Abstract
Ovarian cancer is a heterogeneous disease. Data regarding familial risks for specific proband, age at diagnosis and histology are limited. Such data can assist genetic counseling and help elucidate etiologic differences among various histologic types of ovarian malignancies. By using the Swedish Family-Cancer Database, we calculated relative risks (RRs) for detailed family histories using a two-way comparison, which implied e.g. estimation of RRs for overall ovarian cancer when family history was histology-specific ovarian cancer, and conversely, RRs for histology-specific ovarian cancer when family history was overall ovarian cancer. In families of only mother, only sisters or both mother and sisters diagnosed with ovarian cancer, cancer risks for ovary were 2.40, 2.59 and 10.40, respectively; and were higher for cases diagnosed before the age of 50 years. All histological types showed a familial risk in two-way analyses, except mucinous and sex cord-stromal tumors. RRs for concordant histology were found for serous (2.47), endometrioid (3.59) and mucinous ovarian cancers (6.91). Concordant familial risks were highest for mucinous cancer; for others, some discordant associations, such as endometrioid-undifferentiated (9.27) and serous-undifferentiated (4.80), showed the highest RRs. Familial risks are high for early-onset patients and for those with multiple affected relatives. Sharing of different histological types of ovarian cancer is likely an indication of the complexity of the underlying mechanisms.
Introduction
Ovarian cancer is the seventh most common cancer and the eighth leading cause of cancer-related deaths in women worldwide [1]. The incidence is highest in Eastern and Northern Europe. In Sweden, the incidence has been declining during the last decade [2]. Ovarian cancer is a heterogeneous disease; the most common types are epithelial ovarian cancers and they have been divided into two groups (type I and II) based on distinctive clinicopathologic and molecular genetic features [3]. Type I group includes low-grade serous, low-grade endometrioid, clear cell and mucinous carcinomas, which are indolent and have a good prognosis. While in type II group, tumors are more aggressive and are composed of high-grade serous carcinoma, high- grade endometrioid carcinoma, carcinosarcomas and undifferentiated carcinomas. Non-epithelial ovarian malignancies are far less common and contain sex cord-stromal malignancy; the latter includes thecoma, which is the most prevalent non-epithelial ovarian cancer.
Ovarian cancer risk increases with aging and peaks between the ages of 50 and 80 years [4]. In the general Swedish population, the lifetime risk of ovarian cancer is 1% [2]. Reproductive and menstrual factors are strongly influential regarding ovarian cancer. Factors that can decrease the total number of ovulatory cycles such as pregnancy, breastfeeding and use of oral contraceptives reduce the risk for ovarian cancer. Factors that prolong exposure to ovulation, such as low parity, early menarche and late menopause increase the risk for ovarian cancer [4]. Oral contraceptive use is a confirmed protective factor for ovarian cancer and the widespread use of it during recent decades is considered to be one of the main reasons for the decreasing incidence of ovarian cancer [5]. However, an increased risk for mucinous ovarian cancer was observed in individuals using oral contraceptives [6–8]. The protective effect of high parity has also been confirmed and it is most strongly associated with endometrioid and clear cell types [7]. Other non-reproductive factors, which can influence the risk of ovarian cancer, include smoking and body size (height or body mass index) [9, 10].
Family history is a strong risk factor for ovarian cancer, and the relative risk is estimated to be 2.0 to 4.0 for those that have a first-degree relative affected by the disease [11–15]. The siblings’ risks by age difference are similar, which suggests that the familial clustering of ovarian cancer is mainly heritable [16]. Familial risk is associated with mutations in BRCA1 and BRCA2 genes, which conferred respectively 59% and 16.5% risk of developing ovarian cancer by the age of 70 in the Epidemiological Study of BRCA1 and BRCA2 mutation carriers (EMBRACE) in the UK [17]. Ovarian cancer is also a manifestation in hereditary nonpolyposis colorectal cancer (HNPCC) syndrome which is caused by mutations in mismatch repair (MMR) genes [18]. The related lifetime risk of developing epithelial ovarian cancer is around 12% [19]. Mutations in other genes such as BRIP1, RAD51C and RAD51D also contribute to hereditary ovarian cancer [20]. Each histological type of ovarian cancer harbors distinct mutations. Germline alterations of BRCA1 and BRCA2 were reported to be associated with high-grade serous histology [21, 22], and families with HNPCC syndrome present a tendency towards endometrioid and clear cell types [19, 23, 24].
