other to dopaminemelanin (Fig. 1B). As both branches produce dark pigment, our conclusion that melanin synthesis is blocked at the first step does not preclude the possibility of additional defects further downstream from the bifurcation in one of the two branches. The possibility of such downstream defects was examined in two experiments. First, dopamine, the key substrate in the dopamine-melanin branch (Fig. 1B), was used as the substrate, and all treated specimens of both albino planthopper species were able to produce black pigmentation (Fig. 3J–L; Table 1). The ability of dopamine to produce melanin was blocked by the PO inhibitor PTU (Table 1), confirming PO function downstream in the dopaminemelanin branch (Fig. 1B). Second, to examine the functionality of the DOPA-melanin branch pathway, L-DOPA was used as a substrate in combination with the DDC inhibitor DOPA-methyl-L-alanine (DMA). Black pigment was deposited in all specimens in this experiment (Table 1). Because DMA blocks the dopamine-melanin branch of the pathway, this shows that the DOPA-melanin pathway is functional (Table 1). The results demonstrate that the branch pathways leading from dopamine-to-dopamine melanin and from LDOPA to DOPA-melanin have no lesions and can be functional in both species of cave-adapted planthoppers.
Tyrosinase hydroxylase is present in an albino planthopper
TH is the only enzyme known to function between L-tyrosine and L-DOPA in the insect melanin biosynthesis pathway (True 2003). To determine whether TH is present, we subjected protein extracts of Hawaiian cave planthoppers to SDS/PAGE and Western blot analysis with a cross-reacting monoclonal TH antibody (Fig. 4A). As controls, the Western blots also contained D. melanogaster (Fig. 4B) and A. mexicanus (Fig. 4C) protein extracts. A protein band of the expected molecular mass (58–62 kDa; Stathakis et al. 1999; Gorman and Arakane 2010) was detected in each species. We conclude that TH is present in the Hawaiian cave planthopper species.
Fig. 4.
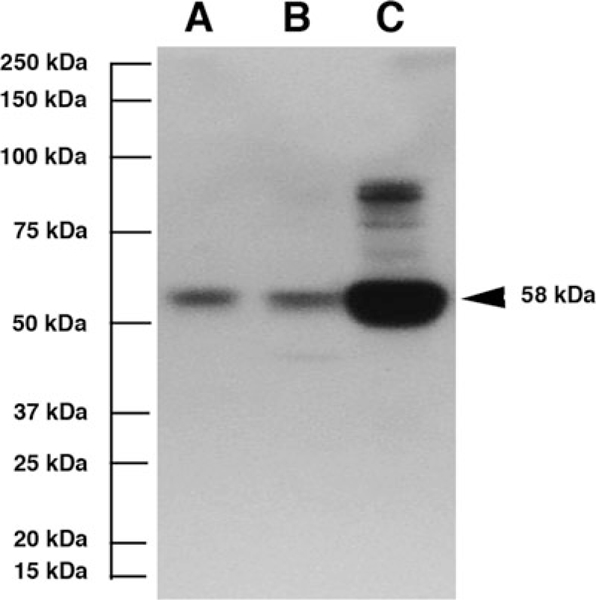
Western blot of proteins extracted from whole adult animals probed with TH antiserum showing immunoreactivebands of 58–62 kDa in (A) the Hawaiian cave planthopper, (B) Drosophila melanogaster and (C) Astyanax mexicanus. The molecular mass scale is indicated on the left and 58-kDa band representing A. mexicanus TH on the right.
DISCUSSION
The results of this study suggest that independently evolved albino planthoppers living in lava or limestone caves are defective in the first step of the melanin synthesis pathway, the conversion of L-tyrosine to L-DOPA. The evidence for this conclusion is that administration of L-DOPA, the second substrate in the pathway, but not L-tyrosine, the first substrate, can rescue pigmentation in both planthopper species. If the planthopper pathway was functional at the first step, exogenously supplied L-tyrosine should have been capable of normal conversion to L-DOPA, as shown in in vitro studies of pigmented insect species (Owen and Bouquillon 1992; Fatahashi and Fugiwara 2005). Blockage upstream of L-tyrosine in the pathway beginning with L-phenylalanine is unlikely because of expected effects on protein synthesis. Furthermore, the L-tyrosine to L-DOPA step is apparently the only lesion in the albino planthopper melanin synthesis pathway because the parallel branches from L-DOPA to DOPA-melanin and dopamine to dopamine-melanin can be functional when animals are provided with appropriate substrates. Accordingly, all of the enzymes and other factors downstream of L-DOPA are likely to be conserved and potentially functional in cave planthoppers. The first step of the pathway is also the single lesion in melanin production in independently evolved populations of the cavefish A. mexicanus (McCauleyetal.2004; Protasetal.2006). We conclude that a critical change(s) resulting in the evolution of albinism has occurred at the beginning of melanin synthesis in divergent lineages of cave-adapted insects and vertebrates.
