Abstract
Malaria is a major infectious disease that still affects nearly half of the world’s population. Information on spatial distribution of malaria vector species is needed to improve malaria control efforts. In this study we used Maximum Entropy Model (MaxEnt) to estimate the potential distribution of Anopheles gambiae sensu lato and its siblings: Anopheles gambiae sensu stricto, and Anopheles arabiensis in Nigeria. Species occurrence data collected during the period 1900–2010 was used together with 19 bioclimatic, landuse and terrain variables. Results show that these species are currently widespread across all ecological zones. Temperature fluctuation from mean diurnal temperature range, extreme temperature and precipitation conditions, high humidity in dry season from precipitation during warm months, and land use and land cover dynamics have the greatest influence on the current seasonal distribution of the Anopheles species. MaxEnt performed statistically significantly better than random with AUC approximately 0.7 for estimation of the Anopheles species environmental suitability, distribution and variable importance. This model result can contribute to surveillance efforts and control strategies for malaria eradication.
Introduction
Anopheles species have plagued the world with malaria for decades and centuries now. An estimated 3.2 billion people worldwide were at risk of malaria in 2014 [1]. In 2016, ninety-one (91) countries and territories in the world had ongoing malaria transmission with estimated 216 million cases of malaria and 445,000 malaria deaths [2]. Fifteen countries accounted for 80% of all malaria cases and deaths globally. Sub-Saharan Africa region was home to 90% of malaria cases and 91% of malaria deaths, globally. Nigeria accounted for the highest proportion of cases globally (27%), followed by the Democratic Republic of the Congo (10%), India (6%) and Mozambique (4%) [2].
Besides Anopheles funestus, Anopheles gambiae is the dominant and most efficient vector of human malaria in the Afrotropical Region [3–5], based on its high abundance, longevity, high propensity for humans feeding, and high vectorial capacity [6,7]. Anopheles gambiae complex herein referred to as Anopheles gambiae sensu lato (s.l.) is made up of eight reproductively isolated species that are almost indistinguishable morphologically: An. arabiensis, An. gambiae sensu stricto (s.s.), An. bwambae, An. melas, An. merus, An. quadriannulatus, An. coluzzii, and An. amharicus [8,9]. Environmental, social and demographic factors such as climate change/variability drive the distribution of the dominant malaria vector species and their parasite transmission [10,11]. They shift in response to changes in temperature and precipitation [12–15]. Onyabe and Conn [4] explained that climatological factors, especially total annual precipitation strongly influence the range and relative abundance of An. arabiensis and An. gambiae within forest zones and savannas [16–18], with An. arabiensis predominating during the dry season and An. gambiae becoming more abundant during the rainy season [19]. In line with recent studies [20,21], Onyabe and Conn [4] observed shifts in species composition of An. arabiensis and An. gambiae s.s. after two years in four of 10 localities in their study, attributing it to random temporal (seasonal) fluctuations. Also, Umar et al. [22] observed low population of female Anopheles mosquitoes during the dry seasons (January to June and October to December), while carrying out assessment of indoor resting density of female anopheline mosquitoes in human dwelling at malaria vector sentinel sites in Bauchi State, Nigeria. According to Umar et al. [22], higher densities of anopheline mosquitoes during the rainy season to a large extent explains the seasonal pattern of clinical cases of malaria, with peak transmission shortly after maximum annual rainfall [19,23].
An understanding of the temporal and spatial determinants of parasite transmission, its seasonal patterns and the dominant vectors implicated in transmission is crucial [24] for the control of vector species [25]. A reliable risk modelling is one of the precautionary means in the framework of public health and management of malaria, especially in view of climate change [26]. Numerous mathematical models have been applied for disease risk modelling [27–30]. Maximum entropy algorithm (MaxEnt)—a type of machine learning technique of ecological niche modelling has been proved to perform well in modelling the distribution of disease vectors and their possible disease transmissions including that of malaria [28–30]. MaxEnt aims to predict potential distribution of biological species from the observation of species occurrences [31,32]. Moffett et al. [28] used MaxEnt to construct niche models for 10 malaria vector species in Africa, and predicted that An. gambiae abundance was highest in West Africa followed by An. arabiensis, An. funestus and An. melas; with human population density as the critical factor determining malaria risk. Similarly, Kulkarni et al. [30] used MaxEnt in Northern Tanzania when they found seasonality of precipitation and maximum annual temperature to have contributed the most to niche models for Anopheles arabiensis and An. funestus s.l. with AUC of 0.989 and 0.991 respectively, cold season precipitation and elevation were also found important for An. gambiae s.s. with AUC of 0.997.
While studies on MaxEnt modelling of the Anopheles species [28–30] predicted potential distribution on all spatial locations within geographic area of interest, without considering ecological zones. The identification of the species distribution based on entomological surveys [18–22] considered the species presence in different ecological zones with respect to absolute locations rather than all spatial locations of interest. In this study, we used MaxEnt for modelling environmental suitability and potential distribution of these dominant malaria mosquitoes in all spatial locations across topographic relief, ecological and regional zones in Nigeria. We also assessed the contributions of bioclimatic and other environmental variables to the occurrence of these Anopheles species.
Materials and methods
Study area
Nigeria is a country in West Africa, located approximately between Latitudes 4o and 14o north of the Equator and between Longitudes 2o 2' and 14o 30' east of the Greenwich Meridian (Fig 1) [33]. It has a total area of 923.77km2 characterised by undulating topographic relief, patterned by valleys created by its river systems (Fig 1) [33,34]. Coastal plains in the south have mean elevation of about 150m above sea level. Northern plains rise to about 600-700m, with Jos Plateau (over 1,500m) within Nigeria’s geographic centre, and Mambilla plateau (over 2,100m) amongst mountains at the border with Cameroon [35,36]. Temperature varies across ecological zones (Fig 1). Tropical at the coast (within Humid forest and Derived savanna) with 10°C and 37°C extreme low and high temperatures respectively, sub-tropical further inland (within Derived and Guinea savannas), and semi-arid in the far north (within Sudan and Sahel savannas) with 6°C and 44°C extreme low and high temperatures respectively [17,34]. Mid Altitude zone of Jos and Mambilla plateaus has average monthly temperatures range of 21–25°C [34]. Annual rainfall ranges from 500mm to 750mm in the north, and 1,200mm to above 4000mm in the south [34]. This diversity in climate conditions across the country affects the spatial epidemiology of malaria mosquitoes, malaria transmission and human vulnerability [24]. About 90% of over 190 million Nigerians are at the risk of malaria [37,38].
