Abstract
Background
One of the key risk factors for cardiovascular disease is hypertension. Hypertension, which leads to heart attacks and strokes, already affects one billion people worldwide, making it a global public health issue. Incidence and prevalence of the condition is on the rise in low- and middle-income countries, with the biggest increase in sub-Saharan Africa and South Africa at the forefront. We examined the prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive patients on ART in a large South African observational cohort.
Methods
We conducted a prospective study of ART naïve adults initiating ART at a public sector HIV clinic in South Africa between April 2004–2017. Patients with diagnosed hypertension at ART initiation were excluded from the incidence analysis. Log-binomial regression was used to estimate predictors of hypertension at ART initiation, while competing risks regression was used to evaluate the relationship between predictors of incident hypertension, accounting for death as a competing risk.
Results
Among 77,696 eligible patients, 22.0% had prevalent hypertension at ART initiation. Of the remaining patients with no hypertension at ART initiation, 8,125 incident hypertension cases were diagnosed over the period of follow-up, corresponding to an incident rate of 5.4 per 100 person-years (95% confidence interval (CI): 5.3–5.6). We found patients ≥40 years of age and patients with a body mass index (BMI) ≥25kg/m2 were at increased risk of both prevalent and incident hypertension. Male patients and those with pre-hypertension at ART initiation had increased hazards of hypertension over the period of follow-up. When assessing the choice of antiretroviral drug in first-line ART, patients initiated on nevirapine were at 27% increased risk of developing hypertension compared to those initiated on efavirenz, while patients who initiated on either zidovudine or stavudine had a 40% increased risk of developing hypertension compared to patients initiated on tenofovir. Patientswith poorer health status at ART initiation (i.e. WHO III/IV stage, low CD4 count, low hemoglobin levels and low BMI) had a decrease risk of prevalent hypertension. We found an inverse relationship in patients with a CD4 count <50 cells/mm3 at ART initiation who had a 25% increased risk of incident hypertension compared to those with a CD4 count ≥350 cells/mm3.
Conclusion
Over 20% of patients in our cohort had hypertension at ART initiation, and 13% of those with normal blood pressure at ART initiation developed hypertension while on ART. Older patients, males, those on nevirapine, zidovudine or stavudine, and those who are overweight/obese should be targeted for frequent blood pressure monitoring and the identification of other cardiovascular risk factors to encourage lifestyle modifications. Additionally, these groups should be offered pharmaceutical therapy to help prevent myocardial infarction, heart failure, stroke, and kidney disease. Further research is needed to determine the level of access and adherence to pharmaceutical treatment for hypertension in this population. Additionally, an HIV-negative comparison population is needed to assess the association of the HIV virus itself with hypertension.
Introduction
One of the key risk factors for cardiovascular disease is hypertension—raised blood pressure. Hypertension, which leads to heart attacks and strokes already affects one billion people worldwide, making it a global public health issue. Researchers have estimated that raised blood pressure currently kills 15 million people every year [1]. In 2017, it was estimated that 80% of the global mortality attributable to high blood pressure would occur in low and middle-income countries [1]. The importance of blood pressure as a modifiable risk factor for cardiovascular disease is well-recognized, and many effective blood pressure lowering treatments are available for the condition. Therefore, hypertension control and prevention of subsequent morbidity and mortality is achievable. However, the incidence and prevalence of the condition is on the rise in low- and middle-income countries, with the biggest increase in sub-Saharan Africa [1] and South Africa at the forefront [2].
South Africa, a middle-income country battling the largest HIV [3] and tuberculosis [4] epidemics in the world, now faces an additional challenge with an increasing burden of non-communicable chronic disease (NCD), particularly for cardiovascular disease. Incidence estimates for hypertension, one of the most common risk factors for cardiovascular disease, are lacking, but evidence indicates the prevalence of hypertension in on the rise in South Africa. In the late 1990s, the prevalence of hypertension in the general population was estimated at approximately 15% [5–7]. In 2008, Bärnighausen et al. reported a prevalence estimate of 33% among adults 15–50 years of age using Demographic Surveillance Area data from 2004 [8]. Berry et al. estimated an adult prevalence of hypertension of 35% using South African National Health and Nutrition Examination Survey data from 2011–2012 [9]. More recently, the 2016 South African Demographic and Health Survey reported 46% of women and 44% of men are hypertensive or taking antihypertensive medication [10]. The prevalence of the condition is expected to rise even faster in the coming years as obesity and sedentary lifestyles, both major risk factors for cardiovascular health, continue to increase [11].
A less-examined contributing factor to the NCD epidemic in South Africa has been the rapid expansion of HIV treatment programs [12–15]. Starting in 2004, antiretroviral therapy (ART) has been offered to HIV-positive individuals who meet a broadening set of eligibility criteria [12], which now includes all infected persons [15]. Access to ART, which has nearly reversed the country’s HIV-related loss of life-expectancy, has resulted in a steady rise in the median age of the HIV-positive population [16]. This, in turn, has increased the HIV-infected population’s risk of HIV and non-HIV related NCDs to a level comparable to that of the HIV-negative population [1]. Currently, hypertension is one of the most common risk factors for cardiovascular disease among HIV-positive individuals, with an estimated prevalence of 5% to 55% in high-income and 9% to 46% in low- and middle-income countries [17]. Studies comparing HIV positive patients to HIV negative controls have reported inconsistent results, with hypertension prevalence in HIV positive populations either comparable to [18], lower than [19,20,21], or higher than [22] their HIV negative counterparts. Some studies have found that HIV-positive individuals in sub-Saharan Africa have lower diastolic and systolic blood pressure than HIV-negative controls, regardless of ART status [19,23]. However, others have suggested an increased risk of hypertension with ART [22], and still others have shown no association between hypertension and HIV, with or without ART [18].
As the HIV-infected population ages and access to ART increases in low- and middle-income countries, the number of patients on ART developing hypertension, and subsequent CVD, will also rise, making identification and management of this condition alongside HIV increasingly important. To help fill gaps in research on this topic in low- and middle-income settings, we examined the prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive patients on ART in a large South African observational cohort.
Methods
Cohort description
This study used data from the Right to Care Clinical HIV cohort [24], which includes HIV positive patients from eight public-sector clinics across South Africa. The clinics began initiating patients onto ART in 2004 at the start of South Africa’s public sector treatment program. HIV care and treatment at the study clinics is provided according to national treatment guidelines [12–15]. As of April 2016, the cohort included 158,374 patients, of whom 119,397 had initiated ART. Patient data, including demographic characteristics, clinical conditions, laboratory test results, and medications (antiretroviral (ARV) and non-ARV) are entered on site by a clinician or a data entry clerk into a live data capturing system called TherapyEdge-HIV™. Weight, height, and systolic and diastolic blood pressure are routinely measured at medical visits.
Eligible patients
All ART-naïve, non-pregnant, HIV-positive adult patients newly initiating standard first-line ART between April 2004 and April 2016 were eligible for the analysis. We excluded patients with missing systolic and diastolic blood pressure measurements at three months prior to one month after ART initiation. The standard first-line regimen until April 2010 was stavudine or zidovudine plus lamivudine and efavirenz; after April 2010, tenofovir or zidovudine replaced stavudine [12–15]. Mortality is routinely recorded in the patient clinical record and confirmed via linkage with the National Vital Registration system [25,26] for patients with a national identification number. For this analysis, loss to follow-up was defined as being at least three months late for the patient’s last scheduled visit to the clinic.
Use of data was approved by the Human Research Ethics Committee of the University of the Witwatersrand (M140201). Approval for analysis of de-identified data was granted by the Institutional Review Board of Boston University (H-29768).
Outcomes
Primary outcomes
Our primary outcomes of interest were prevalent hypertension at ART initiation and incident hypertension on ART. Prevalent hypertension was defined as a single blood pressure (BP) measure of >140/90 mmHg three months prior to and up to one month after ART initiation, or if the patient file had hypertension noted as a condition, or if hypertension drugs had been prescribed in the same time frame. Incident hypertension was defined as a patient having normal blood pressure measurements and no evidence of previous treatment for hypertension prior to or at ART initiation and meeting any of the following criteria after ART initiation [27]:
at least three blood pressure measurements, at least two days apart, which were >140/90 mmHg (or >130/80 mmHg in the presence of co-morbidities), OR
a single blood pressure measure ≥180/110 mmHg, OR
documentation in the patient file of a diagnosis of hypertension more than three months after ART initiation to ensure the patient did not have hypertension at ART initiation, OR
documentation in the patient file of initiation of treatment for hypertension more than three months after ART initiation to ensure the patient did not have hypertension at ART initiation.