Based on human albinisms (Oeting and King 1999), defective melanin pigmentation can be caused by mutations in the tyrosinase gene, which encodes the rate-limiting enzyme in the pathway, and in genes that function upstream or downstream of tyrosinase. If defects throughout the pathway can lead to albinism, then why is the critical lesion restricted to the first step in both cave-adapted insects and vertebrates? There are several possible explanations. First, the gene(s) operating at the first step may be more susceptible to mutation, perhaps due to the large size or genomic location. For example, the oca2 gene, which is responsible for cavefish albinism, covers about 345 KB in a region of frequent chromosomal rearrangements in humans (Yi et al. 2003). Although oca2 genes have only been studied in vertebrates, a similarly large gene involved in L-tyrosine metabolism or processing could be a mutational hotspot in planthoppers as well. Second, this step may be the only place where mutations are allowed because changes in other genes, such as those encoding enzymes involved in innate immunity (see below), would be lethal. Third, blocking the pathway at its beginning could enhance the pool of available L-tyrosine for other metabolic functions that are adaptive for survival in the cave environment. For example, L-tyrosine is also the starting point for synthesis of catecholamines, which have many different physiological and behavioral functions. Fourth, assuming that pigmentation loss is adaptive for life in caves, blocking the initial step of the pathway may be the simplest way to arrest pigmentation because it is the rate-limiting step and it also appears to be a developmental control point in pigmented species. Prior to the formation of a pigmented cuticle during molting, incubation with L-DOPA can cause premature melanin formation in nonalbino insects (Nijhout 1980; Hiruma et al. 1985; Walter et al. 1996), showing that LDOPA is a limiting factor during the normal development of pigmentation. The temporal extension of this developmental control point at later stages of the life cycle would be a convenient way to evolve permanent albinism. None of the explanations provided above are mutually exclusive, and several of them could potentially act in concert to repeatedly target the first step of melanin synthesis for evolutionary change.
Regressive evolution in cave animals has often been attributed to the benefits of conserving energy under conditions of food limitation in cave environments, which lack primary productivity (Culver and Pipan 2009). This explanation seems consistent with blocking melanin synthesis at its initial step because it would prevent potentially useless and energy consuming downstream reactions. However, cave planthoppers feed on roots that penetrate caves from the surface foliage (Howarth 1972, 1987) and are probably not food limited in the sense of most other cave animals. Therefore, energy conservation is not a likely driving force for blocking melanin synthesis at its first step in cave planthoppers. In general, cave planthoppers can provide unique insights into the relationship between energy conservation and regressive evolution,including there duction of eyes, pigmentation, and wings, because they are not subjected to food limitation as are other animals living in caves.
The insect melanin synthesis pathway has two functions in addition to pigment development. First, the pathway is coupled to cuticle formation during molting (Sugumaran 1988; Hopkins and Kramer 1992). Cave planthoppers have a cuticle, but it is completely lacking in melanin. Therefore, it seems likely that there has been a dissociation of pigmentation and cuticle formation during the evolution of albinism. In support of this idea, cuticle development and melanin production are also known to be independent processes in analbinolocuststrain(Malek1957;JonesandSinclair1958), and inhibition of the melanin pathway by PTU injection can lock black pigmentation but not cuticle development during pupation in blowfly larvae (Dennell 1958). Thus, the normal linkage between melanin and cuticle formation may not be obligatory and can be uncoupled in both experimental and evolutionary situations. Second, melanin synthesis has been implicated in innate immunity due to a requirement for some of its enzymes (e.g., PO) in this process, and melanin itself can serve as a structural element to encapsulate invading organisms. Our demonstration that L-DOPA substrate can drive melanin synthesis in cave planthoppers implies that all downstream enzymes that function in both pigmentation and immunity are present and functional. As for the role of melanin itself in an effective immune response, it has been shown that it may not always be required. For example, darkening of encapsulated parasitoid egg infections in Drosophila is due to cell death and not due to extra-cellular melanin deposition (Russo et al. 1996). Therefore, cave planthoppers probably have a normal innate immune system despite the absence of melanin.