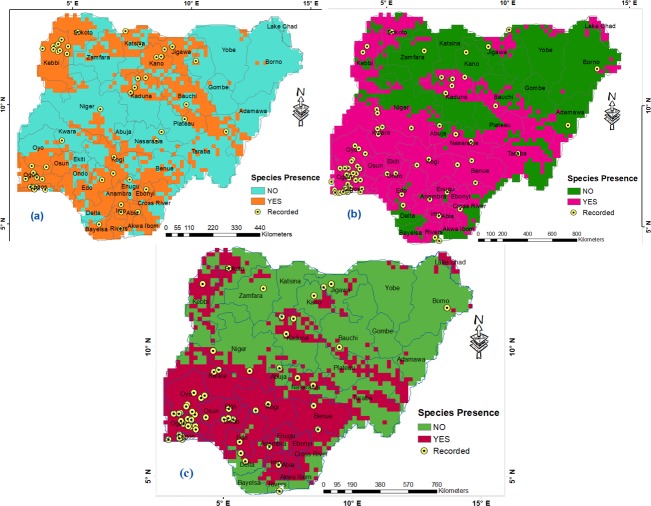
Fig 1. Study area with documented points of Anopheles gambiae species.
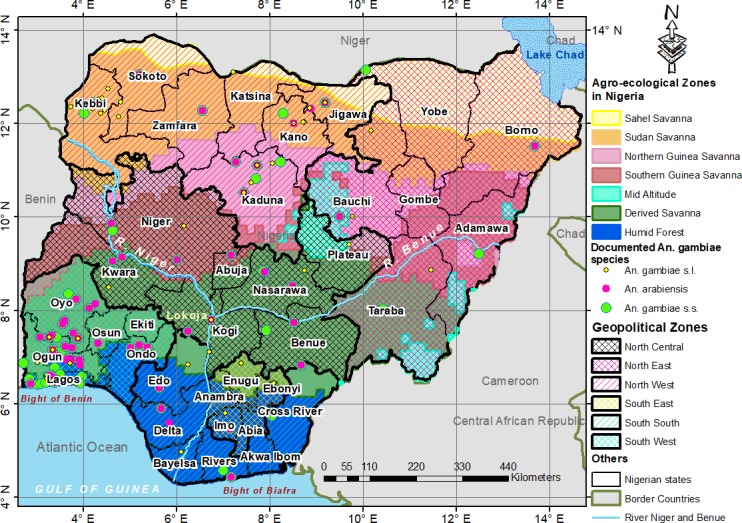
Georeferenced Anopheles species locations reprinted for illustrative purposes only from Okorie et al. [39] under a CC BY 4.0 license, with permission from PLOS ONE.
Modelling procedures and data analysis
Data resources
Malaria vectors data (Occurrence data: 1900–2010) was obtained from Nigeria Anopheles vector database (S1 Table); a comprehensive review of Okorie et al. [39]. Also, 19 bioclimatic variables (1960–1990) with about 1km2 spatial resolution were obtained from WorldClim—Global Climate Data (http://www.worldclim.org), global climate models (GCM)—community climate system model version 4 (CCSM4) [40] to model the impact of current climates on malaria vector species distribution in Nigeria. Additional variables used include land use land cover data with 24 classes obtained from U.S. Geological Survey data release [41], and Digital Elevation Model (DEM) derived from Shuttle Radar Topography Mission (SRTM) 90m obtained from the Consultative Group on International Agricultural Research—Consortium for Spatial Information (CGIAR-CSI) [42].
Model operation
Model operation was carried out as implemented in Maximum entropy algorithm model (MaxEnt) version 3.3.3k, described in detail previously by Phillips et al. [43]. All environmental layers were modified in ArcMap to be at the same extent, and a bias layer was created to provide MaxEnt with background samples to guard against bias in datasets [44]. Locations where Anopheles species were sampled to occur was defined as 1; the remaining pixels that were not sampled had “no data” in the grid [45]. Taking advantage of all available data without having an independent dataset, occurrence data for each species was split twenty-one times into training (75%) and testing (25%) subsets under sub-sample replicated run type. This was done to test the model performance (robustness and predictiveness) given by Area Under the Receiver Operating Characteristic (ROC) Curve or AUC; a plot of sensitivity against specificity which measures the ability of the model to discriminate between sites where a species is present (y = 1) against where it is absent (y = 0) [46–48]. Maximum iterations was increased from 500 (default) to 5000, allowing the model to have adequate time for convergence guarding against over-prediction or under-prediction of the relationships by the model [47]. Other setting options were left at default including regularization of 1 that helped reduced model over-fitting.
Probability of species occurrence predicted by all the predictor environmental variables produced a point-wise mean (model images) [47]. This was classified in ArcMap for current distributions of Anopheles gambiae s.l. and its siblings. This was also classified into suitable and unsuitable habitats with 10 percentile training presence logistic threshold provided by MaxEnt. Suitable areas within ecological zones for the studied mosquito species were generated by MaxEnt based on the entropy of optimal climatic and other environmental conditions that match the empirical average (threshold value) within documented species records. The 10% minimum threshold meant that suitable habitat was defined to include 90% of the data used to develop the model, considering some errors the data used may likely had [47]. Zonal statistics in ArcGIS was used to determine the average distribution density of predicted Anopheles species in all ecological and geopolitical zones, and in each state. Distribution density less than or equal to one (≤1) defines the probability of the species not occurring, greater than one (>1) defines species presence, while 2 represents maximum prevalence of species within a state and each zone. Analysis of percent contribution of each variable and jackknife test of variable importance were used to examine the contributions of environmental variables (Table 1) in defining the Anopheles species suitable habitats [30]. Jackknife test shows the training gain of each variable if the model was run in isolation, and compare it to the training gain with all the variables [47]. Comparing three jackknife plots produced for training gain, test gain and AUC gave very informed evaluations of variables contributions [46].