Secondary outcomes
We assessed three secondary outcomes:
pre-hypertension at ART initiation, defined as a single blood pressure measurement between 120/80 mmHg and 140/90 mmHg three months prior to and up to one month after ART initiation.
elevated blood pressure beyond the initial incident hypertension diagnosis as an indicator of whether patients’ hypertension improved over time (defined as at least three blood pressure measurements, at least two days apart, which were >140/90 mmHg (or >130/80 mmHg in the presence of co-morbidities), or a single blood pressure measure ≥180/110 mmHg).
prevalence of hypertension-related co-morbidities, including hyperlipidemia, hypercholesterolemia, heart failure cardiomyopathy, cardiac arrest, and acute ischemic heart disease.
Analysis
Demographic and clinical characteristics at ART initiation were summarized using descriptive statistics. For prevalent hypertension at ART initiation, we fit a log-binomial model to assess predictors (i.e. age, gender, body mass index (BMI), World Health Organization (WHO) stage of HIV-associated disease progression, CD4 count, and hemoglobin level). For incident hypertension, we fit a competing risk regression [28] model, accounting for death as a competing risk, and evaluated the relationship between incident hypertension and the following characteristics at ART initiation as potential predictors: age, gender, BMI, non-nucleoside reverse transcriptase inhibitor (NNRTI), nucleoside reverse transcriptase inhibitor (NRTI), WHO stage of HIV-associated disease progression, pre-hypertension, CD4 count, and hemoglobin level. Person time was calculated from ART initiation to either: 1) hypertension diagnosis, 2) loss to follow up (censored 3 months after last scheduled visit), 3) death, 4) transfer, or 5) close of the dataset (April 01 2016), whichever occurred first. Only patients without hypertension at ART initiation were considered for the incidence analysis. We fit linear regression models using standardized data results in standardized estimates to assess predictors of systolic and diastolic blood pressure separately. Linear models were adjusted for age, BMI, CD4 count, and hemoglobin level on a continuous scale in addition to categorical variables (i.e. gender, NRTI, NNRTI, WHO stage and pre-hypertension).
As creatinine clearance, a surrogate measure for estimated glomerular filtration rate (eGFR), was not routinely measured until tenofovir was introduced in public sector in April 2010 [13], we ran a second competing risk regression model restricted to patients who initiated after April 2010 adjusting for creatinine clearance. Creatinine clearance was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation which estimates creatinine clearance on the basis of the serum creatinine, race, age, and gender [29]. Creatinine clearance was categorized according to the U.S. National Kidney Foundation’s Kidney Disease Outcome Quality Initiative (K/DOQI) as normal (≥90ml/min), mild (60-89ml/min), moderate (30-59ml/min), and severe (<30ml/min) renal dysfunction [30]. We also adjusted for age, gender, BMI, NNRTI, NRTI, WHO stage, pre-hypertension, CD4 count, and hemoglobin level. Predictors of prevalent and incident hypertension were chosen based on previous peer reviewed studies in HIV-positive populations.
Results and discussion
Cohort description
A total of 80,560 patients were eligible for the analysis. Of those, 77,696 had a blood pressure measurement at ART initiation and were included in our analysis (Table 1). The cohort was predominately female (60.8%), with a median age at ART initiation of 37 years (interquartile range (IQR) 31–44)), median systolic blood pressure of 117 mmHg (106–130), median diastolic blood pressure of 76 mmHg (68–84), and median body mass index of 22.1 kg/m2 (19.5–25.6). Patients were initiated on a standard first-line regimen containing either stavudine (47.7%) or tenofovir (47.7%) plus lamivudine and efavirenz and had a median follow-up time of 22 months (IQR: 6–49 months).
Table 1. Demographic and clinical characteristics of patients on antiretroviral therapy with and without hypertension at ART initiation in Right to Care clinics in South Africa (n = 77,696).
| Characteristic | Unit | Hypertension at ART initiation (N = 17,126); n (%) or median (IQRβ) |
Pre-hypertension at ART initiation (N = 15,009); n (%) or median (IQRβ) |
No hypertension at ART initiation (N = 45,561); n (%) or median (IQRβ) |
|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | median (IQR) | 139 (125–150) | 122 (113–130) | 110 (101–120) |
| Diastolic blood pressure (mm Hg) | median (IQR) | 90 (80–97) | 82 (77–86) | 70 (64–76) |
| Gender | female | 10,079 (58.9%) | 9,116 (60.7%) | 28,024 (61.5%) |
| Age at ART* initiation (years) | median (IQR) | 41 (34–48) | 37 (32–44) | 35 (30–42) |
| 18–24 | 453 (2.6%) | 668 (4.5%) | 3,176 (7.0%) | |
| 25–29 | 1,565 (9.1%) | 2,059 (13.7%) | 7,843 (17.2%) | |
| 30–39 | 6,200 (36.2%) | 6,721 (44.8%) | 20,589 (45.2%) | |
| 40–49 | 5,428 (31.7%) | 3,961 (26.4%) | 10,235 (22.5%) | |
| 50+ | 3,480 (20.3%) | 1,600 (10.7%) | 3,718 (8.2%) | |
| CD4 count at ART* initiation (cells/mm3) | median (IQR) | 144 (66–225) | 127 (54–204) | 115 (45–196) |
| 0–49 | 2,746 (16.0%) | 2,909 (19.4%) | 10,173 (22.3%) | |
| 50–99 | 2,276 (13.3%) | 2,101 (14.0%) | 6,694 (14.7%) | |
| 100–199 | 4,600 (26.9%) | 4,127 (27.5%) | 11,874 (26.1%) | |
| 200–349 | 3,255 (19.0%) | 2,386 (15.9%) | 6,837 (15.0%) | |
| 350+ | 1,084 (6.3%) | 830 (5.5%) | 2,202 (4.8%) | |
| missing | 3,165 (18.5%) | 2,656 (17.7%) | 7,781 (17.1%) | |
| WHO€ stage at ART* initiation | I/II | 7,267 (42.4%) | 5,582 (37.2%) | 15,406 (33.8%) |
| III/IV | 4,005 (23.4%) | 4,218 (28.1%) | 14,804 (32.5%) | |
| missing | 5,854 (34.2%) | 5,209 (34.7%) | 15,351 (33.7%) | |
| Hemoglobin at ART* initiation (g/dL) | median (IQR) | 12.2 (10.6–13.6) | 11.9 (10.3–13.3) | 11.4 (9.8–12.9) |
| <10 | 2,456 (14.3%) | 2,673 (17.8%) | 10,480 (23.0%) | |
| ≥10 | 12,091 (70.6%) | 9,962 (66.4%) | 27,580 (60.5%) | |
| missing | 2,579 (15.1%) | 2,374 (15.8%) | 7,501 (16.5%) | |
| Creatinine clearance (mL/min/1.73m2) | median (IQR) | 119.1 (100.1–132.2) | 123.5 (105.7–135.4) | 126.2 (109.2–137.9) |
| <60 | 391 (2.3%) | 182 (1.2%) | 606 (1.3%) | |
| 60–89 | 978 (5.7%) | 650 (4.3%) | 1,602 (3.5%) | |
| ≥90 | 6,957 (40.6%) | 6,064 (40.4%) | 17,806 (39.1%) | |
| missing | 8,800 (51.4%) | 8,113 (54.1%) | 25,547 (56.1%) | |
| Body mass Index at ART* initiation (kg/m2) | median (IQR) | 23.7 (20.7–27.8) | 22.7 (19.9–26.2) | 21.4 (19.1–24.5) |
| <18 | 940 (5.5%) | 1,154 (7.7%) | 5,395 (11.8%) | |
| 18–24.9 | 6,765 (39.5%) | 6,561 (43.7%) | 21,333 (46.8%) | |
| 25–29.9 | 3,106 (18.1%) | 2,372 (15.8%) | 5,380 (11.8%) | |
| 30–34.9 | 1,379 (8.1%) | 924 (6.2%) | 1,634 (3.6%) | |
| >35 | 787 (4.6%) | 379 (2.5%) | 675 (1.5%) | |
| missing | 4,149 (24.2%) | 3,619 (24.1%) | 11,144 (24.5%) | |
| Tuberculosis at ART* initiation | yes | 1,384 (8.1%) | 1,575 (10.5%) | 5,727 (12.6%) |
| Non-nucleotide reverse transcriptase Inhibitor | efavirenz | 15,817 (92.4%) | 13,769 (91.7%) | 41,158 (90.3%) |
| nevirapine | 1,152 (6.7%) | 1,105 (7.4%) | 3,832 (8.4%) | |
| missing | 157 (0.9%) | 135 (0.9%) | 571 (1.3%) | |
| Nucleoside reverse transcriptase Inhibitor | zidovudine | 852 (5.0%) | 609 (4.1%) | 1,616 (3.5%) |
| stavudine | 8,162 (47.7%) | 7,570 (50.4%) | 24,006 (52.7%) | |
| tenofovir | 8,112 (47.4%) | 6,830 (45.5%) | 19,939 (43.8%) | |
| Time on ART* (months) | median (IQR) | 24.8 (9.2–49.7) | 23.9 (9.0–49.3) | 21.6 (7.4–47.5) |
| Vital status at end of follow-up period | died | 1,480 (8.6%) | 1,276 (8.5%) | 5,047 (11.1%) |
| loss to follow-up | 3,578 (20.9%) | 3,449 (23.0%) | 11,471 (25.2%) | |
| transferred out | 3,959 (23.1%) | 3,609 (24.0%) | 10,838 (23.8%) | |
| alive | 8,109 (47.3%) | 6,675 (44.5%) | 18,205 (40.0%) |
*ART = antiretroviral therapy;
€WHO = World Health Organization;
βIQR = interquartile range.
