Abstract
Hydrocephalus, a disorder of impaired cerebrospinal fluid (CSF) homeostasis, often results from an imbalance between CSF production and reabsorption. Rarely, hydrocephalus is the consequence of CSF hypersecretion in the context of diffuse villous hyperplasia of the choroid plexus (DVHCP). The limited genetic information in previously reported cases suggests a high prevalence of gains of Chromosome 9p in this disease, although the critical genes involved in DVHCP pathogenesis have not been identified. Here, we report a patient with syndromic hydrocephalus with DVHCP associated with a novel 9p24.3-11.2 triplication and 15q13.2-q13.3 microdeletion. We review the clinical, radiological, and pathological features of DVHCP, as well as its surgical management. A better understanding of the genetic basis of DVHCP could spur the development of rational, targeted nonsurgical hydrocephalus treatments.
Keywords: hydrocephalus, moderate global developmental delay
INTRODUCTION
Hydrocephalus is broadly defined as an abnormal dilation of the cerebral ventricles due to impaired cerebrospinal fluid (CSF) homeostasis, often the result of imbalance in CSF production and absorption (Rekate 2008; Kahle et al. 2016). This disorder is frequently caused by obstructed CSF flow and/or impaired absorption of CSF into systemic circulation. In rare cases, hydrocephalus is the result of CSF hypersecretion by the choroid plexus epithelium in pathologies such as choroid plexus hyperplasia (CPH), choroid plexus papilloma (CPP), and choroid plexus carcinoma (CPC) (Rickert and Paulus 2001; Karimy et al. 2016).
Hydrocephalus due to CSF hypersecretion in diffuse villous hyperplasia of the choroid plexus (DVHCP) has been reported in only 25 patients (Davis 1924; Welch et al. 1983; Bucholz and Pittman 1991; Hirano et al. 1994; Norman et al. 1995; Britz et al. 1996; Philips et al. 1998; D'Ambrosio et al. 2003; Fujimoto et al. 2004; Aziz et al. 2005; Iplikcioglu et al. 2006; Tamburrini et al. 2006; Smith et al. 2007; Puvabanditsin et al. 2008; Warren et al. 2009; Cataltepe et al. 2010; Anei et al. 2011; Hallaert et al. 2012; Hong et al. 2015; Cox et al. 2016; Boxill et al. 2018). Cytogenetic analyses of only eight of these patients have been reported: three patients with tetrasomy 9p (Norman et al. 1995; Hallaert et al. 2012; Boxill et al. 2018), four patients with trisomy 9p (Norman et al. 1995; Hallaert et al. 2012; Boxill et al. 2018), and one patient with monosomy 1p36 (Puvabanditsin et al. 2008).
Here, we report a patient with syndromic hydrocephalus and DVHCP associated with a novel 9p24.3-11.2 triplication and 15q13.2-q13.3 microdeletion. We also discuss the diagnostic evaluation and management of hydrocephalus due to DVHCP.
RESULTS
Case Presentation and Management
The patient was born by Cesarean section at 39 wk of gestation to a 34-yr-old G3P2001 mother following a pregnancy notable for maternal genitourinary Group B streptococcal infection and the presence of IgM and IgG antibodies to cytomegalovirus (CMV) in maternal serum. The mother had previously lost a 3-yr-old female child diagnosed with twice relapsing acute megakaryoblastic leukemia and concentric left ventricular hypertrophy in the absence of a discernible cardiac defect on transthoracic echocardiogram. Ultrasound of the patient obtained at 21 wk of gestation showed dilated lateral ventricles (11 mm in diameter), indicating mild fetal ventriculomegaly on this otherwise normal evaluation. Subsequent ultrasound evaluations at 27 wk and 34 wk of gestation demonstrated affirmed ventriculomegaly. Head ultrasound obtained shortly after birth revealed continued dilatation of the cerebral ventricles and prominent choroid plexi in the lateral and third ventricles.
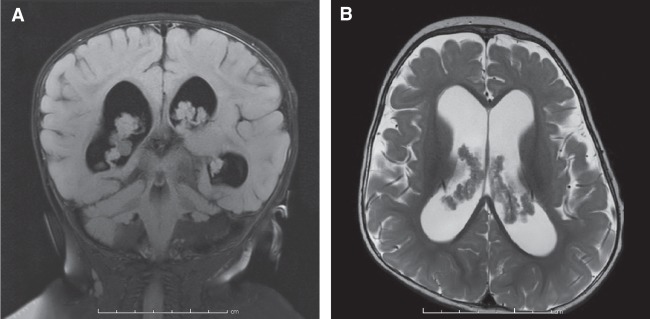
At 5 mo, the patient came to the attention of our pediatric neurosurgical service after a surveillance magnetic resonance imaging (MRI) of the head showed markedly dilated lateral and third ventricles with prominence of the extra-axial spaces in the frontal and temporal regions, abnormal prominence of the choroid plexus, and two choroid plexus cysts with no evidence of aqueductal stenosis or other intraventricular obstructions (Fig. 1). Initial examination by our team did not reveal clinical signs of hydrocephalus (i.e., full and bulging fontanel, progressive macrocephaly) but was notable for positional plagiocephaly and dysmorphic facial features (flattened left facial structures and asymmetrically set ears). The patient also had sensorineural hearing loss of the left ear and global developmental delay. A diagnosis of hydrocephalus associated with diffuse villous hyperplasia of the choroid plexus (DVHCP) was made. In the setting of no clinical signs of hydrocephalus and subsequent MRI evaluations showing no significant increase in ventriculomegaly, the patient was managed conservatively with surveillance MRIs with a plan to pursue CSF diversion surgery if indicated. The patient experienced delays in attaining developmental milestones: he sat independently at 12 mo, pulled to his knees at 16 mo, and possessed a vocabulary of fewer than 10 words at 21 mo.