Table 1. Environmental variables used.
| Code | Bioclimatic/Ecological variables |
|---|---|
| BIO1 | Annual Mean Temperature |
| BIO2 | Mean Diurnal Range (Mean of monthly (max temp—min temp)) |
| BIO3 | Isothermality (BIO2/BIO7)*100 |
| BIO4 | Temperature Seasonality (standard deviation *100) |
| BIO5 | Maximum Temperature of Warmest Month |
| BIO6 | Minimum Temperature of Coldest Month |
| BIO7 | Temperature Annual Range (BIO5-BIO6) |
| BIO8 | Mean Temperature of Wettest Quarter |
| BIO9 | Mean Temperature of Driest Quarter |
| BIO10 | Mean Temperature of Warmest Quarter |
| BIO11 | Mean Temperature of Coldest Quarter |
| BIO12 | Annual Precipitation |
| BIO13 | Precipitation of Wettest Month |
| BIO14 | Precipitation of Driest Month |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) |
| BIO16 | Precipitation of Wettest Quarter |
| BIO17 | Precipitation of Driest Quarter |
| BIO18 | Precipitation of Warmest Quarter |
| BIO19 | Precipitation of Coldest Quarter |
| LULC_NIG | Land use Land cover of Nigeria |
| DEM_NIG | Digital Elevation Model of Nigeria (Land Surface Terrain) |
Note
* denotes multiplication.
Results
Potential suitable areas for the occurrence and distribution of An. gambiae s.l. and its siblings
The result of MaxEnt modelling predicted that the approximately 85,000 square kilometre (km2) Humid forest and 204,000km2 Derived savanna are highly suitable for the occurrence and distribution of An. gambiae s.l., An. gambiae s.s. and An. Arabiensis; with prevalence between 65% and 71% (Fig 2; Table 2). This makes all the states in South West, South East, South South and parts of North Central regions within the two ecological zones highly suitable for the Anopheles gambiae species (Fig 2; Table 2). The estimated 25,000 km2 Mid Altitude zone of Jos and Mambilla plateaus, and highlands along boundary with Cameron (Fig 2) seem less suitable with prevalence between 57% and 59% (Table 2), just as parts of Mangrove and Fresh water swamp forests within the Humid forest, especially along the deltas within Delta, Bayelsa, Rivers and Akwa Ibom states (Fig 2). Also, highlands within Niger, Kwara, Oyo, Ondo, Ekiti, Edo, Kogi, Enugu and Anambra states appear less suitable for these species especially An. gambiae s.l. Moreover, Sudan savanna and parts of Northern Guinea savanna in the North Western region are highly suitable for the occurrence of An. gambiae s.l. (Table 2), while North Eastern region landmass seems less suitable (Fig 2A). Sokoto and Kebbi states in the North West within Sudan savanna are highly suitable for the occurrence of An. gambiae s.s. and An. arabiensis, just as Kaduna South / Kaduna Central and parts of Jigawa, Kano and Zamfara states (with large less suitable landmass). Similar to An. gambiae s.l., landmass in Sahel, Sudan, Northern and Southern Guinea savannas within North Eastern region appear less suitable for An. gambiae s.s. and An. Arabiensis (Fig 2; Table 2). However, areas within Lake Chad, Bama and Kala/Balge districts of Borno state appear suitable for An. gambiae s.s. and An. arabiensis, just as Yobe state boundary with Niger Republic, and considerable landmass from Taraba, Adamawa, Gombe, through Plateau to Bauchi state (Fig 2).
Fig 2.
Suitable habitats for Anopheles species in Nigeria: (a) An. gambiae s.l., (b) An. gambiae s.s. and (c) An. arabiensis. Georeferenced Anopheles species locations reprinted for illustrative purposes only from Okorie et al. [39] under a CC BY 4.0 license, with permission from PLOS ONE.
Table 2. Ecological zones suitability and distribution of Anopheles species.
| Ecological zone | Estimated Area (km2) | Mean Distribution Density per sq. km | Prevalence (%) | ||||
|---|---|---|---|---|---|---|---|
| An. gambiae s.l. | An. gambiae s.s. | An. arabiensis | An. gambiae s.l. | An. gambiae s.s. | An. arabiensis | ||
| Sahel savanna | 80,277.79 | 1.19 | 1.15 | 1.12 | 59.38 | 57.71 | 56.12 |
| Sudan savanna | 152,500.02 | 1.34 | 1.18 | 1.16 | 67.21 | 58.96 | 58.08 |
| Northern Guinea savanna | 91,944.45 | 1.24 | 1.20 | 1.17 | 61.96 | 60.22 | 58.57 |
| Southern Guinea savanna | 109,444.46 | 1.20 | 1.24 | 1.20 | 60.06 | 61.88 | 59.82 |
| Mid Altitude | 25,000.00 | 1.17 | 1.18 | 1.16 | 58.45 | 59.02 | 57.90 |
| Derived savanna | 204,166.69 | 1.30 | 1.39 | 1.36 | 65.18 | 69.26 | 68.24 |
| Humid forest | 85,277.79 | 1.43 | 1.35 | 1.36 | 71.47 | 67.59 | 67.79 |
Note: distribution density = 1km-2 is equivalent to prevalence = 50% (unsuitable zone and species absence, designated with a green square); >1km-2 ≡ >50% (suitable zone and species presence); and 2km-2 ≡ 100% (highly suitable zone with maximum prevalence of species, designated with a red square).