Based on the above, it can be hypothesized that histology-specific etiology may exist in ovarian cancers. There are limited data regarding familial risk for specific histologic types of ovarian malignancy; such data may help elucidate etiologic differences among the various histologic types of ovarian malignancy and assist clinical genetic counseling. In this study, we use the recent national Swedish Family-Cancer Database, which included 16.1 million individuals, to estimate the familial risks of ovarian cancer by age at diagnosis, proband type (mother or sisters) and histology.
Methods
The Swedish Family-Cancer Database (FCD) includes all people born in Sweden since 1932 (offspring generation) together with their biological parents (parental generation) [25]. The latest version of FCD contains 16.1 million individuals among which nearly 2.0 million were cancer patients recorded to the end of 2015. The 3-digital codes of the 7th revision of the International Classification of Diseases (ICD-7) were used to identify ovarian cancers. Histological types of ovarian cancers were classified according to Systemized Nomenclature of Medicine (SNOMED) codes since 1993. The follow-up for cancer in offspring generation (8.8 million individuals) commenced from the beginning of 1958 (for histological analysis it was started in 1993), the birth year, or the immigration year, whichever came latest. The follow-up was terminated when a person was diagnosed with cancer, emigrated or died, or at the end of 2015, whichever came first.
In this study, all the incident cases of ovarian cancers reported between 1958 and 2015 were analyzed. The world standard population was used to calculate age-standardized incidence [26]. For familial risk analysis by proband type, first-degree relatives (including parents and/or siblings), who were affected by ovarian cancers, were considered as family history. However, in the present study only mother-sister, sister-sister and mother-two sisters family history were taken into consideration. Familial risk for individuals diagnosed < 50 years and ≥ 50 years old were estimated separately. A two-way comparison was employed to estimate relative risks (RRs) for overall (histology-specific) ovarian cancer when family history was histology-specific ovarian cancer, and conversely, RRs for histology-specific ovarian cancer when family history was overall (histology-specific) ovarian cancer. The reference group was individuals without a family history of ovarian cancer, i.e. unaffected relatives.
Poisson regression model was employed to estimate RRs and corresponding confidence intervals (CIs) for 5%, 1% and 0.1% significance levels. Trend tests were performed by modeling the three proband types (only mother, only sisters and mother and sisters) as a continuous covariate. Potential confounders, including age group, calendar period, residential area and socioeconomic status as well as parity were added to the model as covariates. SAS version 9.4 was used to perform the statistical analysis.
The study was approved by the Ethical Committee of Lund University.
Results and discussion
A total of 46,015 ovarian cancer cases were found in our database and of these 11,301 cases were in the offspring generation diagnosed at age 0–83 years, for which RRs were calculated. The age-standardized incidence per 100 000 person-years (Fig 1) in the six periods were the following: 12.6 (1958–1970), 14.7 (1971–1980), 13.4 (1981–1990), 11.2 (1991–2000), 8.9 (2001–2010) and 7.3 (2011–2015). Since the 1970s, the incidence of invasive ovarian cancer has declined in Sweden for a number of reasons, mainly due to the widespread use of oral contraceptives [27]. As for histological type, apart from sex cord-stromal ovarian cancer, incidence of all the other types has decreased. In 2011–2015, the highest incidence was noted for serous (4.23) and endometriod (0.65) types. It has been reported that oral contraceptive use may increase the risk of mucinous ovarian cancer, as opposed to other histologies [6]. Yet the incidence of mucinous ovarian cancer still decreased during 1993–2015, which suggests complex influence of many factors.
Fig 1. Age-standardized incidence in different time period for overall and eight histological types of invasive ovarian cancer.
Since the record of SNOMED was started in 1993, the periods for subtypes only included 1993–2000, 2001–2010 and 2011–2015.
The median ages at diagnosis for all ovarian cancers were 63 for the period 1958–2015 and 65 for the period 1993–2015. Ages at diagnosis and proportion of different histological types are displayed in Table 1. Sex cord-stromal type had the lowest age at diagnosis and median ages at diagnosis of other types were all over 60. Age-specific incidence data during 1993 to 2015 are shown in S1 Fig. The maximal incidences for overall and the most common histology-specific ovarian cancers were in the group 70–74 years, which is similar to a Danish report of the period 1978–2002 [28], but relatively younger than the report from the USA in 2011 and older than the report from South Korea during 1999–2012 [29, 30].