Figure 1.
Magnetic resonance images demonstrating ventriculomegaly and diffuse villous hyperplasia of the choroid plexus. (A) Coronal T2-FLAIR magnetic resonance image through the dorsal midbrain and fourth ventricle demonstrating significant ventriculomegaly and bilaterally hypertrophied and cystic choroid plexuses. (B) Axial T2 weighted magnetic resonance image further highlighting significant dilation of the lateral ventricles and strikingly enlarged choroid plexuses bilaterally.
Genetic Analysis
Whole-exome sequencing (WES) was performed on DNA obtained from buccal swab samples from the nuclear kindred (I-1, I-2, and II-2) (see family pedigree in Fig. 2). Bioinformatic analysis was performed to identify extremely rare (ExAC MAF < 2 × 10−5), damaging variants (loss-of-function or missense mutations predicted deleterious by MetaSVM). Candidate mutations were confirmed by Sanger sequencing of all living family members (I-1, I-2, II-1, II-2); however, none of the rare variants identified segregated with the affected proband. Cytogenetic analysis was subsequently pursued.
Figure 2.
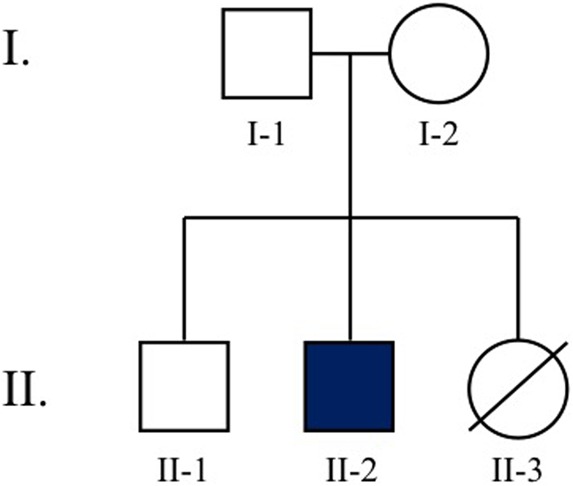
Pedigree of the proband's nuclear kindred. II-2 is our patient. II-3 passed away during a hospitalization for neutropenic fever following a relapse of acute megakaryoblastic leukemia (AML/M7).
Chromosome analysis of cells in metaphase from cultured stimulated lymphocytes of the proband's peripheral blood showed a supernumerary isodicentric chromosome fusing at 9q12 in most cells with very few others showing a normal male complement, suggesting a mosaic pattern in this patient. The karyotype was described as mos 47, XY, +idic(9) (q12)/46, XY. Concurrent array comparative genomic hybridization (aCGH; Agilent 400K) analysis on high molecular weight DNA extracted from peripheral blood cells of the proband revealed an XY male with a 44.055 Mb triplication of 9q24.3-p11.2 (Chr 9:204,193-44,259,464) and a 1.567Mb deletion at 15q13.2-q13.3 (Chr 15:30,943,903-32,510,863). The large triplication of 9p24.3-p11.2 covered the entire short arm of Chromosome 9, consistent with the supernumerary isodicentric chromosome fusing at 9q12 on karyotyping (Table 1).
Table 1.
9p triplication variant table
| Chromosome segment | Chr:position (GRCh37/hg19) | HGVS DNA | Type of variant | Predicted effect | Parent of origin | Observed effect |
|---|---|---|---|---|---|---|
| 9p24.3-11.2 | 9:204,193-44,259,464 | n/a | Triplication | Pathogenic | n/a | Hydrocephalus, facial dysmorphism, sensorineural hearing loss, global developmental delay |
n/a, not available.
The 1.57-Mb microdeletion at 15q13.2-q13.3 includes FAN1, MTMR10, TRPM1, KLF13, OTUD7A, and CHRNA7 genes and has been previously associated with Chromosome 15q13.3 microdeletion syndrome (OMIM # 612001) (Lowther et al. 2015). Fluorescent in situ hybridization (FISH; using dual color probes for the CDKN2A gene at 9p21 and the D9Z3 locus at 9q12; Cytocell Inc.) showed an abnormal pattern of four signals for the CDKN2A probe and three signals for the D9Z3 probe in 94.5% of the peripheral blood leukocytes examined, confirming the mosaic pattern of tetrasomy 9 in this patient. The proband's parents declined cytogenetic studies, hence the nature of the alteration (de novo vs. transmitted) could not be assessed, although one certainly could use WES data and genetic analysis tools like CONIFER and xHMM to determine the triplication (Cai et al. 2017).
DISCUSSION
We present a patient with hydrocephalus due to diffuse villous hyperplasia of the choroid plexus (DVHCP), dysmorphic facial features (flattening of left facial structures and asymmetry of head), and global developmental delay. Genetic testing identified tetrasomy 9p (triplication 9p24.3-p11.2) and a 1.57-Mb deletion at 15q13.2-q13.3, the former being very likely associated with DVHCP in this patient. In some older reports, DVHCP has been termed “choroid plexus hypertrophy” or “bilateral choroid plexus papilloma,” although these are distinct entities. DVHCP refers to a diffuse “bilateral” enlargement of the choroid plexus due to an increase in the “number” of choroid plexus epithelial cells. Villous hypertrophy suggests an “enlargement” in the cells with little to “no change in the number” of cells, and CPP describes benign, often discrete, “neoplastic” masses that grow from the villous epithelium of the choroid plexus.