Prevalent hypertension
More than a fifth of the cohort (n = 17,126, 22.0%) had prevalent hypertension at ART initiation. As expected, older patients (40–49 –hazard ratio (HR): 1.49; 95% confidence interval (CI):1.42–1.54 and >50–2.00; 1.91–2.11) and patients classified as overweight or obese (BMI 25–29.9 kg/m2 –HR: 1.39; 95% CI: 1.32–1.45; 30–34.9 kg/m2 –HR: 1.70; 95% CI: 1.60–1.81; and ≥35 kg/m2 –HR: 1.96; 95% CI: 1.83–2.10) had a higher prevalence of hypertension at ART initiation. However, individuals initiating ART with poorer health status (i.e. WHO staging III/IV, and hemoglobin <10g/dL) had lower prevalent hypertension (Table 2).
Table 2. Predictors of prevalent hypertension in patients on ART (n = 77,696).
| Variable | Number with baseline hypertension (%) | Unadjusted Risk Ratio (95% CI¥) | Adjusted Risk Ratio (95% CI¥) |
|---|---|---|---|
| Age | |||
| 18–24 | 453 (10.5%) | 0.57 (0.52–0.62) | 0.60 (0.52–0.69) |
| 25–29 | 1,565 (13.6%) | 0.74 (0.70–0.78) | 0.73 (0.68–0.79) |
| 30–39 | 6,200 (18.5%) | Reference | Reference |
| 40–49 | 5,428 (27.7%) | 1.49 (1.45–1.54) | 1.49 (1.42–1.56) |
| 50+ | 3,480 (39.6%) | 2.14 (2.07–2.21) | 2.00 (1.91–2.11) |
| Gender | |||
| Female | 10,079 (21.3%) | Reference | Reference |
| Male | 7,047 (23.1%) | 1.08 (1.05–1.11) | 1.13 (1.08–1.18) |
| Body mass index (kg/m2) at ART* initiation | |||
| <18 | 940 (12.6%) | 0.64 (0.60–0.69) | 0.72 (0.67–0.78) |
| 18–24.9 | 6,765 (19.5%) | Reference | Reference |
| 25–29.9 | 3,106 (28.6%) | 1.47 (1.41–1.52) | 1.39 (1.32–1.45) |
| 30–34.9 | 1,379 (35.0%) | 1.79 (1.71–1.88) | 1.70 (1.60–1.81) |
| ≥35 | 787 (42.7%) | 2.19 (2.07–2.32) | 1.96 (1.83–2.10) |
| WHO€ stage | |||
| I/II | 7,267 (25.7%) | Reference | Reference |
| III/IV | 4,005 (17.4%) | 0.68 (0.65–0.70) | 0.84 (0.80–0.87) |
| CD4 count at ART* initiation (cells/μl) | |||
| 0–49 | 2,746 (17.3%) | 0.66 (0.62–0.70) | 0.93 (0.85–1.01) |
| 50–99 | 2,276 (20.6%) | 0.78 (0.73–0.83) | 0.99 (0.91–1.08) |
| 100–199 | 4,600 (22.3%) | 0.85 (0.80–0.90) | 1.01 (0.94–1.10) |
| 200–349 | 3,255 (26.1%) | 0.99 (0.93–1.05) | 1.08 (1.00–1.18) |
| 350+ | 1,084 (26.3%) | Reference | Reference |
| Hemoglobin at ART* initiation (g/dL) | |||
| > = 10 | 12,091 (24.4%) | Reference | Reference |
| <10 | 2,456 (15.7%) | 0.65 (0.62–0.67) | 0.79 (0.75–0.84) |
*ART = antiretroviral therapy;
€WHO = World Health Organization;
¥CI = confidence interval. All variables in the adjusted model are displayed in the table.
Incident hypertension
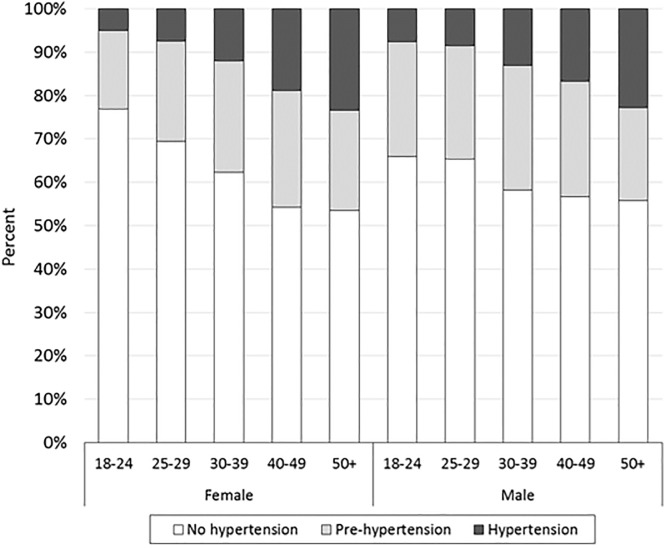
For our assessment of incident hypertension while on ART, we excluded patients with hypertension at ART initiation (n = 17,126), leaving a final sample of 60,570. Of these, 75.2% (n = 45,561) had normal blood pressure and 24.8% (n = 15,009) patients were pre-hypertensive. There were 8,125 (13.4%) incident hypertension cases diagnosed at a median of 13 months (IQR: 9–230) after ART initiation. The overall incidence rate for this cohort was 5.44 per 100 person years (95% CI: 5.32–5.56). Men had a higher rate of incident hypertension (6.25 per 100 person years; 95%CI: 6.04–6.46) than women (4.98 per 100 person years; 95%CI: 4.84–5.12) overall. By age, men had higher incidence of pre-hypertension and hypertension than women until the oldest two age groups (40–49 and ≥50 years), where women showed higher rates than men (Fig 1). Results from the linear regression models indicate that a change of one standard deviation in the in BMI resulted in a 0.175 (95% CI: 0.142, 0.208) standard deviation increase in the systolic blood pressure and a 0.251 (0.218, 0.285) standard deviation increase in the diastolic blood pressure (Table 3). Additionally, a change of one standard deviation in the in BMI resulted in a 0.209 (95% CI: 0.196, 0.222) standard deviation increase in diastolic blood pressure.
Fig 1. Proportion of study population with incident hypertension and pre-hypertension after ART initiation, stratified by age and gender (N = 60,570).