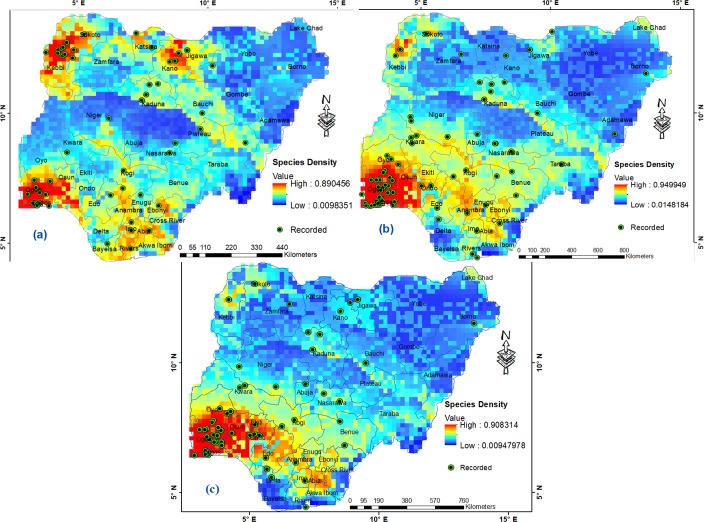
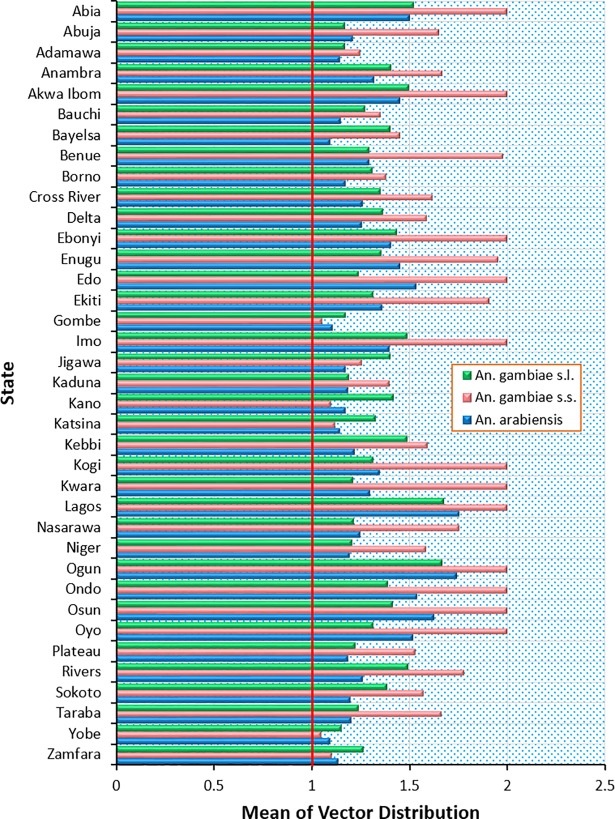
With respect to their environmental suitability, An. gambiae s.l., An. gambiae s.s. and An. arabiensis are widespread in Humid forest, Derived savanna; less distributed in Northern and Southern Guinea savannas, and least distributed in Sahel savanna and Mid Altitude zones (Table 2; Fig 3). While An. gambiae s.l. is widespread in Sudan savanna, An. gambiae s.s. and An. arabiensis record high presence only within west of Sudan savanna (Table 2; Fig 3). Unlike An. gambiae s.l., An. gambiae s.s. and An. arabiensis with similar distribution pattern are widespread within Lake Chad region of Sahel savanna, but record limited presence in Fresh water and Mangrove swamp forests of the Niger Delta region (Fig 3). In terms of regional zones, An. gambiae s.l. is more widespread in the South East, followed by South West and South South (Table 3); with highest mean distribution density in Lagos followed by Ogun and Abia States, while Yobe state records the lowest among all states (Figs 3A and 4). North East has the lowest mean distribution density of An. gambiae s.l. (Table 3). The boundary line on the mean distribution density graph defines presence and absence condition for each Anopheles species from zonal statistics (Fig 4). An. gambiae s.s. is highly prevalent in South Western and South Eastern regions of Nigeria (Table 3; Fig 4). It is more widespread in North Central region than South South and North West, and lowest in North East (Table 3). As a dominant Anopheles species [6,7], An. arabiensis exists in all states in Nigeria, highest in Lagos state and lowest in Bayelsa state (Fig 4). South West records highest prevalence of An. arabiensis, followed by South East, South South, North Central, North West, and lowest in North East (Table 3).
Fig 3.
Potential distribution of Anopheles species in Nigeria: (a) An. gambiae s.l., (b) An. gambiae s.s. and (c) An. arabiensis. Georeferenced Anopheles species locations reprinted for illustrative purposes only from Okorie et al. [39] under a CC BY 4.0 license, with permission from PLOS ONE.
Table 3. Anopheles species prevalence by regions under current climate.
| Geopolitical zone | Estimated Area (km2) | Mean Distribution Density per sq. km. | Prevalence (%) | ||||
|---|---|---|---|---|---|---|---|
| An. gambiae s.l. | An. gambiae s.s. | An. arabiensis | An. gambiae s.l. | An. gambiae s.s. | An. arabiensis | ||
| South South | 68,888.90 | 1.38 | 1.29 | 1.29 | 69.10 | 64.46 | 64.65 |
| South East | 23,611.11 | 1.44 | 1.42 | 1.41 | 71.94 | 71.12 | 70.75 |
| South West | 61,666.67 | 1.43 | 1.57 | 1.59 | 71.29 | 78.28 | 79.70 |
| North Central | 186,388.91 | 1.24 | 1.30 | 1.25 | 61.75 | 64.85 | 62.51 |
| North East | 227,500.02 | 1.20 | 1.17 | 1.14 | 59.79 | 58.48 | 57.05 |
| North West | 177,222.24 | 1.34 | 1.20 | 1.18 | 67.16 | 60.07 | 58.95 |
Note: distribution density = 1km-2 is equivalent to prevalence = 50% (species absence, designated with a green square); >1km-2 ≡ >50% (species presence); and 2km-2 ≡ 100% (maximum prevalence of species, designated with a red square).
Fig 4. Mean distribution density of An. gambiae s.l., An. gambiae s.s., and An. arabiensis in Each Nigerian State.
Note: if 1 ≡ 0 (species do not occur); then >1 = species occur; and 2 = maximum species prevalence.
Variability exists in the spatial distributions of the Anopheles species (Fig 3) among states (Fig 4) across topographic relief, ecological and geopolitical regions based on the diversity in climate conditions across the country, which affects the spatial epidemiology of these Anopheles species and malaria transmission [24]. Matching the pattern of malaria parasite prevalence [25], distribution density of the Anopheles species increases from the sub-tropical Middle Belt region to the tropical southern regions with high rainfall and coastal plains (Fig 3). Especially South West and South East where Lagos state, smallest by landmass but the most populated, and most urbanised [39,49] records highest prevalence of An. gambiae s.l., An. gambiae s.s. and An. arabiensis, matching about 1.2 million confirmed cases of malaria in 2016 [50].