Since the first reported case of DVHCP (Davis 1924), there have been only 25 other reported patients (including ours) (Table 2; Welch et al. 1983; Bucholz and Pittman 1991; Hirano et al. 1994; Norman et al. 1995; Britz et al. 1996; Philips et al. 1998; D'Ambrosio et al. 2003; Fujimoto et al. 2004; Aziz et al. 2005; Iplikcioglu et al. 2006; Tamburrini et al. 2006; Smith et al. 2007; Puvabanditsin et al. 2008; Warren et al. 2009; Cataltepe et al. 2010; Anei et al. 2011; Hallaert et al. 2012; Hong et al. 2015; Cox et al. 2016; Boxill et al. 2018). The majority of these cases were diagnosed in children, and a few cases diagnosed in utero, suggesting DVHCP may be a congenital disease with an underlying genetic etiology. Cytogenetic testing has been reported in nine of these patients (including ours): monosomy 1p36 in one patient (Puvabanditsin et al. 2008), trisomy 9p in four patients (Norman et al. 1995; Hallaert et al. 2012; Boxill et al. 2018), and tetrasomy 9p in four patients (our patient is the fourth) (Norman et al. 1995; Boxill et al. 2018).
Table 2.
Previous DVHCP case reports in the literature
| Reference | Patient's age at diagnosis, sex | Radiographic findings | Initial procedure | Histological findings | Complication | Subsequent procedures | CSF production rate (ml/day) | Genetics |
|---|---|---|---|---|---|---|---|---|
| Davis 1924a | 15 mo, M | |||||||
| Welch et al. 1983 | In utero, F | SE | VPS | NCP | Ascites | VAS, BCP | ||
| Bucholz and Pittman 1991 | 1 mo, F | SE | VPS | NCP | Ascites | EC | ||
| Hirano et al. 1994 | 7 yr, F | SE | VPS | NCP | Shunt failure | EVD, BCP | 2000 | |
| Norman et al. 1995a | 6 mo, M | SE | VPS | NCP | Tetrasomy 9p | |||
| 3 mo, F | SE, CF | VPS | Trisomy 9p | |||||
| Britz et al. 1996 | 3 mo, M | SE | VPS | Ascites | EVD, VAS | 900 | ||
| Philips et al. 1998 | 13 mo, F | SE, CF | VPS | NCP | Ascites | VPS, EC | ||
| D'Ambrosio et al. 2003 | 7 mo, F | SE | VPS | NCP | Ascites | VPS, BCP | 1200 | |
| Fujimoto et al. 2004 | 20 mo, M | SE, lobular | VPS | NCP | Ascites | UCP, VPS | ||
| Aziz et al. 2005 | 11 yr, M | SE | VPS | NCP | Ascites | EVD, VPS, BCP | 2650 | |
| Iplikcioglu et al. 2006 | 5 yr, F | SE | VPS | Ascites | VAS | |||
| Tamburrini et al. 2006 | 24 mo, F | SE | VPS | NCP | Ascites | EVD, EC, BCP, VPS | 2000 | |
| Smith et al. 2007 | 15 mo, F | SE | VPS | NCP | Ascites | EVD, BCP, VPS | 1400 | |
| Puvabanditsin et al. 2008 | in utero, M | SE | Monosomy 1p36 | |||||
| Warren et al. 2009 | 8 d, M | SE | VPS | NCP | Ascites | EC, BCP, VPS | ||
| Cataltepe et al. 2010 | in utero, M | SE | VPS, EC | NCP | Ascites | EVD, VPS, BCP, VAS | 2000 | |
| Anei et al. 2011 | 8 mo, F | SE | VPS | Ascites | EVD, VAS | 1500 | ||
| Hallaert et al. 2012 | 3 yr, F | SE | VPS | NCP | Ascites | VPS, EVD, EC | >2000 | Tetrasomy 9p |
| Hong et al. 2015 | 32 yr, M | SE | EC, VPS | |||||
| Cox et al. 2016 | 40 yr, M | EC | NCP | |||||
| 41 yr, M | EC + SPF | NCP | ||||||
| Boxill et al. 2018 | 2.5 mo, M | SE | VPS | Ascites | VAS | 500 | Trisomy 9p | |
| 2 yr, Fb | VPS | Ascites, CSF leakage | VAS | Trisomy 9p | ||||
| 4 yr, Fb | Trisomy 9p | |||||||
| 2 yr, F | SE | Tetrasomy 9p | ||||||
| Present case | 5 mo, M | SE, CF | Tetrasomy 9p |
M, male; F, female; SE, symmetric enlargement; CF, cystic formation; NCP, normal choroid plexus; VPS, ventriculoperitoneal shunt; VAS, ventriculoatrial shunt; EC, endoscopic coagulation; EVD, external ventricular drain; SPF, septum pellucidum fenestration; BCP, bilateral choroid plexectomy.
aPostmortem report.
bTwins.