Table 3. Linear regression analysis on systolic and diastolic blood pressure showing both normal and standardrized parameter estimates of predictors (n = 60,570).
| Variable | Parameter estimates | Standardized parameter estimates | |||
|---|---|---|---|---|---|
| Unadjusted Unstandardized β estimate (95% CI¥) |
Adjusted unstandardized β estimate (95% CI¥) |
Unadjusted standardized β estimate (95% CI¥) |
Adjusted standardized β estimate (95% CI¥) |
||
| Systolic blood pressure | |||||
| Age (per 1 year increase) | -0.021 (-0.037, -0.007) | -0.064 (-0.088, -0.039) | -0.012 (-0.020, -0.004) | -0.034 (-0.47, -0.021) | |
| Gender | Female | Reference | Reference | Reference | Reference |
| Male | 1.483 (1.210, 1.756) | 2.208 (1.764, 2.652) | 0.043 (0.035, 0.051) | 0.065 (0.052, 0.078) | |
| Body mass index (per kg/m2 increase) | .054 (0.043, 0.065) | 0.216 (0.175, 0.257) | 0.044 (0.035, 0.053) | 0.175 (0.142, 0.208) | |
| Pre-hypertension at ART* initiation | No | Reference | Reference | Reference | Reference |
| Yes | 4.291 (3.98, 4.598) | 3.167 (2.684, 3.651) | 0.111 (0.103, 0.119) | 0.082 (0.070, 0.095) | |
| NNRTIα | efavirenz | Reference | Reference | Reference | Reference |
| nevirapine | -0.183 (-0.668, 0.303) | 0.182 (-0.621, 0.984) | -0.003 (-0.011, 0.005) | 0.003 (-0.010, 0.016) | |
| NRTIΩ | tenofovir | Reference | Reference | Reference | Reference |
| zidovudine | -1.361 (-2.083, -0.638) | -2.304 (-3.532, -1.076) | -0.015 (-0.024, -0.007) | -0.026 (-0.040, -0.012) | |
| stavudine | -2.959 (-3.230, -2.688) | -2.506 (-2.947, -2.065) | -0.089 (-0.097, -0.081) | -0.075 (-0.089, -0.062) | |
| CD4 count (per 100 cells/μl) | 0.006 (-0.002, 0.013) | -0.0002 (-0.011, 0.0107) | 0.007 (-0.002, 0.016) | -0.0002 (-0.013, 0.013) | |
| WHO€ stage | I/II | Reference | Reference | Reference | Reference |
| III/IV | -2.914 (-3.244, -2.584) | -1.891 (-2.320, -1.463) | -0.088 (-0.097, -0.078) | -0.057 (-0.070, -0.044) | |
| Hemoglobin (per 1 g/dL increase) | 0.061 (0.044, 0.079) | 0.025 (-0.0001, 0.049) | 0.031 (0.022, 0.040) | 0.013 (-0.0001, 0.025) | |
| Diastolic blood pressure | |||||
| Age (per 1 year increase) | 0.487 (0.463, 0.511) | 0.447 (0.407, 0.487) | 0.161 (0.153, 0.169) | 0.148 (0.135, 0.161) | |
| Gender | Female | Reference | Reference | Reference | Reference |
| Male | 2.265 (1.821, 2.708) | 2.168 (1.444, 2.892) | 0.041 (0.033, 0.049) | 0.039 (0.026, 0.052) | |
| Body mass index (per kg/m2 increase) | 0.073 (0.055, 0.092) | 0.503 (0.436, 0.571) | 0.037 (0.027, 0.046) | 0.251 (0.218, 0.285) | |
| Pre-hypertension at ART* initiation | No | Reference | Reference | Reference | Reference |
| Yes | 14.238 (13.749, 14.726) | 13.088 (12.299, 13.876) | 0.227 (0.220, 0.235) | 0.209 (0.196, 0.222) | |
| NNRTIα | efavirenz | Reference | Reference | Reference | Reference |
| nevirapine | -0.229 (-1.018, 0.560) | 1.588 (0.278, 2.897) | -0.002 (-0.010, 0.006) | 0.016 (0.003, 0.029) | |
| NRTIΩ | tenofovir | Reference | Reference | Reference | Reference |
| zidovudine | 5.477 (4.303, 6.650) | 6.597 (4.593, 8.601) | 0.038 (0.030, 0.046) | 0.046 (0.032, 0.060) | |
| stavudine | 4.964 (4.524, 5.404) | 7.752 (7.033, 8.471) | 0.092 (0.084, 0.100) | 0.143 (0.130, 0.157) | |
| CD4 count (per 100 cells/μl) | 0.009 (-0.021, 0.003) | 0.014 (-0.004, 0.032) | 0.007 (-0.002, 0.016) | 0.010 (-0.003, 0.024) | |
| WHO€ stage | I/II | Reference | Reference | Reference | Reference |
| III/IV | -2.404 (-2.951, -1.857) | -0.870 (-1.569, -0.170) | -0.044 (-0.055, -0.034) | -0.016 (-0.029, -0.003) | |
| Hemoglobin (per 1 g/dL increase) | 0.055 (0.027, 0.083) | 0.012 (-0.028, 0.052) | 0.017 (0.008, 0.026) | 0.004 (-0.009, 0.016) | |
*ART = antiretroviral therapy;
€WHO = World Health Organization;
¥CI = confidence interval;
αNNRTI = Non-Nucleoside Reverse Transcriptase Inhibitor;
ΩNRTI = Nucleoside reverse transcriptase inhibitor. All predictors are measured at ART initiation. Model adjusted for gender, body mass index, age, nucleoside reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, WHO stage, CD4 count at ART initiation, hemoglobin levels at ART initiation.
Similar to prevalent hypertension, we found that patients ≥40 years of age and those classified as overweight or obese were at increased hazards of hypertension during their time on ART (Table 4). Male patients (HR: 1.23; 95% CI: 1.14–1.32) and those with pre-hypertension (HR: 2.05; 95% CI: 1.92–2.19) at ART initiation also had increased hazards of hypertension over the period of follow-up (Table 4). Patients with a CD4 count <50 cells/mm3 at ART initiation had a 25% increased hazards of developing hypertension compared to those with a CD4 count ≥350 cells/mm3. When assessing the choice of NNRTI in first-line ART, patients initiated on nevirapine were at 27% increased hazards of developing hypertension compared to patients initiated on efavirenz (HR: 1.27; 95% CI: 1.13–1.43), while patients who initiated on either zidovudine or stavudine had a 40% increased hazards of developing hypertension compared to patients initiated on tenofovir (Table 4).
Table 4. Predictors of incident hypertension in patients on ART (n = 60,570).