What is the critical molecule(s) involved in melanin synthesis that is defective incave planthoppers? According to the consensus pathway for insects (True 2003), the only enzyme known to be involved in the first step of melanin synthesis is TH, which converts L-tyrosine to L-DOPA. Mutations in the pale (TH) gene cause pigment reduction in Drosophila (Neckameyer and White 1993), and pale is a candidate gene associated with a pigment loss QTL in A. aquaticus (Protas et al. 2011). However, TH is also required for cuticle development, neural functions, and immunity, and its absence would be expected to be lethal (Gorman et al. 2007; Gorman and Arakane 2010). This reasoning is consistent with our demonstration of a TH immunoreactive protein in Hawaiian cave planthoppers. Although cave planthopper TH could be mutated without a detectable change in protein size, the results open the possibility that an unknown factor functioning at the first step of the pathway could be responsible for cave planthopper albinism. This factor could modulate TH activity or make L-tyrosine available for conversion into LDOPA by TH to initiate melanin production. If the latter, then this situation would be analogous to that in cavefish where mutations in the oca2 gene, which has been postulated to be involved in L-tyrosine transport (Toyofuku et al. 2002), may cause albinism by limiting L-tyrosine availability (Protas et al. 2006). Thus, cave planthoppers and cavefish might converge not only in the position in which melanin synthesis is blocked but also in the physiological and molecular mechanisms underlying albinism.
Animals in phyla ranging from Porifera to Chordata have become adapted to life in caves and consequently have reduced or lost melanin pigmentation. In addition, other animals living in lightless conditions such as the deep sea, soils, and parasites in the bodies of their hosts have evolved albinism. Here, we have shown that albinism has appeared in two independently evolved cave planthoppers by a convergent defect in the first step in melanin synthesis, the same place in melanogenesis in which a lesion is found in independently evolved cavefish populations, indicating repeated evolutionary targeting of the first step of the pathway. To fully understand the convergence of albinism, it will be important to determine the position of the defect in melanin formation in a wider array of albino cave animals and in animals living in other lightless environments.
Acknowledgments
This research was supported by Croatian MSES Grant 098– 0982913–2874 to H. C. and an NSF grant IBN-05384 and a´ University of Maryland Research Achievement and Scholarship Award to W. R. J. We thank Branko Jalžić and David Jeffery for´ assistance with animal collection (State of Hawaii collection permit FMH10–219 and Republic of Croatia, Ministry of Culture collection permit KL: UP/1–612–07/10–33/1115, URBR: 532–08–01– 04/4–10–02), Boris Krstinic for assistance with photography, and´ Osvin Pecar, Principal of Mljet National Park, for logistical supportˇ during field trips. Two anonymous reviewers are thanked for their thoughtful comments concerning this manuscript.
REFERENCES
- Barr TC Jr. 1968. Cave ecology and the evolution of troglobites. Evol. Biol. 2: 35–102. [Google Scholar]
- Culver DG, and Pipan T 2009. Biology of Caves and Other Subterranean Habitats. Oxford University Press, Oxford. [Google Scholar]
- Dennell R 1958. The amino acid metabolism of a developing insect cuticle: the larval cuticle and puparium of Calliphora vomitoria. II. The non-specific hydroxylation of aromatic amino-acids and the production of polyphenols by the cuticle. Proc. Roy. Soc. London B 148: 280–284. [DOI] [PubMed] [Google Scholar]
- Fatahashi R, and Fugiwara H 2005. Melanin-synthesis enzymes coregulate stage-specific larval cuticular markings in the swallowtail butterfly, Papilio xuthus. Dev. Genes Evol. 215: 519–529. [DOI] [PubMed] [Google Scholar]
- Fennah RG 1973. The cavernicolous fauna of Hawaiian lava tubes, 4. Two new blind Oliarus. Pac. Insects 1: 181–184. [Google Scholar]
- Gorman MJ, An C, and Kanost MR 2007. Characterization of tyrosine hydroxylase from Manduca sexta. Insect Biochem. Mol. Biol. 37: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, and Arakane Y 2010. Tyrosine hydroxylase is required for cuticle sclerotization and pigmentation in Tribolium casteneum. Insect Biochem. Mol. Biol. 40: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, and Lane D 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hiruma K, Riddiford LM, Hopkins TL, and Morgan TD 1985. Roles of dopa decarboxylase and phenoloxidase in the melanization of the tobacco hornworm and their control by 20-hydroxyecdysone. J. Comp. Physiol. B 155: 659–669. [DOI] [PubMed] [Google Scholar]