The high prevalence of gains of Chromosome 9p among patients with DVHCP suggests that 9p contains gene(s) that are associated with the pathogenesis of DVHCP and subsequent hydrocephalus. Interestingly, none of the genes implicated in regulation of choroid plexus development in murine models has a homolog located on Chromosome 9p in the human genome (Liddelow 2015; Boxill et al. 2018). As in previous reports of DVHCP and CPP with well-defined locations of chromosomal breakpoints, the 9p24.3-p11.2 triplication in our patient covered the entire short arm of Chromosome 9, making it impossible to propose a critical region of 9p involved in DVHCP pathogenesis (Shapiro et al. 1985; Norman et al. 1995; Boxill et al. 2018). Future investigations may pursue identification of this critical region of 9p in attempt to identify gene(s) within that chromosomal segment that regulate choroid plexus development and are implicated in the pathogenesis of DVHCP.
Norman and colleagues raised suspicion of a relationship between Chromosome 9p abnormalities and abnormal growth of the choroid plexus when they reported on duplication of 9p in a patient with CPH and another patient with CPP (Norman et al. 1995). Hallaert et al. subsequently reported on a 3-yr-old child with DVHCP who had been diagnosed with tetrasomy 9p at 7 mo of age (Hallaert et al. 2012). Most recently, Boxill et al. reported on a 2-yr-old patient with hydrocephalus due to DVHCP who had been diagnosed with tetrasomy 9p at 11 mo of age (Boxill et al. 2018).
Tetrasomy 9p occurs from either isodicentric 9p (as in our patient) or pseudodicentric 9p duplications. There have been fewer than 70 reported cases of tetrasomy 9p (El Khattabi et al. 2015; Fremond et al. 2015). Patients are typically mosaics for the chromosomal aberration and demonstrate considerable clinical heterogeneity, ranging from impaired physical and mental development to severe intellectual disability and growth delay (El Khattabi et al. 2015). Dysmorphic facial features may include microcephaly, microretrognathia, hypertelorism, low-set ears, and bulbous nose. Several of the reported patients have demonstrated cardiac defects, renal anomalies, and joint dislocations. As in trisomy 9p, central nervous system (CNS) anomalies in tetrasomy 9p include Dandy–Walker malformation (with secondary obstructive hydrocephalus) and corpus callosum anomalies (Cazorla Calleja et al. 2003; Boxill et al. 2018). The 9pter-p11.2 region of the chromosome has been proposed as critical for development of Dandy–Walker malformations in gains of 9p (Chen et al. 2005).
Aberrations involving Chromosome 9p have been reported in other pathologies of the choroid plexus, emphasizing the likely association of genes on 9p with regulation of choroid plexus growth. Using comparative genomic hybridization (CGH), Rickert et al. (2002) found that 50% of CPPs and 33% of CPCs demonstrated gains of Chromosome 9p (i.e., +9p), and patients with CPC harboring +9p had significantly longer survival than those with CPC without gains of 9p. Future studies may investigate the mechanisms by which chromosomal aberrations involving 9p confer neoplastic features to the choroid plexus.
Our patient's aCGH also identified a 1.57-Mb deletion at 15q13.2-13.3, which has not been reported in the previous cases of DVHCP in tetrasomy 9p and may be contributory to the phenotype of our patient with syndromic hydrocephalus and developmental delay. This previously described microdeletion has a highly variable phenotype and is predominantly associated with neurologic dysfunction including developmental and intellectual disability, epilepsy, autism spectrum disorders, schizophrenia, and ADHD; other major congenital malformations are rarely observed (Lowther et al. 2015). Our patient had global developmental delay and dysmorphic facies—features that may be attributed to the microdeletion—but he has not been diagnosed with epilepsy or autism and demonstrates no behavioral concerns on evaluation by his neurologist. Interestingly, similar symptoms of the microdeletion are observed in carriers with single gene deletions of CHRNA7 (located at 15q13.3), suggesting a central role for this gene in the 15q13.3 microdeletion phenotype (Hoppman-Chaney et al. 2013). CHRNA7 encodes the alpha 7 subunit of the neuronal nicotinic acetylcholine receptor that mediates rapid synaptic transmission; mutations in this gene have been implicated in diseases affecting memory, among others (Baranowska and Wiśniewska 2017; Yadav et al. 2017).
Most cases of DVHCP are diagnosed by neuroimaging. Although there are currently no established radiological criteria for determining “normal” choroid plexus or diagnosing DVHCP, it is noteworthy that previously reported cases have identified symmetric enlargement of the bilateral choroid plexus that very rarely may be associated with cyst formation or lobulation on MRI (Philips et al. 1998; Fujimoto et al. 2004). Bilateral CPP, on the other hand, often appears as an asymmetric enlargement of the choroid plexuses and is more likely to be lobulated and/or associated with cyst formation (Erman et al. 2003). Anei et al. (2011) demonstrated the utility of thallium-201 SPECT in the workup of DVHCP, observing a symmetrically increased uptake in the bilateral choroid plexuses in both early and delayed images. In spite of current limitations in using CT, MRI, and SPECT in diagnosing DVHCP, these radiologic tools are invaluable in the initial evaluation of patients presenting with signs and symptoms of hydrocephalus that may be attributed to DVHCP, villous hypertrophy, CPP, or CPC.