| Variable | Number of events/person years | Incidence rate/100 person years (95% CI¥) | Crude hazard ratio (95% CI¥) | Adjusted hazard ratio (95% CI¥) |
|---|---|---|---|---|
| Gender | ||||
| Female | 4,699/83,557 | 5.62 (5.46–5.79) | Reference | Reference |
| Male | 3,426/47,144 | 7.27 (7.03–7.51) | 1.22 (1.17–1.28) | 1.23 (1.14–1.32) |
| Age (years) at ART* initiation | ||||
| 18–24 | 208/7,406 | 2.81 (2.44–3.22) | 0.50 (0.43–0.57) | 0.54 (0.44–0.67) |
| 25–29 | 761/21,818 | 3.49 (3.24–3.74) | 0.64 (0.60–0.70) | 0.74 (0.66–0.82) |
| 30–39 | 3,396/62,283 | 5.45 (5.27–5.64) | Reference | Reference |
| 40–49 | 2,532/30,528 | 8.29 (7.97–8.62) | 1.49 (1.41–1.57) | 1.45 (1.35–1.57) |
| ≥50 | 1,228/8,665 | 14.17 (13.39–14.99) | 2.15 (2.01–2.30) | 1.99 (1.80–2.20) |
| Body mass index (kg/m2) at ART* initiation | ||||
| <18 | 674/13,430 | 5.02 (4.65–5.41) | 0.72 (0.66–0.78) | 0.72 (0.65–0.80) |
| 18.0–24.9 | 3,756/64,355 | 5.84 (5.65–6.03) | Reference | Reference |
| 25.0–29.9 | 1,304/17,556 | 7.43 (7.03–7.84) | 1.34 (1.25–1.43) | 1.38 (1.27–1.51) |
| 30.0–34.9 | 515/5,232 | 9.84 (9.01–10.73) | 1.74 (1.58–1.91) | 1.87 (1.64–2.13) |
| ≥35 | 215/1,958 | 10.98 (9.56–12.55) | 1.88 (1.63–2.16) | 2.01 (1.66–2.45) |
| Pre-hypertension at ART* initiation | ||||
| No | 4,805/100,828 | 4.77 (4.63–4.90) | Reference | Reference |
| Yes | 3,320/29,873 | 11.11 (10.74–11.50) | 2.32 (2.22–2.43) | 2.05 (1.92–2.19) |
| Non-Nucleoside Reverse Transcriptase Inhibitor | ||||
| efavirenz | 7,335/117,486 | 6.24 (6.10–6.39) | Reference | Reference |
| nevirapine | 679/11,346 | 5.98 (5.54–6.45) | 1.00 (0.93–1.09) | 1.27 (1.13–1.43) |
| Nucleoside Reverse Transcriptase Inhibitor | ||||
| tenofovir | 2,368/41,118 | 5.76 (5.53–6.00) | Reference | Reference |
| zidovudine | 379/5,171 | 7.33 (6.61–8.11) | 1.32 (1.18–1.48) | 1.41 (1.18–1.69) |
| stavudine | 5,378/84,412 | 6.37 (6.20–6.54) | 1.17 (1.11–1.23) | 1.42 (1.31–1.54) |
| CD4 count at ART* initiation (per 100 cells/μl) | ||||
| 0–49 | 1,900/27,708 | 6.86 (6.55–7.17) | 1.09 (0.96–1.24) | 1.25 (1.03–1.50) |
| 50–99 | 1,327/19,613 | 6.77 (6.41–7.14) | 1.15 (1.01–1.31) | 1.15 (0.95–1.39) |
| 100–199 | 2,238/38,678 | 5.79 (5.55–6.03) | 1.08 (0.95–1.22) | 1.05 (0.87–1.26) |
| 200–349 | 1,102/18,751 | 5.88 (5.54–6.23) | 1.08 (0.95–1.23) | 1.13 (0.93–1.37) |
| 350+ | 0,279/4,945 | 5.64 (5.00–6.34) | Reference | Reference |
| WHO€ stage at ART* initiation | ||||
| I/II | 3,175/49,997 | 6.35 (6.13–6.58) | Reference | Reference |
| III/IV | 2,613/42,053 | 6.21 (5.98–6.46) | 0.86 (0.81–0.90) | 1.00 (0.93–1.07) |
| Hemoglobin at ART* initiation (g/dL) | ||||
| <10 | 1,690/25,104 | 6.73 (6.41–7.06) | 0.90 (0.85–0.96) | 1.06 (1.00–1.12) |
| ≥10 | 5,472/87,415 | 6.26 (6.10–6.43) | Reference | Reference |
*ART = antiretroviral therapy;
€WHO = World Health Organization;
¥CI = confidence interval. All predictors are measured at ART initiation. Model adjusted for gender, body mass index, age, nucleoside reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, WHO stage, CD4 count at ART initiation, hemoglobin levels at ART initiation. Fine and Gray’s competing risk regression used controlling for death as a competing risk for incident hypertension
When limiting the cohort to those patients who initiated ART after the South African national guidelines called for the replacement of stavudine with tenofovir in first-line ART (April 1, 2010), we found that patients with moderate/severe renal insufficiency (creatinine clearance <60 ml/min) had a 70% increase in the hazards of hypertension compared to those with normal renal function (creatinine clearance ≥90 ml/min) (HR: 1.70; 95% CI: 1.18–2.46) (Table 5).
Table 5. Predictors of incident hypertension in patients on ART after April 2010 when tenofovir was introduced into first-line ART (n = 32,919).
| Variable | Number of events/person years | Incidence rate/100 person years (95% CI¥) | Crude hazard ratio (95% CI¥) | Adjusted hazard ratio (95% CI¥) |
|---|---|---|---|---|
| Gender | ||||
| Female | 1,781/31,985 | 5.57 (5.31–5.83) | Reference | Reference |
| Male | 1,426/19,936 | 7.15 (6.79–7.53) | 1.28 (1.19–1.37) | 1.48 (1.28–1.71) |
| Age (years) at ART* initiation | ||||
| 18–24 | 81/2,970 | 2.73 (2.17–3.39) | 0.50 (0.40–0.63) | 0.38 (0.22–0.67) |
| 25–29 | 235/8,477 | 2.77 (2.43–3.15) | 0.54 (0.47–0.62) | 0.58 (0.44–0.76) |
| 30–39 | 1,229/23,659 | 5.19 (4.91–5.49) | Reference | Reference |
| 40–49 | 1,054/13,121 | 8.03 (7.56–8.53) | 1.63 (1.50–1.77) | 1.55 (1.33–1.80) |
| ≥50 | 608/3,693 | 16.46 (15.18–17.83) | 2.76 (2.50–3.05) | 2.18 (1.78–2.67) |
| Body mass index (kg/m2) at ART* initiation | ||||
| <18 | 205/3,958 | 5.18 (4.49–5.94) | 0.85 (0.73–0.99) | 0.88 (0.68–1.12) |
| 18.0–24.9 | 1,257/23,553 | 5.34 (5.05–5.64) | Reference | Reference |
| 25.0–29.9 | 525/7,352 | 7.14 (6.54–7.78) | 1.38 (1.2.4–1.53) | 1.65 (1.39–1.95) |
| 30.0–34.9 | 240/2,602 | 9.22 (8.09–10.47) | 1.77 (1.54–2.04) | 2.35 (1.87–2.95) |
| ≥35 | 103/1,020 | 10.09 (8.24–12.24) | 1.92 (1.57–2.35) | 2.15 (1.50–3.07) |
| Pre-hypertension at ART* initiation | ||||
| No | 2,477/45,945 | 5.39 (5.18–5.61) | Reference | Reference |
| Yes | 730/5,976 | 12.22 (11.35–13.14) | 2.62 (2.44–2.81) | 2.72 (2.38–3.11) |
| Non-nucleoside reverse transcriptase inhibitor | ||||
| efavirenz | 2,951/48,374 | 6.10 (5.88–6.32) | Reference | Reference |
| nevirapine | 239/3,249 | 7.36 (6.45–8.35) | 1.20 (1.05–1.37) | 1.91 (1.46–2.50) |
| Nucleoside reverse transcriptase inhibitor | ||||
| tenofovir | 2,262/38,939 | 5.81 (5.57–6.05) | Reference | Reference |
| zidovudine | 123/1,770 | 6.95 (5.77–8.29) | 1.12 (0.93–1.36) | 1.20 (0.75–1.93) |
| stavudine | 822/11,211 | 7.33 (6.84–7.85) | 1.31 (1.21–1.42) | 1.33 (1.13–1.58) |
| CD4 count at ART* initiation (cells/μl) | ||||
| 0–49 | 555/8,174 | 6.79 (6.24–7.38) | 1.16 (0.97–1.38) | 1.23 (0.91–1.67) |
| 50–99 | 440/6,277 | 7.01 (6.37–7.70) | 1.28 (1.06–1.53) | 1.19 (0.87–1.63) |
| 100–199 | 664/11,359 | 5.85 (5.41–6.31) | 1.19 (1.01–1.42) | 1.10 (0.83–1.46) |
| 200–349 | 795/14,031 | 5.67 (5.28–6.07) | 1.16 (0.97–1.38) | 1.09 (0.82–1.45) |
| 350+ | 157/2,873 | 5.46 (4.64–6.39) | Reference | Reference |
| WHO€ stage at ART* initiation | ||||
| I/II | 1,134/18,724 | 6.06 (5.71–6.42) | Reference | Reference |
| III/IV | 729/11,365 | 6.41 (5.96–6.90) | 0.96 (0.88–1.06) | 1.03 (0.89–1.19) |
| Hemoglobin at ART* initiation (g/dL) | ||||
| <10 | 622/8,861 | 7.02 (6.48–7.59) | 1.01 (0.92–1.10) | 1.24 (1.04–1.47) |
| ≥10 | 2,127/36,047 | 5.90 (5.65–6.16) | Reference | Reference |
| Creatinine Clearance (mL/min/1.73m2) | ||||
| <60 | 84/512 | 16.40 (13.08–20.31) | 2.11 (1.69–2.64) | 1.70 (1.18–2.46) |
| 60–89 | 234/2,043 | 11.46 (10.03–13.02) | 1.81 (1.58–2.08) | 1.34 (1.06–1.70) |
| ≥90 | 1,844/32,827 | 5.62 (5.36–5.88) | Reference | Reference |
*ART = antiretroviral therapy;
€WHO = World Health Organization;
¥CI = confidence interval. All predictors are measured at ART initiation. Model adjusted for gender, BMI, age, NRTI, NNRTI, WHO stage, CD4 count at ART initiation, hemoglobin levels at ART initiation, creatinine clearance. Fine and Gray’s competing risk regression used controlling for death as a competing risk for incident hypertension
Clinical management and outcomes among patients receiving treatment for hypertension
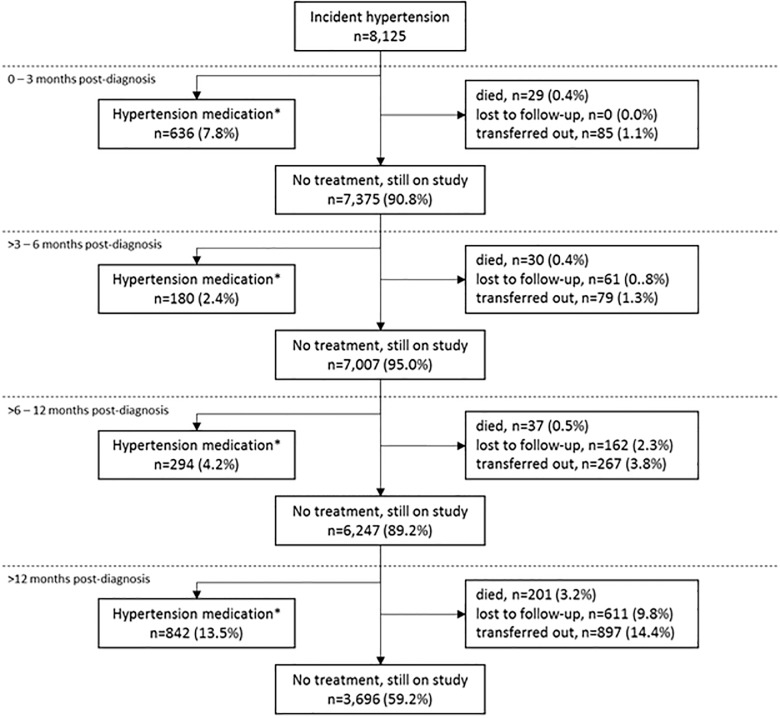