Table 1. Age at diagnosis in overall and different histological types of ovarian cancer.
| Histological types* | Number of cases (%) | Age at diagnosis Median (Q1, Q3) |
|---|---|---|
| Alla | 46015 | 63 (53, 72) |
| Allb | 16273 (100) | 65 (55, 74) |
| Undifferentiated | 411 (2.5) | 65 (57, 75) |
| Clear cell | 769 (4.7) | 61 (52, 70) |
| Endometrioid | 1760 (10.8) | 62 (52, 72) |
| Serous | 7404 (45.5) | 65 (57, 74) |
| Mucinous | 1269 (7.8) | 62 (50, 73) |
| Sex cord-stromal c | 457 (2.8) | 56 (44, 67) |
| Others d | 4203 (25.8) | 66 (55, 77) |
* All the ovarian cancer cases with histological type information were diagnosed in the period 1993–2015.
a: Ovarian cancer cases diagnosed in the period 1958–2015.
b: Ovarian cancer cases diagnosed in the period 1993–2015.
c: Sex cord-stromal was represented by thecoma.
d: Others include histological types of other ovarian cancers such as papillary and germ cell ovarian cancers as well as unspecified ovarian cancers
Serous ovarian cancer accounted for the 45.50% of all the ovarian malignancies, followed by endometrioid type (10.82%). The two proportions are slightly different from the results of the ovarian cancer patients diagnosed during 1993–1990, showing 38.4% for serous and 12.4% for endometrioid types [31]. The changes may be due to the influence of the use of molecular markers for subtypes to regroup some high-grade endometrioid cancer with high-grade serous cancer [32]. Alteration is also observed for the mucinous type, decreasing from 9.7% to 7.8% [31], probably resulted from the application of immunohistochemical staining that can distinguish the primary mucinous ovarian cancer from the metastatic gastrointestinal malignancies [32].
The total number of familial cases was 807 among daughters and mothers; among them a total of 487 were daughters. Thus 4.3% (487/11,301) of invasive ovarian cancer cases were familial in Sweden. The overall familial risk of ovarian cancer was 2.51 (2.29–2.75, P <0.001). Table 2 shows that in families of only mother, only sister and both mother and sisters diagnosed with ovarian cancer, familial risks were 2.40, 2.59 and 10.40, respectively, and all of them were significant at the 0.001 level. Risk was higher with a sororal family history, compared to maternal family history, implying recessive inheritance or shared environmental factors among sisters. When considering the cases diagnosed before the age of 50 years the risks increased up to 2.74, 3.86 and 16.05, respectively (P <0.001 for all). While for cases over 50 years old, the respective familial risks were lower, 2.22, 2.12 and 8.33, but they were still highly significant (P <0.001). Notably, the risks for mother and sister history were equal in the older age group, in contrast to the younger counterpart, which indicates that the excess sororal risk only influenced early onset cases with possible interactions with sex-related hormone levels.
Table 2. Familial risk of ovarian cancer in daughters by proband type and age of diagnosis.
| Age | Cases without family history | Only mother | Only sisters | Mother and sister | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | RR | 95%CI | N | RR | 95%CI | N | RR | 95%CI | P-trend | ||
| < 50 | 3872 | 120 | 2.74 | 2.29–3.26 | 63 | 3.86 | 3.01–4.96 | 6 | 16.05 | 7.20–35.74 | < .0001 |
| ≥50 | 6942 | 195 | 2.22 | 1.93–2.56 | 95 | 2.12 | 1.73–2.60 | 8 | 8.33 | 4.16–16.67 | < .0001 |
| All | 10814 | 315 | 2.40 | 2.14–2.68 | 158 | 2.59 | 2.21–3.03 | 14 | 10.40 | 6.16–17.57 | < .0001 |
Bolding, italic and underlining indicate that the 95% CI, 99% CI and 99.9% CI did not overlap with 1.00 respectively.
Familial risk was very high when both the mother and the sister were diagnosed with ovarian cancer, which may be related to high penetrant dominant effects. For the high-risk group of RR 10.40, histology was available only for ten patients; five were serous, two were non-specified adenocarcinomas and the remaining three were diverse histologies (clear cell, endometrioid and undifferentiated). Five of eight specific histologies were serous which may suggest an association with BRCA1/2 [21, 22]. In a previous UK study, it was estimated that BRCA1 and BRCA2 mutations account for about 24% of the familial risk of epithelial ovarian cancer among first-degree relatives, and in the remaining cases familial relative risk was estimated at 2.24 [15]. That study reported a higher familial risk for serous than non-serous cases, most likely associated with BRCA mutations; in the present analyses no marked differences were noted. An unknown factor in population-based studies on ovarian cancer without data on mutation status is the lack of information on ovariectomies. Removal of ovaries in mutation carriers would obviously suppress familial risk for serous cancers.