Definitive diagnosis of DVHCP requires obtaining a specimen for histological evaluation. Previous case reports of histological findings in DVHCP all identify normal choroid plexus—several fronds of connective tissue and blood vessels surrounded by a single layer of cuboidal-to-columnar epithelial cells—with minimal-to-no mitosis or pleiomorphism and no invasion into brain parenchyma (Welch et al. 1983; Bucholz and Pittman 1991; Hirano et al. 1994; Philips et al. 1998; D'Ambrosio et al. 2003; Fujimoto et al. 2004; Aziz et al. 2005; Smith et al. 2007; Warren et al. 2009; Cataltepe et al. 2010; Hallaert et al. 2012). D'Ambrosio et al. (2003) suggest the utility of the MIB-1 index (a marker of cell proliferation) in differentiating between normal choroid plexus (as in DVHCP), CPP, and CPC. In normal choroid plexus the MIB-1 index is nearly 0%; in CPP and CPC the MIB-1 indices are 0.2%–17.42% and 4.14%–29.7%, respectively (Vajtai et al. 1996; Carlotti et al. 2002). Some authors argue that this overlap between MIB-1 indices of DVHCP, CPP, and CPC suggests a potential for DVHCP to progress to CPP and then to CPC, a hypothesis yet to be proven (Anei et al. 2011).
In his classic 1924 report, Davis implicated CSF overproduction as the cause of hydrocephalus in his patient (Davis 1924). This hypothesis is supported by the communicating hydrocephalus noted in most patients with DVHCP and reports of CSF overproduction as the cause of hydrocephalus is CPP (Eisenberg et al. 1974). Some authors, however, have suggested that the hydrocephalus in DVHCP may partly be the result of obstruction of CSF flow by the enlarged choroid plexus (Hallaert et al. 2012; Cox et al. 2016). Further, bleeding from the highly vascularized choroid plexus can obstruct CSF flow and impair CSF absorption as has been proposed in some models of posthemorrhagic hydrocephalus (Strahle et al. 2012). One readily observes that reported patients with DVHCP have demonstrated markedly increased CSF production rates, ranging from 500ml/day to >2000ml/day (Table 2).
As in most cases of hydrocephalus, CSF diversion procedures constitute the initial treatment for DVHCP, although some patients have needed subsequent endoscopic cauterization or choroid plexectomy. Placement of ventriculoperitoneal shunts (VPS) is highly associated with development of ascites in patients with DVHCP, presumably because of the peritoneum's inability to resorb CSF at the rate of CSF hypersecretion (Table 2; Welch et al. 1983; Bucholz and Pittman 1991; Britz et al. 1996; Philips et al. 1998; D'Ambrosio et al. 2003; Fujimoto et al. 2004; Aziz et al. 2005; Iplikcioglu et al. 2006; Tamburrini et al. 2006; Smith et al. 2007; Warren et al. 2009; Cataltepe et al. 2010; Anei et al. 2011; Hallaert et al. 2012). In a few patients, placement of ventriculoatrial shunts (VASs) results in successful management of the disease (Iplikcioglu et al. 2006; Boxill et al. 2018). Choroid plexectomy was first proposed by Dandy for management of communicating hydrocephalus (Dandy 1918). Both unilateral and bilateral choroid plexectomies have proven effective in a few reported cases with the latter being more popular (Welch et al. 1983; Hirano et al. 1994; D'Ambrosio et al. 2003; Aziz et al. 2005; Tamburrini et al. 2006; Smith et al. 2007; Warren et al. 2009; Cataltepe et al. 2010). The less invasive endoscopic cauterization of the choroid plexus has also been effectively used in DVHCP, in spite of concerns of not being able to sufficiently ablate deeper feeding vessels of the choroid plexus (Philips et al. 1998; Hallaert et al. 2012). Future studies may compare the efficacies and side effect profiles of these different treatment modalities. Given the high frequency of failure of CSF diversion measures in patients with hydrocephalus due to CPH, it is imperative that clinicians correctly diagnose the disease to aid in determining effective therapy. One study reported a 13-months latency between onset of hydrocephalus and definitive diagnosis of DVHCP (Hallaert et al. 2012).
CSF diversion techniques like VPS and VAS remain the standard of care in most types of hydrocephalus and have not markedly changed in more than five decades because of our limited understanding of the pathogenesis of different types of hydrocephalus (McAllister 2012; McAllister et al. 2015). The high prevalence of gains of Chromosome 9p among patients with hydrocephalus due to DVHCP strongly suggests an association of this chromosomal aberration with the disease. Patients with DVHCP should be considered for genetic testing with particular attention to detecting +9p aberrations. Further, future investigations should characterize the critical region(s) on Chromosome 9p that are involved in the disease pathogenesis. Not only will that advance our understanding of the regulatory mechanisms in choroid plexus development, but that will also create opportunities to develop pharmacotherapies that may treat the disease better than invasive CSF diversion surgeries.
METHODS
Subjects and Sample Collection
All study procedures and protocol comply with Yale University's Human Investigation Committee and Human Research Protection Program. Written consent from parents was obtained prior to genetic studies. Our patient and his participating family members provided buccal swab samples (Isohelix SK-2S DNA buccal swab kits), peripheral blood samples, medical records, neuroimaging studies, and phenotype data. All reported genomic coordinates reference the 2009 human genome assembly (GRCh37/hg19).
Cytogenetics
FISH was performed on peripheral leukocytes using dual color probes for CDKN2A gene at 9p21 and the D9Z3 locus at 9q12 (Cytocell Inc.). Chromosome microarray analysis was performed on an array of 400,000-oligonucleotide copy number and single-nucleotide polymorphism (SNP) probes covering the whole-genome (Agilent 400K CGH/SNP), using high molecular weight DNA extracted from the patient's peripheral blood specimen.