Of the 8,125 incident hypertension cases, 24.0% (n = 1,952) received medical treatment for elevated blood pressure at the same clinic during the course of follow-up (Fig 2). Of these, 32.6% (n = 636) were treated within 3 months, 9.2% (n = 180) within 3–6 months, 15.0% (n = 294) within 6–12 months, and 43.1% (n = 842) more than 12 months after hypertension diagnosis (Fig 2). Of the 1,952 who received treatment, 4.1% (n = 79) died, 10.6% (n = 207) were lost to follow-up, 16.7% (n = 325) transferred out, and 68.7% (n = 1,341) remained in care at their original clinics after the incident hypertension event while on ART. More than half (58.2%) of the treated patients who had sufficient follow-up blood pressure data (n = 1,546/1,952) went on to have elevated blood pressure at a median of 5 months (IQR 3–9 months) after receiving hypertension medication. A small fraction of these patients (n = 35, 1.8%) developed 37 co-morbidities between them related to hypertension, including 26 (70.3%) cases of hyperlipidemia/ hypercholesterolemia, 7 (18.9%) of hypertensive heart disease, 2 (5.4%) of cardiomyopathy, and 2 (5.4%) of congestive heart failure.
Fig 2. Clinical management of incident hypertension amongst patients on antiretroviral therapy.
Hypertension medication includes the following: amlodipine besylate, atenolol, captopril, carvedilol, enalapril maleate, enalaprilat, felodipine, hydralazine hydrochloride, indapamide, lisinopril, metoprolol tartrate, nifedipine, nimodipine, nisoldipine, perindopril erbumine, prazosin hydrochloride, propranolol hydrochloride, verapamil hydrochloride, valsartan, simvastatin.
Clinical management and outcomes among patients not receiving treatment for hypertension
The remaining 6,173 (76.0%) with incident hypertension did not get medical treatment during follow-up. The frequency of mortality (4.8%; n = 298), loss to follow up (13.5%; n = 834) and transfers out of the clinic (22.2%; n = 1,371) were higher than in the population of patients who received treatment for hypertension. 53.9% of these untreated patients with sufficient follow-up blood pressure data (n = 4,849/6,173) still had elevated blood pressure at a median of 6 months (IQR: 5–13) after their initial incident hypertension diagnosis, and 1.6% (n = 96) of them had 98 co-morbidities between them related to hypertension: 93 (94.9%) cases of hyperlipidemia/ hypercholesterolemia, 1 (1.0%) of heart failure, 3 (3.1%) of cardiomyopathy and 1 (1.0%) of cardiac arrest.
As treatment programs for HIV are scaled up globally and deaths from AIDS-defining conditions decline, the management of NCDs, such as hypertension, is emerging as an essential part of HIV care in low- and middle-income countries. Longitudinal data on incident hypertension in HIV patients in sub-Saharan Africa are scarce. With over 75,000 HIV-positive patients on ART over 12 years of follow-up, our cohort is one of the largest to date to assess prevalent and incident hypertension, associated risk factors, treatment, and control of the condition in a middle-income setting. As such, our work adds to the evidence base on hypertension and its risk factors in settings of both high HIV prevalence and the non-HIV risk factors common in middle-income countries.
In the present study, we found a prevalence of hypertension of 22% at ART initiation, consistent with previously published estimates from other sub-Saharan countries [19]. Incidence of hypertension in our cohort was 13% over a median of 22 months of follow up, corresponding to an overall rate of hypertension of 5.4 per 100 person-years. While incidence estimates among HIV-positive populations are scarce, our results are lower than studies conducted in low-income populations in sub-Saharan Africa (12.0 cases per 100 person-years (95% CI: 7.2–15.0) in Tanzania [31]; 11.2 cases per 100 person-years in Uganda (95% CI: 10.2–12.2)) [32] that had similar prevalence. When comparing our rates to the HIV-negative population, our estimates appear to be substantially lower than previous studies out of South Africa have reported. Estimates from the Heart of Soweto Study reported an incidence rate of 24% over 5 years of follow-up [23].
Risk factors for hypertension are well-characterized, and our findings were generally consistent with previous work [32–34]. We found that males, patients age >40 years, those with body mass index ≥25 kg/m2, and patients with pre-hypertension at ART initiation were at increased hazards of incident hypertension. Patients with pre-hypertension were twice as likely to experience incident hypertension, underscoring the need to monitor these patients closely. We also found that patients with low CD4 count at ART initiation were at increased hazards of incident hypertension. Previous research showed patients with a nadir CD4 cell count <50 cells/mm3 had a 150% (adjusted odds ratio [OR], 2.48; 95%CI: 1.27–4.83) increase in the odds of hypertension compared to those with higher CD4 cell counts [35]. A potential biological mechanism explaining this association is that low CD4 cell count has been associated with chronic immune activation and persistent microbial translocation [35–39]. Ingjerd et al. demonstrated a strong association between microbial translocation, measured by increased levels of lipopolysaccharide before initiation of ART, and subsequent sustained hypertension [40]. However, this association was inverted when assessing hypertension at ART initiation, where we show that more advanced disease (WHO stage III/IV, low CD4 and low Hb) was protective against prevalent hypertension. Weight loss and lower levels of triglycerides, most frequently found in patients with CD4 lymphocyte counts of less than 200 cells/mm3 [41,42], could be reflected in the lower prevalence of incident hypertension in patients on ART. Our linear regression models, which used standardized data to assess predictors of systolic and diastolic blood pressure, showed minimal effect of any risk factors.
Consistent with previous studies [43,44], we observed a 30% increased hazards of hypertension among patients on nevirapine compared to efavirenzand a 40% increased hazards of hypertension in patients on stavudine or zidovudine vs tenofovir. Although we were unable to assess the association of incident nephrotoxicity on incident hypertension, we did assess the association of hypertension with renal function at ART initiation and found that patients with moderate/severe renal insufficiency (HIV-associated, not ART related, nephropathy) at treatment initiation had a 55% increased hazard of incident hypertension compared to those with normal renal function. As two potential pathways that lead to increased risk of hypertension risk amongst HIV patients, researchers speculate that ART effects body mass index or immune reconstitution [42,45]. Studies have also shown that exposure to tenofovir has been associated with accelerated eGFR decrease in the first 6 to 12 months after ART initiation among African HIV patients with no or mild renal dysfunction at initiation of ART [46]. The possible impact of secondary nephrotoxicity on hypertension rates deserves consideration in view of the large proportion of patients receiving tenofovir-based ART since it became standard first-line ART in 2010 in many low- and middle-income countries [47].