- Hopkins TL, and Kramer KJ 1992. Insect cuticle sclerotization. Annu. Rev. Entomol. 37: 273–302. [Google Scholar]
- Howarth FG 1972. Cavernicoles in lava tubes on the island of Hawaii. Science 175: 325–326. [DOI] [PubMed] [Google Scholar]
- Howarth FG 1987EvolutionaryecologyofAeolianandsubterranean habitats in Hawai’i. Trends Evol. Ecol. 2: 220–223. [DOI] [PubMed] [Google Scholar]
- Jeffery WR 2006. Regressive evolution of pigmentation in the cavefish Astyanax. Is. J. Evol. Ecol. 52: 405–422. [Google Scholar]
- Jones BM, and Sinclair W 1958. Induction of melanin patterns and hardening as separate processes in the cuticle of albino locusts with internally absorbed phenol substrates. Nature 181: 926–927. [Google Scholar]
- Juan C, Guzik MT, Damia J, and Cooper SJB 2010. Evolution in caves: Darwin’s “wrecks of ancient life” in the molecular era. Mol. Ecol. 19: 3865–3880. [DOI] [PubMed] [Google Scholar]
- Malek SRA 1957. Sclerotization and melanization: two independent processes in the cuticle of the dessert locust. Nature 180: 23. [Google Scholar]
- Marmaras VJ, and Lampropoulou M 2009. Regulator and signaling in insect haemocyte immunity. Cell Signal 21: 186–195. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Hixon E, and Jeffery WR 2004. Evolution of pigment cell regression in the cavefish Astyanax: a late step in melanogenesis. Evol. Dev. 6: 209–218. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, and White K 1993. Drosophila tyrosinse hydroxylase is encoded by the pale locus. J. Neurogenet. 8: 189–199. [DOI] [PubMed] [Google Scholar]
- Nijhout HF 1980OntogenyofthecolorpatternonthewingsofPrecis coenia (Lepidoptera: Nymphalidae). Dev. Biol. 80: 275–288. [DOI] [PubMed] [Google Scholar]
- Oeting WS, and King RA 1999. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum. Mutations 13: 99–113. [DOI] [PubMed] [Google Scholar]
- Owen MD, and Bouquillon AI 1992. The synthesis of Ldihydroxyphenylalanine (L-DOPA) in the cerebral ganglion of the cockroach, Periplaneta americana L. Insect Biochem. Mol. Biol. 22: 193–198. [Google Scholar]
- Porter M, and Crandall K 2003. Lost along the way: the significance of evolution in reverse. Trends Ecol. Evol. 18: 541–547. [Google Scholar]
- Protas ME, et al. 2006. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat. Genet. 38: 107– 111. [DOI] [PubMed] [Google Scholar]
- Protas ME, and Patel NH 2008. Evolution of coloration patterns. Annu. Rev. Cell Dev. Biol. 24: 425–446. [DOI] [PubMed] [Google Scholar]
- Protas ME, Trontelj P, and Patel NH 2011. Genetic basis of eye and pigment loss in the cave crustacean, Asellus aquaticus. Proc. Natl. Acad. Sci. USA 108: 5702–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley PA 1997. Melanin. Int. J. Biochem. Cell Biol. 11: 1235–1239. [DOI] [PubMed] [Google Scholar]
- Russo J, Dupas S, Frey F, Carton Y, and Brehelin M 1996. Insect immunity: early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology 112: 135–142. [DOI] [PubMed] [Google Scholar]
- Sforza R, Bourgoin T, Wilson SW, and Boudon-Padieu E 1999. Field observations, laboratory rearing and description of immature stagesoftheplanthopperHyalesthesobsoletus(Hemiptera:Cixiidae). Eur. J. Entomol. 96: 409–418. [Google Scholar]
- Stathakis DG, Burton DY, McIvor WE, Krishnakumar S, Wright TRF, and O’Donnell JM 1999. The catecholamine up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics 153: 361– 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran M 1988. Molecular mechanisms for cuticle sclerotization. Adv. Insect Physiol. 21: 179–229. [Google Scholar]
- Toyofuku K, et al. 2002. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pig. Cell Res. 15: 217–224. [DOI] [PubMed] [Google Scholar]
- True JR 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18: 640–647. [Google Scholar]
- Vavricka CJ, Christensen BM, and Li J 2010. Melanization in living organisms: a perspective of species evolution. Protein Cell 1: 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter ME, Zeineh LL, Black BC, McIvor WE, Wright TRF, and Biesmann H 1996. Catecholamine metabolism and in vitro induction of premature cuticle melanization in wild type and pigmentation mutants of Drosophila melanogaster. Arch. Insect Biochem. Physiol. 31: 219–233. [DOI] [PubMed] [Google Scholar]
- Yi Z, et al. 2003. A 122.5-kilobase deletion of the P gene underlies the high prevalence of oculocutaneous albinism type 2 in the Navajo population. Am. J. Hum. Genet. 72: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