Exome Sequencing and Variant Calling
DNA was isolated from buccal swab samples in accordance with manufacturer protocol. Whole-exome sequencing was performed using the IDT xGen capture reagent followed by 99 base paired-end sequencing on the Illumina HiSeq 2000 instrument at the Yale Center for Genome Analysis as previously described (Timberlake et al. 2016). The average depth of coverage of the whole-exome sequencing data was 54.2×, with greater than 8× coverage in the 94% of the target region for exome capture (see Supplemental Table 1 for WES metrics and coverage).
Sequence reads were mapped and aligned to the GRCh37/hg19 human reference genome using Burrows–Wheeler Aligner-MEM (Li and Durbin 2009). In accordance with GATK Best Practices recommendations, the data were further processed using Genome Analysis Toolkit (GATK) base quality score recalibration (McKenna et al. 2010), indel realignment, duplication marking and removal, and base quality score recalibration (DePristo et al. 2011; Van der Auwera et al. 2013). Single-nucleotide variants and small insertions and deletions were called using GATK Haplotype Caller and annotated using ANNOVAR (Wang et al. 2010), NHLBI exome variant server (Fu et al. 2013), 1000 Genomes (Genomes Project et al. 2015), dbSNP (Sherry et al. 2000), and gnomAD and ExAC databases (Lek et al. 2016).
The sporadic or autosomal recessive mode of inheritance exhibited in our cohort's pedigrees led us to prioritize de novo, compound heterozygous, and homozygous variants. Variants were filtered for predicted deleteriousness and conservation using a series of in silico prediction algorithms, including Meta-analytic support vector machine (MetaSVM) and Meta-analytic logistic regression (MetaLR) (Dong et al. 2015), Polymorphism Phenotyping (PolyPhen) (Adzhubei et al. 2010), Combined Annotation-Dependent Depletion (CADD) (Kircher et al. 2014), Sorting Intolerant From Tolerant (SIFT) (Kumar et al. 2009; Ng and Henikoff 2003), conservation across 46 orthologs (cons46diff) (Cromer et al. 2012; Stuart et al. 2015), Likelihood Ratio Test (LRT) (Chun and Fay 2009), MutationTaster (Schwarz et al. 2010), Functional Analysis Through Hidden Markov Models (FATHMM) (Reva et al. 2011), FATHMM-Multiple Kernel Learning (FATHMM-MKL) , FATHMM-Coding, Protein Variant Effect Analyzer, Variant Effect Scoring Tool (VEST3), Mendelian Clinically Applicable Pathogenicity (M-CAP), deleterious annotation of genetic variants using neural networks (DANN), Genomic Evolutionary Rate Profiling (GERP++ and GERP++ GT2), phylogenetic P-values (phyloP100way and phyloP20way), phastCons100way and phastCons20way, Site-specific Phylogenetic analysis (SiPhy), REVEL, and MPC.
Kinship Analysis
Pedigree information and participant relationships were confirmed utilizing pairwise PLINK identity-by-descent (IBD) calculation (Purcell et al. 2007).
ADDITIONAL INFORMATION
Data Deposition and Access
The sequencing data for the kindred reported in this manuscript have been deposited in the NCBI database of Genotypes and Phenotypes (dbGaP) under accession number phs000744. The variant has been uploaded to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) under accession number SCV000803381.
Ethics Statement
All procedures comply with Yale University's Human Investigation Committee and Human Research Protection Program. Written consent from the proband's parents was obtained prior to genetic testing.
Acknowledgments
We sincerely appreciate the parents of our patient for agreeing to have us present this case to the medical community. We also thank our colleagues who helped manage the patient presented in this report.
Funding
C.F. and P.A. are supported by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA). K.T.K. is supported by the Hydrocephalus Foundation, March of Dimes, and NIH. None of these organizations had a role in study design, data collection, analysis, or interpretation.