In low- and middle-income countries, many people do not seek treatment for hypertension because it is prohibitively expensive due to the cost of the drugs. Close to 25% of patients in our cohort who had incident hypertension during follow up observation received medication for the condition. Of these, close to 60% had high blood pressure five months after treatment, suggesting that either the medication was not effective or was not adhered to appropriately. More than half of the remaining 75% of patients with incident hypertension, and no documentation of treatment for the condition, went on to have normal blood pressure measurements within six months of their diagnosis. The goal of the national guidelines for monitoring and treatment of hypertension is to decrease blood pressure to <140/90 mmHg in patients being treated with antihypertensive medication regardless of cardiovascular risk factors and underlying co-morbidities [48]. Further research is needed to closely examine the monitoring and treatment of the condition in patients receiving long-term ART.
Our findings should be considered alongside the study’s limitations. First, because our study reports data from large, public sector government HIV clinics, our results may not be generalizable to the overall population. Second, lack of documentation of blood pressure measurements, clinical hypertension diagnosis, and/or prescribed antihypertensive medication could cause us to underestimate the proportion of patients prevalent and incident hypertension in the sample. This missingness could be the result of poor data capturing at the clinic level or due to the fact that monitoring and treatment of NCDs occurs separately from HIV care so a known hypertensive patient on treatment may not be reflected in the HIV data. Third, this lack of accurate information on prescribed hypertensive medication could explain why 58.2% of patients that received treatment for incident hypertension and went on to have elevated blood pressure at a median of 5 months after receiving hypertension medication, while 53.2% of those that did not receive treatment had elevated blood pressure measurement in a median of 6 months after being diagnosed hypertensive. Fourth, patients that were initiated onto the older generation of antiretroviral drugs (nevirapine, stavudine or zidovudine) could have had contraindications to efavirenz or tenofovir, placing them at higher hazards of hypertension. Since information on conditions that would indicate contraindication to the newer antiretrovirals was not available, we were unable to control for this and may be overestimating the hazards of hypertension in patients on those antiretrovirals. Fifth, we show that more advanced disease (WHO stage III/IV, low CD4, and low Hb) is protective against prevalent hypertension, while low BMI (<18 kg/m2) is protective against incident hypertension. This might be related to BMI, as when adjusting for BMI our estimates are slightly attenuated, but still remain significant. It may be that BMI is not as accurate a marker of body fat as waist measurement would be [49] and adjusting for waist could nullify the effect association of makers of advanced disease. Alternatively, these indicators of poorer health status by WHO classification could be associated with malnutrition, in which low blood pressure is common, implying that our cohort differs from healthy HIV-negative cohorts in that it contains malnourished, HIV-infected patients, both at the onset of ART and over a life time of treatment. Six, as our estimates of prevalent hypertension are among HIV-positive patients that survive to initiate ART, survivor bias result in an underestimate of prevalence, biasing our results downwards. Finally, as we do not have access to HIV-negative population for comparison, we are unable to assess any association between HIV status and hypertension. However, the 2016 South African Demographic and Health Survey reported estimated a hypertension prevalence of over 40% among black South Africans older than 15 years of age [10], roughly 20% higher than the estimated 22% we reported in this study and consistent with previous research from sub-Saharan Africa [9,23].
Conclusion
Over 20% of patients in our cohort had hypertension at ART initiation, and 13% of those with normal blood pressure at ART initiation developed hypertension while on ART. Older patients, males, those on nevirapine, zidovudine or stavudine, and those who are overweight/obese should be targeted for frequent blood pressure monitoring and the identification of other cardiovascular risk factors to encourage lifestyle modifications. Additionally, these at-risk populations should be offered pharmaceutical therapy to help prevent myocardial infarction, heart failure, stroke, and kidney disease. Further research is needed to determine the level of access and adherence to pharmaceutical treatment for hypertension in this population. Additionally, an HIV-negative comparison population is needed to assess the association of the HIV virus itself with hypertension.
Acknowledgments
We express our gratitude to Right to Care, the nongovernmental organization supporting the study site through a partnership with the United States Agency for International Development, and to the staff of the participating clinics. We also thank the provincial and national Departments of Health for providing for the care of the patients under the Comprehensive Care Management and Treatment program and the HIV/AIDS conditional grant. Most of all we thank the patients attending the clinic for their continued trust in the treatment provided at the clinic.
Data Availability
The data used in this study is owned by the study site and National Department of Health (South Africa) and governed by the Human Research Ethics Committee (University of Witwatersrand, Johannesburg, South Africa). All relevant data is included in the paper and supplementary tables. The full data are available from the Health Economics and Epidemiology Research Office for researchers who meet the criteria for access to confidential data and have approval from the owners of the data (information@heroza.org).
Funding Statement
Funding was provided 5T32AI052074-13 from the National Institutes of Health and by USAID under the terms of the Cooperative Agreement 674-A-00-08-0000-700 to Right to Care and INROADS AID-674-A-12-00029. This study is made possible by the generous support of the American people through the United States Agency for International Development and the National Institutes of Health. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, the United States government or the Right to Care Clinics. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Noncommunicable Disease. Progress Monitor 2017. [http://apps.who.int/iris/bitstream/handle/10665/258940/9789241513029eng.pdf;jsessionid=1C1BA5558A13F32641747BB22410DF56?sequence=1.]
- 2.Statistics South Africa. (2016) Mortality and causes of death in South Africa, 2016. Findings from death notification. [http://www.statssa.gov.za/publications/P03093/P030932016.pdf.]
- 3.UNAIDS (2017) ‘Ending AIDS: Progress towards 90-90-90 targets’. [http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf.]
- 4.WHO South Africa Country Profile. Tuberculosis. Generated: 2017-12-10. [https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=ZA&LAN=EN&outtype=html.]
- 5.Daniels A, Hoffman M, Lombard C, Steyn K, Levitt N, Katzenellenbogen J. Blood pressure and social support observations from Mamre, South Africa, during social and political transition. J Hum Hypertens. 1999;13: 689–693. [DOI] [PubMed] [Google Scholar]
- 6.Metcalf CA, Hoffman MN, Steyn K, Katzenellenbogen JM, Fourie JM. Design and baseline characteristics of a hypertension intervention program in a South African village. J Hum Hypertens. 1996;10:21–26. [PubMed] [Google Scholar]
- 7.Steyn K, Gaziano TA, Bradshaw D, Laubscher R, Fourie J. Hypertens in South African adults: results from the Demographic and Health Survey, 1998. J Hypertens. 2001;19:1717–1725. [DOI] [PubMed] [Google Scholar]
- 8.Bärnighausen T, Welz T, Hosegood V, Batzing-Feigenbaum J, Tanser F, et al. (2008) Hiding in the shadows of the HIV epidemic: obesity and hypertension in a rural population with very high HIV prevalence in South Africa. J Hum Hypertens 22: 236–239 10.1038/sj.jhh.1002308 [DOI] [PubMed] [Google Scholar]
- 9.Berry KM, Parker WA, Mchiza ZJ, Sewpaul R, Labadarios D, Rosen S, Stokes A. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Glob Health. 2017. August 16;2(3):e000348 10.1136/bmjgh-2017-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.South Africa Demographic and Health Survey 2016 Key Indicators Report National Department of Health Pretoria, South Africa South African Medical Research Council Cape Town, South Africa The DHS Program ICF Rockville, Maryland, USA May 2017.
- 11.Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Department of Health, Republic of South Africa (2004). The South African Antiretroviral Treatment Guidelines. [http://southafrica.usembassy.gov/media/2004-doh-art-guidelines.pdf].
- 13.National Department of Health, Republic of South Africa (2010). The South African Antiretroviral Treatment Guidelines. [http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf].
- 14.National Department of Health, Republic of South Africa (2013). The South African Antiretroviral Treatment Guidelines [http://www.auruminstitute.org/phocadownload/guidelines-short.pdf].
- 15.Pillay Y. Implementation of the Universal Test and Treat Strategy for HIV-positive patients and differentiated care for stable patients. August 2016. http://www.sahivsoc.org/Files/22%208%2016%20Circular%20UTT%20%20%20Decongestion%20CCMT%20Directorate%20(2).pdf. Accessed: January 2018.