Environmental variables contributions in defining Anopheles gambiae species distributions
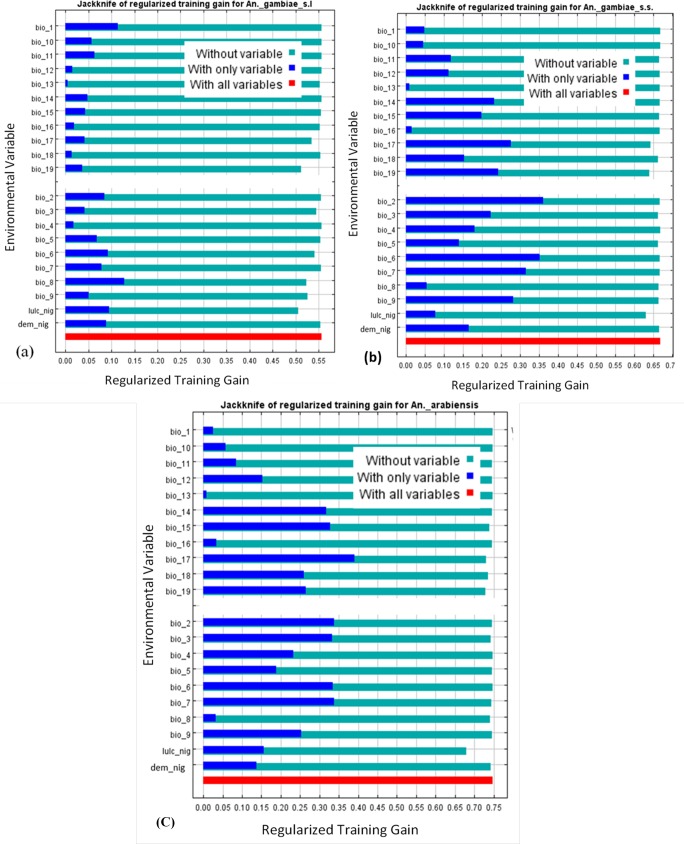
Mean temperature of wettest quarter (bio_8) is the environmental variable with highest gain to the MaxEnt model of An. gambiae s.l. when used in isolation, which therefore appears to have the most useful information by itself (Fig 5A). Bio_8 demonstrates how mean temperatures (28°C minimum and 30°C maximum) [51] during the wettest three months (June—August) of the year may affect seasonal distributions of An. gambiae s.l. Other environmental variables that influence the occurrence and distribution of An. gambiae s.l. when used together with all other environmental variables are minimum temperature of coldest month (bio_6) (23°C, August), precipitation of coldest quarter (bio_19) (June—August, about 211mm in the arid north to above 2000mm in the coastal south), annual mean temperature (bio_1) 33°C, and precipitation of driest quarter (bio_17) (December—February, 0mm in arid north to 240mm in coastal south) (Fig 5A) [52]. Also, mean diurnal range (bio_2) (7–16°C) [52] is the major environmental variable which defines suitable habitats for An. gambiae s.s. in isolation, and constitutes the relevance of temperature fluctuation on spatial distribution of An. gambiae s.s. Other variables which appear very pivotal in the occurrence and distribution of An. gambiae s.s. when used alongside all other environmental variables are minimum temperature of coldest month (bio_6), temperature annual range (bio_7) (28–36°C), mean temperature of driest quarter (bio_9) (20°C minimum and 35°C maximum), precipitation of driest quarter (bio_17), precipitation of coldest quarter (bio_19) and precipitation of driest month (bio_14) (January, not exceeding 27mm in the wet southern coast) (Fig 5B) [51,52]. Precipitation of driest quarter (bio_17) is the major environmental variable that influences the occurrence and seasonal distributions of An. arabiensis in isolation (Fig 5C). Precipitation of coldest quarter (bio_19), precipitation seasonality (bio_15), mean diurnal range (bio_2), mean temperature of driest quarter (bio_9) and precipitation of warmest quarter (bio_18) (March to May, about 10mm in the arid north to 700mm in the coastal south) combine with all other environmental variables to delineate suitable habitats for the occurrence and distribution of An. arabiensis (Fig 5C). However, land use land cover map reflecting high urbanisation, increased population density and anthropogenic activities is the environmental variable that would decrease the gain the most for all three Anopheles species if omitted. It has the most information that is not present in the other variables. The critical influence of land use land cover dynamics in the occurrence and distribution dynamics of An. gambiae s.l., An. gambiae s.s. and An. arabiensis is expressed in their high distribution density in highly populated/urbanised states with increased anthropogenic activities including Abia, Akwa Ibom, Anambra, Enugu, Imo, Kano, Lagos, Ogun, Ondo, Osun, Oyo, Rivers and Sokoto states (Fig 4) [13].
Fig 5.
Jackknife test for (a) An. gambiae s.l. (b) An. gambiae s.s. (c) An. Arabiensis.
MaxEnt model performance
MaxEnt recorded a fair performance for 21 replicate runs of An. gambiae s.l., An. gambiae s.s., and An. arabiensis with average test AUC of 0.713, 0.699, and 0.713 respectively. The value of AUC determines the performance of the model; AUC of 0.5 implies that the model was no better than random, while an AUC of 1 indicates a perfect prediction. In essence, AUC values tend to be higher for species with narrow ranges relative to the study area described by the environmental data. A behaviour that is an artifact of the AUC statistic, but does not necessarily mean that the models are better [46].