Competing Interest Statement
The authors have declared no competing interest.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. 2010. A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anei R, Hayashi Y, Hiroshima S, Mitsui N, Orimoto R, Uemori G, Saito M, Sato M, Wada H, Hododuka A, et al. 2011. Hydrocephalus due to diffuse villous hyperplasia of the choroid plexus. Neurol Med Chir (Tokyo) 51: 437–441. [DOI] [PubMed] [Google Scholar]
- Aziz AA, Coleman L, Morokoff A, Maixner W. 2005. Diffuse choroid plexus hyperplasia: an under-diagnosed cause of hydrocephalus in children? Pediatr Radiol 35: 815–818. [DOI] [PubMed] [Google Scholar]
- Baranowska U, Wiśniewska RJ. 2017. The α7-nACh nicotinic receptor and its role in memory and selected diseases of the central nervous system. Postepy Hig Med Dosw (Online) 71: 633–648. [DOI] [PubMed] [Google Scholar]
- Boxill M, Becher N, Sunde L, Thelle T. 2018. Choroid plexus hyperplasia and Chromosome 9p gains. Am J Med Genet A 176: 1416–1422. [DOI] [PubMed] [Google Scholar]
- Britz GW, Kim DK, Loeser JD. 1996. Hydrocephalus secondary to diffuse villous hyperplasia of the choroid plexus. Case report and review of the literature. J Neurosurg 85: 689–691. [DOI] [PubMed] [Google Scholar]
- Bucholz RD, Pittman T. 1991. Endoscopic coagulation of the choroid plexus using the Nd:YAG laser: initial experience and proposal for management. Neurosurgery 28: 421–426; discussion 426–427. [PubMed] [Google Scholar]
- Cai Y, Patterson KE, Reinier F, Keesecker SE, Blue E, Bamshad M, Haddad J Jr. 2017. Copy number changes identified using whole exome sequencing in nonsyndromic cleft lip and palate in a Honduran population. Birth Defects Res 109: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotti CGJ, Salhia B, Weitzman S, Greenberg M, Dirks PB, Mason W, Becker LE, Rutka JT. 2002. Evaluation of proliferative index and cell cycle protein expression in choroid plexus tumors in children. Acta Neuropathol 103: 1–10. [DOI] [PubMed] [Google Scholar]
- Cataltepe O, Liptzin D, Jolley L, Smith TW. 2010. Diffuse villous hyperplasia of the choroid plexus and its surgical management. J Neurosurg Pediatr 5: 518–522. [DOI] [PubMed] [Google Scholar]
- Cazorla Calleja M, Verdú A, Félix V. 2003. Dandy–Walker malformation in an infant with tetrasomy 9p. Brain Dev 25: 220–223. [DOI] [PubMed] [Google Scholar]
- Chen CP, Chen CP, Shih JC. 2005. Association of partial trisomy 9p and the Dandy–Walker malformation. Am J Med Genet A 132A: 111–112. [DOI] [PubMed] [Google Scholar]
- Chun S, Fay JC. 2009. Identification of deleterious mutations within three human genomes. Genome Res 19: 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JT, Gaglani SM, Jusué-Torres I, Elder BD, Goodwin CR, Haynes MR, Blitz AM, Rigamonti D. 2016. Choroid plexus hyperplasia—a possible cause of hydrocephalus in adults. Neurology 87: 2058–2060. [DOI] [PubMed] [Google Scholar]
- Cromer MK, Starker LF, Choi M, Udelsman R, Nelson-Williams C, Lifton RP, Carling T. 2012. Identification of somatic mutations in parathyroid tumors using whole-exome sequencing. J Clin Endocrinol Metab 97: E1774–E1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio AL, O'Toole JE, Connolly ES Jr, Feldstein NA. 2003. Villous hypertrophy versus choroid plexus papilloma: a case report demonstrating a diagnostic role for the proliferation index. Pediatr Neurosurg 39: 91–96. [DOI] [PubMed] [Google Scholar]
- Dandy WE. 1918. Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg 68: 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE. 1924. A physio-pathologic study of the choroid plexus with the report of a case of villous hypertrophy. J Med Res 44: 521–534.511. [PMC free article] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, et al. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, Liu X. 2015. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet 24: 2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg HM, McComb JG, Lorenzo AV. 1974. Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J Neurosurg 40: 381–385. [DOI] [PubMed] [Google Scholar]
- El Khattabi L, Jaillard S, Andrieux J, Pasquier L, Perrin L, Capri Y, Benmansour A, Toutain A, Marcorelles P, Vincent-Delorme C, et al. 2015. Clinical and molecular delineation of Tetrasomy 9p syndrome: report of 12 new cases and literature review. Am J Med Genet A 167: 1252–1261. [DOI] [PubMed] [Google Scholar]
- Erman T, Gocer AI, Erdogan S, Tuna M, Ildan F, Zorludemir S. 2003. Choroid plexus papilloma of bilateral lateral ventricle. Acta Neurochir (Wien) 145: 139–143; discussion 143. [DOI] [PubMed] [Google Scholar]
- Fremond ML, Gitiaux C, Bonnet D, Guiddir T, Crow YJ, de Pontual L, Bader-Meunier B. 2015. Mosaic tetrasomy 9p: a mendelian condition associated with pediatric-onset overlap myositis. Pediatrics 136: e544–e547. [DOI] [PubMed] [Google Scholar]
- Fu W, O'Connor TD, Jun G, Kang HM, Abecasis G, Leal SM, Gabriel S, Rieder MJ, Altshuler D, Shendure J, et al. 2013. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493: 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Matsushita H, Plese JP, Marino R Jr. 2004. Hydrocephalus due to diffuse villous hyperplasia of the choroid plexus. Case report and review of the literature. Pediatr Neurosurg 40: 32–36. [DOI] [PubMed] [Google Scholar]
- Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, et al. 2015. A global reference for human genetic variation. Nature 526: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallaert GG, Vanhauwaert DJ, Logghe K, Van den Broecke C, Baert E, Van Roost D, Caemaert J. 2012. Endoscopic coagulation of choroid plexus hyperplasia. J Neurosurg Pediatr 9: 169–177. [DOI] [PubMed] [Google Scholar]
- Hirano H, Hirahara K, Asakura T, Shimozuru T, Kadota K, Kasamo S, Shimohonji M, Kimotsuki K, Goto M. 1994. Hydrocephalus due to villous hypertrophy of the choroid plexus in the lateral ventricles. Case report. J Neurosurg 80: 321–323. [DOI] [PubMed] [Google Scholar]