- 16.Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013. February 22; 339(6122):961–5. 10.1126/science.1230413 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KA, Peer N, Mills EJ, et al. Burden, determinants, and pharmacological management of hypertension in HIV-Positive patients and populations: a systematic narrative review. AIDS Rev 2015;17:83–95. [PubMed] [Google Scholar]
- 18.Ogunmola OJ, Oladosu OY, Olamoyegun AM. Association of hypertension and obesity with HIV and antiretroviral therapy in a rural tertiary health center in Nigeria: a cross-sectional cohort study. Vascular Health and Risk Management. 2014;10:129–137. 10.2147/VHRM.S58449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2012;42(6):1754–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sliwa K, Carrington MJ, Becker A et al. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012;33:866–74. 10.1093/eurheartj/ehr398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart S, Libhaber E, Carrington M, Damasceno A, Abbasi H, Hansen C, Wilkinson D, Sliwa K. The clinical consequences and challenges of hypertension in urban-dwelling black Africans: insights from the Heart of Soweto Study. Int J Cardiol. 2011. January 7;146(1):22–7. 10.1016/j.ijcard.2009.05.061 [DOI] [PubMed] [Google Scholar]
- 22.van Zoest RA, Wit FW, Kooij KW, van der Valk M, Schouten J, Kootstra NA, Wiersinga WJ, Prins M, van den Born BJ, Reiss P; AGEhIV Cohort Study Group. Higher Prevalence of Hypertension in HIV-1-Infected Patients on Combination Antiretroviral Therapy Is Associated With Changes in Body Composition and Prior Stavudine Exposure. Clin Infect Dis. 2016. July 15;63(2):205–13. 10.1093/cid/ciw285 [DOI] [PubMed] [Google Scholar]
- 23.Schutte AE, Schutte R, Huisman HW, van Rooyen JM, Fourie CM, Malan NT, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. Int J Epidemiol 2012; 41(4): 1114–23. 10.1093/ije/dys106 [DOI] [PubMed] [Google Scholar]
- 24.Fox MP, Maskew M, Brennan AT, Evans D, Onoya D, Malete G, MacPhail P, Long L, Sanne I. Cohort Profile: The Right to Care Clinical HIV Cohort, Johannesburg, South Africa. BMJ Open 2017;7:e015620 10.1136/bmjopen-2016-015620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garenne M, Collinson MA, Kabudula CW, Gómez-Olivé FX, Kahn K, Tollman S. Completeness of birth and death registration in a rural area of South Africa: the Agincourt health and demographic surveillance, 1992–2014. Glob Health Action. 2016; 9: 10.3402/gha.v9.32795 Published online 2016 Oct 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timaeus I, Dorrington R, Bradshaw D, and Nannan N (2002) Mortality trends in South Africa 1985–2000: From apartheid to AIDS. Cape Town, South African Medical Research Council. [Google Scholar]
- 27.Seedat YK, Rayner BL, Veriava Y, Hypertension guideline working group. South African hypertension practice guideline 2014. Cardiovasc J Afr. 2014. Nov-Dec;25(6):288–94. 10.5830/CVJA-2014-062 Review. Erratum in: Cardiovasc J Afr. 2015 Mar-Apr;26(2):90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine Jason P. and Gray Robert J. A Proportional Hazards Model for the Sub distribution of a Competing Risk. JSTOR. p.496–509. [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 31.Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, Furrer H, Hatz C, Tanner M, Battegay M, Letang E; KIULARCO Study Group. Incidence and risk factors for hypertension among HIV patients in rural Tanzania—A prospective cohort study. PLoS One. 2017. March 8;12(3):e0172089 10.1371/journal.pone.0172089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okello S, Kanyesigye M, Muyindike WR, Annex BH, Hunt PW, Haneuse S, Siedner MJ. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens. 2015. October;33(10):2039–45. 10.1097/HJH.0000000000000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiébaut R, El-Sadr WM, Friis-Møller N, Rickenbach M, Reiss P, Monforte AD, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients D:A:D. Antivir Ther. 2005; 10: 811–823. [DOI] [PubMed] [Google Scholar]
- 34.Cooper RS, Forrester TE, Plange-Rhule J, Bovet P, Lambert EV, Dugas LR, Cargill KE, Durazo-Arvizu RA, Shoham DA, Tong L, Cao G, Luke A. Elevated hypertension risk for African-origin populations in biracial societies: modeling the Epidemiologic Transition Study. J Hypertens. 2015. March;33(3):473–80; discussion 480–1. 10.1097/HJH.0000000000000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helelo TP, Gelaw YA, Adane AA. Prevalence and associated factors of hypertension among adults in Durame Town, Southern Ethiopia. PLoS One. 2014. November 21;9(11):e112790 10.1371/journal.pone.0112790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manner IW, Trøseid M, Oektedalen O, Baekken M, Os I. Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens (Greenwich). 2013. February;15(2):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis.2009;199:1177–1185. 10.1086/597476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis.2003;187:1534–1543. 10.1086/374786 [DOI] [PubMed] [Google Scholar]
- 39.Fernandez S, Price P, McKinnon EJ, et al. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol.2006;120:163–170. 10.1016/j.clim.2006.04.570 [DOI] [PubMed] [Google Scholar]
- 40.Ingjerd W. Manner1,2, Dag Kvale1,2, Morten Baekken1, Maria Pedersen3, Susanne Dam Poulsen3, Ingrid Os1,2, Marius Troseid11Oslo University Hospital, Oslo, Norway 2University of Oslo, Oslo, Norway, 3Rigshospitalet, Copenhagen, Denmark. Microbial translocation independently predicts future hypertension in HIV-infected individuals. Paper #814. 19th Conference on Retroviruses and Opportunistic Infections; 2012. 19th Conference on Retroviruses and Opportunistic nections.
- 41.Calza L, Manfredi R, Chiodo F. Dyslipidemia associated with antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother 2004, 53:10–4. 10.1093/jac/dkh013 [DOI] [PubMed] [Google Scholar]
- 42.Kotler DP: HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008, 49 (Suppl. 2): S79–S85. [DOI] [PubMed] [Google Scholar]
- 43.Shaffer D, Hughes MD, Sawe F, Bao Y, Moses A, Hogg E, Lockman S, Currier J. Cardiovascular disease risk factors in HIV-infected women after initiation of lopinavir/ritonavir- and nevirapine-based antiretroviral therapy in Sub-Saharan Africa: A5208 (OCTANE). J Acquir Immune Defic Syndr. 2014. June 1;66(2):155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatleberg CI, Ryom L, d’Arminio Monforte A, Fontas E, Reiss P, Kirk O, et al. Impact of Antiretroviral Drugs on Hypertension in HIV-positive Persons: D:A:D Study. Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, USA, February 2015.
- 45.Currier J, Scherzer R, Bacchetti P, Heymsfield S, Lee D, Sidney S, Tien PC: Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008, 48 (1): 35–43. 10.1097/QAI.0b013e318164227f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulenga L, Musonda P, Mwango A, Vinikoor MJ, Davies MA, Mweemba A, Calmy A, Stringer JS, Keiser O, Chi BH, Wandeler G, IeDEA-Southern Africa. Effect of baseline renal function on tenofovir-containing antiretroviral therapy outcomes in Zambia. Clin Infect Dis. 2014. May;58(10):1473–80. 10.1093/cid/ciu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. 2010 revision. http://www.who.int/hiv/pub/arv/adult2010/en/. Accessed: January 2018. [PubMed]
- 48.Beckett NS, Peters R, Fletcher AE. et al. HYVET study group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:2417–2428. [DOI] [PubMed] [Google Scholar]
- 49.Majane OH, Norton GR, Maseko MJ, Makaula S, Crowther N, Paiker J, Thijs L, Brooksbank R, Sareli P, Staessen JA, Woodiwiss AJ. The association of waist circumference with ambulatory blood pressure is independent of alternative adiposity indices. J Hypertens. 2007;25(9):1798–806. 10.1097/HJH.0b013e3281e6666f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study is owned by the study site and National Department of Health (South Africa) and governed by the Human Research Ethics Committee (University of Witwatersrand, Johannesburg, South Africa). All relevant data is included in the paper and supplementary tables. The full data are available from the Health Economics and Epidemiology Research Office for researchers who meet the criteria for access to confidential data and have approval from the owners of the data (information@heroza.org).