Discussion
In agreement with previous studies [4,17,23,39], the model results suggested that An. gambiae s.l., An. gambiae s.s., and An. arabiensis are widespread across all ecological zones in Nigeria, where they co-exist in sympatric relationship [24]. The combinations of soil, landform and climatic characteristics within ecological zones define distinct distribution of the modelled Anopheles species [36], and they predominantly occur in Humid forest, Guinea savannas and Sudan savanna regions [4,39,53–56]. The high environmental suitability of the Derived savanna and Humid forest within southern and parts of North Central regions [28,39] is influenced by human settlement patterns, topographical and climatic conditions of the regions [11,28,57]. Total annual precipitation, random temporal fluctuations, climate seasonality and land use land cover dynamics strongly influenced the range, relative abundance and ecological adaptability of the dominant members of the An. gambiae complex, in line with previous findings [4,16,28,30]. The highest mean distribution density of An. gambiae s.s. amongst other species corroborates with the results of Bruce-Chwatt [17] and Okwa et al. [18] who reported An. gambiae s.s. as the most efficient and most widespread within the gambiae complex [58]. Its high abundance is highly associated with the mean diurnal temperature range that increases the species sensitivity to changes in climates, leading to widespread presence of the species [52,59]. In accordance with Oyewole et al. [19], the combined contributions of environmental variables favour higher distribution of An. gambiae s.s. in wet season than An. arabiensis, while precipitation during dry and warm months (high humidity in dry season) favour higher distribution of An. arabiensis than An. gambiae s.s. in dry season [20,21,30]. An. arabiensis preference of warmer climates [19,30] possibly impacts its limited presence in the cold swamps within the Humid forest, and the high suitability of Sahel savanna localities, especially Lake Chad basin area of Borno state enhanced by high relative humidity from the lake [53].
The low suitability of areas within Mid Altitude zone may be attributed to the comparatively cold weather of the highlands associated with average monthly temperatures 21–25°C [14,34], relatively below the model optimum temperatures of 23–35°C for rapid population expansion of the An. gambiae species [12]. As derived from the model, the high suitability of Lagos state for the malaria vector species can also be attributed to high temperature and precipitation in that area of the Humid forest [12]. The complex nature of the society, poor planning and lack of infrastructure in expanding slum areas, rapid population expansion and industrial activities make the climate warmer and create conditions highly suitable for malaria vector reproduction, survival and increased biting rates, exacerbating malaria transmission in Lagos state [11,14,28]. The influence of seasonal rainfall variability and high tropical temperatures on the extent and unbalanced distribution of the modelled mosquito species observed in most part of the country also agrees with the findings of Oyewole et al. [19] and Umar et al. [22], in relation to unbalanced and seasonal malaria transmission. The observed gradient in the distribution density from coastal south to arid north, shows that vector abundance is greatest in areas with consistently high temperatures and in any case, small mean diurnal temperature range and consistent precipitation. This is in line with the observation of Dimitrov and Morton [52] who reported that entomological inoculation rate was highest in the coastal areas and lowest in the northeast.
MaxEnt performance was better than random [45] with AUC values less than those obtained in similar studies [28,30]. This may be attributable to large ranges of the documented species [46] relative to the study area (especially in the North Eastern part of the country), resulting in increased sampling bias [44,60,61], which may influence the model performance [46,47]. However, according to Lobo et al. [62], an accurate model for widespread species (just as the ones modelled in this study) where the probability of presence increases steadily with predictor values have low AUC values, denoting the true generalist nature of the species distribution. It is important to note that suitable areas with low distribution density of the modelled Anopheles gambiae species may likely experience widespread prevalence with high distribution density, species migration and invasion [63], if there is a change in any of the environmental variables identified in this study as crucial to their distribution pattern. This will lend credence to the prediction of ecological models, that the distribution of world biomes is likely to shift as a result of changes in climate system associated with increased warming [64], since An. gambiae s.l., An. gambiae s.s., and An. arabiensis highly flourish with warm climate [12,65]. Thus, the propensity of future malaria transmission in Nigeria is expected to be higher with seasonal spatial shifts due to climate change and altered weather patterns; influencing the range (both latitude and altitude), intensity, and seasonality of vectors [11–15,65,66].
Conclusions
In this paper we used Maxent in modelling environmental suitability and distribution of dominant Anopheles gambiae species in Nigeria. We also assessed the contributions and importance of bioclimatic and other environmental variables to the model. Results showed that the species are more prevalent within the Humid forest and the Derived savanna, but most prevalent in South Western and South Eastern geopolitical zones within the two ecological zones. This is particularly worrisome in highly populated and urbanised Lagos state which recorded highest distribution density of all three species. Our results also showed that land use dynamics become very critical for the occurrence and distribution of the three dominant species of Anopheles, while seasonal rainfall, temperature fluctuations and high humidity during warm weather (dry season) drive the occurrence and seasonal distribution of the Anopheles species and potential malaria transmission. The derived MaxEnt model was successful in defining potential suitable habitats for the occurrence and distribution of the Anopheles species, and estimated variable importance. This result might be useful in predicting the variability of malaria vector distribution across ecological gradients and in understanding the potential causes of its severity from an environmental point of view in tropical regions.