- Hong Y, Xu Q, Maimaiti YS, Ye J, Chen G. 2015. Diffuse choroid plexus hyperplasia (CPH) associated with multiple malformations. Acta Neurologica Belgica 115: 387–388. [DOI] [PubMed] [Google Scholar]
- Hoppman-Chaney N, Wain K, Seger PR, Superneau DW, Hodge JC. 2013. Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. Clin Genet 83: 345–351. [DOI] [PubMed] [Google Scholar]
- Iplikcioglu AC, Bek S, Gokduman CA, Bikmaz K, Cosar M. 2006. Diffuse villous hyperplasia of choroid plexus. Acta Neurochir (Wien) 148: 691–694. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Kulkarni AV, Limbrick DD, Warf BC. 2016. Hydrocephalus in children. Lancet 387: 788–799. [DOI] [PubMed] [Google Scholar]
- Karimy JK, Duran D, Hu JK, Gavankar C, Gaillard JR, Bayri Y, Rice H, DiLuna ML, Gerzanich V, Marc Simard J, et al. 2016. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg Focus 41: E10. [DOI] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. 2014. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46: 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. 2016. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA. 2015. Development of the choroid plexus and blood–CSF barrier. Front Neurosci 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther C, Costain G, Stavropoulos DJ, Melvin R, Silversides CK, Andrade DM, So J, Faghfoury H, Lionel AC, Marshall CR, et al. 2015. Delineating the 15q13.3 microdeletion phenotype: a case series and comprehensive review of the literature. Genet Med 17: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JP II. 2012. Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med 17: 285–294. [DOI] [PubMed] [Google Scholar]
- McAllister JP II, Williams MA, Walker ML, Kestle JR, Relkin NR, Anderson AM, Gross PH, Browd SR, Hydrocephalus Symposium Expert Panel. 2015. An update on research priorities in hydrocephalus: overview of the third National Institutes of Health–sponsored symposium “Opportunities for Hydrocephalus Research: Pathways to Better Outcomes.” J Neurosurg 123: 1427–1438. [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. 2003. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 31: 3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MG, Harrison KJ, Poskitt KJ, Kalousek DK. 1995. Duplication of 9P and hyperplasia of the choroid plexus: a pathologic, radiologic, and molecular cytogenetics study. Pediatr Pathol Lab Med 15: 109–120. [DOI] [PubMed] [Google Scholar]
- Philips MF, Shanno G, Duhaime AC. 1998. Treatment of villous hypertrophy of the choroid plexus by endoscopic contact coagulation. Pediatr Neurosurg 28: 252–256. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvabanditsin S, Garrow E, Patel N, D'Ella A, Zaafran A, Phattraprayoon N, Davis SE. 2008. Choroid plexus hyperplasia and monosomy 1p36- report of new findings. J Child Neurol 23: 922–925. [DOI] [PubMed] [Google Scholar]
- Rekate HL. 2008. The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal Fluid Res 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva B, Antipin Y, Sander C. 2011. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res 39: e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert CH, Paulus W. 2001. Tumors of the choroid plexus. Microsc Res Tech 52: 104–111. [DOI] [PubMed] [Google Scholar]
- Rickert CH, Wiestler OD, Paulus W. 2002. Chromosomal imbalances in choroid plexus tumors. Am J Pathol 160: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. 2010. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7: 575–576. [DOI] [PubMed] [Google Scholar]
- Shapiro SD, Hansen KL, Littlefield CA. 1985. Brief clinical report: non-mosaic partial tetrasomy and partial trisomy 9. Am J Hum Genet 20: 271–276. [DOI] [PubMed] [Google Scholar]
- Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. 2000. dbSNP—the NCBI database of genetic variation. Nucleic Acids Res 29: 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZA, Moftakhar P, Malkasian D, Xiong Z, Vinters HV, Lazareff JA. 2007. Choroid plexus hyperplasia: surgical treatment and immunohistochemical results. Case report. J Neurosurg 107: 255–262. [DOI] [PubMed] [Google Scholar]
- Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. 2012. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res 3: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, Choi M, Dharwadkar P, Torres F, Girod CE, et al. 2015. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet 47: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburrini G, Caldarelli M, Di Rocco F, Massimi L, D'Angelo L, Fasano T, Di Rocco C. 2006. The role of endoscopic choroid plexus coagulation in the surgical management of bilateral choroid plexuses hyperplasia. Child's Nerv Syst 22: 605–608. [DOI] [PubMed] [Google Scholar]
- Timberlake AT, Choi J, Zaidi S, Lu Q, Nelson-Williams C, Brooks ED, Bilguvar K, Tikhonova I, Mane S, Yang JF, et al. 2016. Two locus inheritance of non-syndromic midline craniosynostosis via rare SMAD6 and common BMP2 alleles. Elife 5: e20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajtai I, Varga Z, Aguzzi A. 1996. MIB-1 immunoreactivity reveals different labelling in low-grade and in malignant epithelial neoplasms of the choroid plexus. Histopathology 29: 147–151. [PubMed] [Google Scholar]
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, et al. 2013. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 43: 11 10 11–11 10 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DT, Hendson G, Cochrane DD. 2009. Bilateral choroid plexus hyperplasia: a case report and management strategies. Child's Nerv Syst 25: 1617–1622. [DOI] [PubMed] [Google Scholar]
- Welch K, Strand R, Bresnan M, Cavazzuti V. 1983. Congenital hydrocephalus due to villous hypertrophy of the telencephalic choroid plexuses. Case report. J Neurosurg 59: 172–175. [DOI] [PubMed] [Google Scholar]
- Yadav R, Deepshikha D, Srivastava P. 2017. Homology modeling and protein interaction map of CHRNA7 neurogenesis protein. Ann Neurosci 24: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.