Supporting information
Reused from Okorie et al. [39] under a CC BY 4.0 license, with permission from PLOS ONE.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files. Occurrence data was obtained from Nigeria Anopheles vector database and can be found on: https://doi.org/10.1371/journal.pone.0028347.s001.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. World Malaria Report 2015 Geneva: World Health Organization; 2015. 280p. [Google Scholar]
- 2.World Health Organization. World Malaria Report 2017 Geneva: World Health Organization; 2017. 196p. [Google Scholar]
- 3.CDC. Anopheles Mosquitoes. Centers for Disease Control and Prevention: Global Health. Division of Parasitic Diseases and Malaria 2015, October 21. Available from: http://www.cdc.gov/malaria/about/biology/mosquitoes/
- 4.Onyabe DY, Conn JE. The Distribution of Two Major Malaria Vectors, Anopheles gambiae and Anopheles arabiensis, in Nigeria. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2001; 96(8):1081–1084. [DOI] [PubMed] [Google Scholar]
- 5.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasites and Vectors. 2010; 3(117). 10.1186/1756-3305-3-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takken W, Scott TW. Ecological Aspects for Application of Genetically Modified Mosquitoes Dordrecht: Kluwer Academic Publishers; 2003; 75–90. [Google Scholar]
- 7.Autino B, Noris A, Russo R, Castelli F. Epidemiology of Malaria in Endemic Areas. Meditter J Hematol Infect Dis. 2012; 4(1):e2012060 10.4084/MJHID.2012.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region) In Publications of the South African Institute for Medical research Johannesburg; 1987. p. 55. [Google Scholar]
- 9.Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) In Publications of the South African Institute for Medical Research Johannesburg; 1968. p. 54. [Google Scholar]
- 10.Adepoju KA, Akpan GE. Historical Assessment of Malaria Hazard and Mortality in Nigeria—Cases and Deaths: 1955–2015. Int J Environ. Bioener. 2017; 12(1): 30–46. [Google Scholar]
- 11.Tolulope O. Spatio–Temporal Clustering of Malaria Morbidity in Nigeria (2004–2008). J Sci Res. 2014; 13:99–113. [Google Scholar]
- 12.Martens WJ, Niessen LW, Rotmans J, Jetten TH, McMichael AJ. Potential Impact of Global Climate Change on Malaria Risk. Environ Health Perspect. 1995; 103(5): 458–464. 10.1289/ehp.95103458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khasnis AA, Nettleman MD. REVIEW ARTICLE: Global Warming and Infectious Disease. Arch Med Res. 2005; 36: 689–696. 10.1016/j.arcmed.2005.03.041 [DOI] [PubMed] [Google Scholar]
- 14.Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proceedings of the National Academy of Sciences of the United States of America. 2006; 103(15): 5829–5834. 10.1073/pnas.0508929103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostfeld RS. Climate change and the distribution and intensity of infectious diseases. Ecol. 2009; 90(4): 903–905. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay SW, Parson L, Thomas CJ. Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc R Soc London B. 1998; 265:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce-Chwatt LJ. Malaria in Nigeria. Bull. World Hlth Org. 1951; 4:301–327. [PMC free article] [PubMed] [Google Scholar]
- 18.Okwa OO, Akinmolayan FI, Carter V, Hurd H. Transmission dynamics of malaria in four selected ecological zones of Nigeria in the rainy season. Ann Afr Med, 2009; 8(1):1–9. 10.4103/1596-3519.55756 [DOI] [PubMed] [Google Scholar]
- 19.Oyewole IO, Ibidapo CA, Oduola AO, Obansa JB, Awolola TS. Anthropophilic mosquitoes and malaria transmission in a tropical rain forest area of Nigeria. acta SATECH. 2005; 2(1):6–10. [Google Scholar]
- 20.Awolola TS, Oyewole IO, Koekemoer LL, Coetzee M. Identification of three members of Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. Roy Soc Trop Med Hyg. 2005; 99:525–531. [DOI] [PubMed] [Google Scholar]
- 21.Amadi EC, Harry SI, Ogbalu OK. Prelimnary Survey on TheAbundance of Mosquito Species and Transmission of Plasmodium to Humans in Bille, Oporoama, Sama and Tema Communities of Rivers State, Nigeria. IOSR J. of Phar. and Bio. Sci. 2015; 10(6 Ver. II):82–86. [Google Scholar]
- 22.Umar A, Kabir BG, Abdullahi MB, Barde A, Misau AA, Sambo ML, et al. Assessment of Indoor Resting Density of Female Anopheline Mosquitoes in Human Dwelling at Malaria Vector Sentinel Sites in Bauchi State, Nigeria. Adv Stud Biol. 2015; 7(7):323–333. Available from: http://dx.doi.org/10.12988/asb.2015.5210 [Google Scholar]
- 23.Okorie PN, Popoola K, Awobifa OM, Ibrahim KT, Ademowo GO. Species composition and temporal distribution of mosquito populations in Ibadan, Southwest Nigeria. J Entomol Zoo Stud 2014; 2(4):164–169. [PMC free article] [PubMed] [Google Scholar]
- 24.National Malaria Control Programme, suNMaP, World Health Organization and the INFORM Project. A description of the epidemiology of malaria to guide the planning of control in Nigeria. A report prepared for the Federal Ministry of Health, Nigeria, the Roll Back Malaria Partnership and the Department for International Development, UK. November, 2013.
- 25.World Health Organization. World Malaria Report 2016 Geneva: World Health Organization; 2016. 186p. [Google Scholar]
- 26.Dalitz MK. Autochthone Malaria im mitteldeutschen Raum. Dissertation, Martin-LutherUniversität, Halle-Wittenberg Universität. Halle-Wittenberg. 2005.
- 27.Siettos CI, Russo L. Mathematical modeling of infectious disease dynamics. Virulence. 2013; 4(4):295–306. 10.4161/viru.24041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moffett A, Shackelford N, Sarkar S. Malaria in Africa: Vector Species’ Niche Models and Relative Risk Maps. PLoS ONE. 2007; 2(9):e824 10.1371/journal.pone.0000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley DH, Klein TA, Kim HC, Sames WJ, Wilkerson RC, Rueda LM. Geographic Distribution and Ecology of Potential Malaria Vectors in the Republic of Korea. J Med Entomol. 2009; 46(3):680–692. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni MA, Desrochers RE, Kerr JT. High Resolution Niche Models of Malaria Vectors in Northern Tanzania: A New Capacity to Predict Malaria Risk? PLoS ONE. 2010; 5(2): p. e9396 10.1371/journal.pone.0009396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson AT. Ecologic Niche Modeling and Spatial Patterns of Disease Transmission. Emerg Infect Dis. 2006; 12(12): p1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity Distrib. 2011; 17:43–57. [Google Scholar]
- 33.Amadi SO, Udo SO, Ewona IO. Trends and Variations of Monthly Mean Minimum and Maximum Temperature Data over Nigeria for the Period 1950–2012. IJPAP. 2014; 2(4):1–27. [Google Scholar]
- 34.Metz HC (Ed.). Nigeria: A Country Study. Washington: GPO for the Library of Congress. 1991. Available from: http://countrystudies.us/nigeria/33.htm
- 35.USAID. Nigeria Environmental Analysis Final Report. Biodiversity and Sustainable Forestry (BIOFOR) Indefinite Quantity Contract (IQC); 2002. USAID BIOFOR IQC No.: LAG-I-00-99-00013-00.
- 36.FAO Soils Bulletin 73. Agro-Ecological Zoning Guidelines. Soil Resources, Management and Conservation Service. FAO Land and Water Development Division. Food and Agriculture Organization of the United Nations, Rome. 1996. Available from: http://www.fao.org/docrep/w2962e/w2962e-03.htm#P229_15540
- 37.United Nations, Department of Economic and Social Affairs, Population Division (UN-DESA). World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. 2017; Working Paper No. ESA/P/WP/248.
- 38.Owoseye A. World Malaria Day: Nigerians Warned to Stop Using Chloroquine for Malaria. Treatment. Premium Times. 25 Apr 2017. Available from: http://allafrica.com/stories/201704260043.html Cited 9 Nov 2017.
- 39.Okorie PN, McKenzie FE, Ademowo OG, Bockarie M, Kelly-Hope L. Nigeria Anopheles Vector Database: An Overview of 100 Years’ Research. PLoS ONE. 2011; 6(12):e28347 10.1371/journal.pone.0028347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WorldClim—Global Climate Data: Free climate data for ecological modelling and GIS. Download. Available from: http://www.worldclim.org/download.html.
- 41.Tappan GG, Cushing WM, Cotillon SE, Mathis ML, Hutchinson JA, Dalsted KJ. West Africa Land Use Land Cover Time Series: U.S. Geological Survey data release. 2016. Available from: 10.5066/F73N21JF [DOI]
- 42.CGIAR-CSI. SRTM 90m Digital Elevation Data. The CGIAR Consortium for Spatial Information (CGIAR-CSI). Jan 2017. Available from: http://srtm.csi.cgiar.org/SELECTION/inputCoord2.asp
- 43.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006; 190: 231–259. Available from: 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- 44.Fourcade Y, Engler JO, Rödder D, Secondi J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE. 2014; 9(5): e97122 10.1371/journal.pone.0097122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altamiranda-Saavedra M, Arboleda S, Parra JL, Peterson AT, Correa MM. Potential distribution of mosquito vector species in a primary malaria endemic region of Colombia. PLoS ONE. 2017; 12(6): e0179093 Available from: 10.1371/journal.pone.0179093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips SJ. A Brief Tutorial on Maxent. 2017. Available from: http://biodiversityinformatics.amnh.org/open_source/maxent/
- 47.Young N, Carter L, Evangelista P. A MaxEnt Model v3.3.3e Tutorial (ArcGIS v10). Natural Resource Ecology, Colorado State University, USA. 2011.
- 48.Hanley JA, McNeil BJ. The meaning and use of the area under a Receiver. Operating Characteristic (ROC) curve. Radiology, 1982; 143:29–36. 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 49.Sunday O, Ajewole A. Implications of the Changing Pattern of Land cover of the Lagos Coastal Area of Nigerian. AEJSR, 2006; (1):31–37. IDOSI Publications. Available from: www.idosi.org/aejsr/1(1)06/7.pdf [Google Scholar]
- 50.Vanguard. Malaria: Lagos State records 1.2m cases in 2016 –Commissioner. Vanguard Media Limited, Nigeria. 4 May 2017. Available from: https://www.vanguardngr.com/2017/05/malaria-lagos-state-records-1-2m-cases-2016-commissioner/ Cited 10 Nov 2017.
- 51.World Guides. Nigeria Weather, When to Go and Climate Information (Nigeria, NG, West Africa). TravelSmart Ltd. 2016. Available from: http://www.world-guides.com/africa/west-africa/nigeria/nigeria_weather.html Cited 7 June 2018.
- 52.Dimitrov NB, Morton DP. Combinatorial Design of a Stochastic Markov Decision Process In: Saltzman MJ, Chinneck JW, Kristjansson B, editors. Operations Research and Cyber-Infrastructure. New York: Springer; 2009. P. 167–193. 10.1007/978-0-387-88843-9 [DOI] [Google Scholar]
- 53.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979; 73:483–497. [DOI] [PubMed] [Google Scholar]
- 54.Amajoh CN. A review of Malaria Vector behaviour in Nigeria. Abstracts of two days National symposium on malaria in Nigeria held at Nigerian, 4th to 5th November 1997 Yaba, Lagos: Institute of Medical Research; 1997. [Google Scholar]
- 55.Coetzee M, Craig M, le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000; 16:74–77. [DOI] [PubMed] [Google Scholar]
- 56.Onyabe DY, Vajime CG, Nock IH, Ndams IS, Akpa AU, Alaribe AA, et al. The distribution of M and S molecular forms of Anopheles gambiae in Nigeria. Trans R Soc Trop Med Hyg. 2003; 97:605–608. [DOI] [PubMed] [Google Scholar]
- 57.Siteti MC, Injete SD, Wanyonyi WA. Malaria parasite species prevalence and transmission dynamics at selected sites in the Western highlands of Kenya. CHRISMED J Health Res. 2016; 3:45–50. [Google Scholar]
- 58.Olayemi IK, Ande AT, Odeyemi MO, Ibemesi G Emmanuel R. Temporal Ecologic Adaptability of the Principal Vector of Malaria, Anopheles gambiae s.l. (Diptera: Culicidae), in Northcentral Nigeria. App Sci Report. 2014; 5(3):110–117. doi: 10.15192/PSCP.ASR.2014.1.3.110117 [Google Scholar]
- 59.Briga M, Verhulst S. Large diurnal temperature range increases bird sensitivity to climate change. Sci Report. 2015; 5:16600 10.1038/srep16600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syfert MM, Smith MJ, Coomes DA. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE. 2013; 8(2): e55158 10.1371/journal.pone.0055158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008; 31:161–175. [Google Scholar]
- 62.Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr. 2007. 10.1111/j.1466-8238.2007.00358.x [DOI] [Google Scholar]
- 63.Peterson AT. Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect Dis. 2009; 9(59). 10.1186/1471-2334-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.IPCC. The Regional Impacts of Climate Change: An Assessment of Vulnerability, (Eds Watson R. T., Zinyowera M. C., and Moss R. H.), Cambridge University Press, Cambridge, UK: 1998. [Google Scholar]
- 65.IPCC. Climate Change 1995 Impacts, Adaptations and Mitigation of Climate Change: Scientific-Technical Analyses. Contribution of Working Group II to the Second Assessment Report of the Intergovernmental Panel on Climate Change. (RT Watson, MC Zinyowera, RH Moss, DJ Dokken, Eds.) New York, USA: Press Syndicate of the University of Cambridge. 1995.
- 66.Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000; 289:1763–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reused from Okorie et al. [39] under a CC BY 4.0 license, with permission from PLOS ONE.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Occurrence data was obtained from Nigeria Anopheles vector database and can be found on: https://doi.org/10.1371/journal.pone.0028347.s001.