Familial associations of ovarian cancer with histology- specific ovarian cancers are displayed in Table 3. Overall ovarian cancer risk was associated with family history of ovarian cancers of undifferentiated (4.79, P <0.001) > endometrioid (3.81, P <0.001) > sex cord-stromal (2.72) > mucinous (2.21, P <0.01) > clear cell (2.16) and > serous (2.15, P <0.001) type. On the other hand, i.e. for specific histological types of ovarian cancer when probands had any ovarian cancer, increased risks were found in all but mucinous and sex cord-stromal types. The order of RRs was undifferentiated (5.45, P <0.001) > serous (2.96, P <0.001) > endometrioid (2.81, P < 0.001) and > clear cell (1.67). In comparison, the international Ovarian Cancer Cohort Consortium found the overall familial risk was 1.48 in the combined cohort covering 5,584 invasive ovarian cancer patients and only serous ovarian cancer was observed to be associated with the family history of 1.61 [7]. The differences between the present familial risks (2.51, excess familial risk 1.51) and those reported by the Ovarian Cancer Cohort Consortium (1.48, excess risk 0.48) are vast. The authors of the international consortium did not compare their risk estimates to the reported values from the literature and we can only speculate that family history was underreported in that study [11–15]. Self-reported family histories tend to be unreliable and for ovarian cancer the positive predictive value of correct reporting was only 69% in a published pooled analysis [33]. Among epithelial ovarian cancers, serous, endometrioid and clear cell tumors are proposed to display Muellerian phenotype according to their origins [3]. Therefore, we did the similar analysis as Table 3 in S1 Table by only including undifferentiated, clear cell, endometrioid and serous types in overall ovarian cancers. However, no interesting results were found.
Table 3. Familial associations of overall ovarian cancer with histology-specific ovarian cancer.
| Histological type | Overall risk of ovarian cancer in daughters | Risk of histology-specific ovarian cancer in daughters | ||||||
|---|---|---|---|---|---|---|---|---|
| N1 | N2 | RR | 95% CI | N1 | N2 | RR | 95% CI | |
| Undifferentiated | 8841 | 9 | 4.79 | 2.49–9.21 | 175 | 18 | 5.45 | 3.36–8.86 |
| Clear cell | 8843 | 7 | 2.16 | 1.03–4.54 | 496 | 15 | 1.67 | 1.00–2.80 |
| Endometrioid | 8821 | 29 | 3.81 | 2.65–5.49 | 951 | 48 | 2.81 | 2.10–3.75 |
| Serous | 8780 | 70 | 2.15 | 1.70–2.73 | 3934 | 215 | 2.96 | 2.58–3.40 |
| Mucinous | 8838 | 12 | 2.21 | 1.26–3.90 | 708 | 18 | 1.51 | 0.94–2.41 |
| Sex cord-stromal | 8846 | 4 | 2.72 | 1.02–7.25 | 295 | 5 | 1.10 | 0.46–2.66 |
N1: Number of cases without family history in first-degree relatives; N2: Number of cases with family history in first-degree relatives;
Bolding, italic and underlining indicate that the 95% CI, 99% CI and 99.9% CI did not overlap with 1.00 respectively;
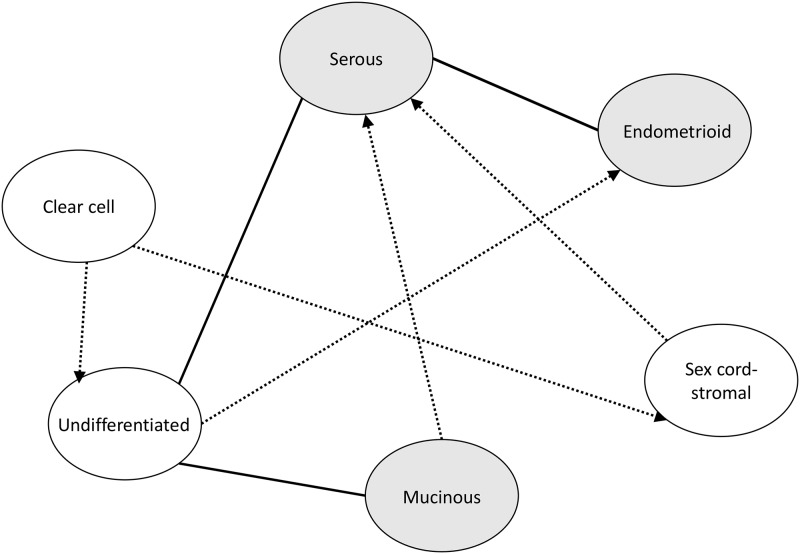
Table 4 shows familial associations among different histological types of ovarian cancers in cases and probands and those associations are summarized in S2 Fig. Risk of undifferentiated ovarian cancer increased when a first-degree relative was diagnosed with clear cell (15.44, P <0.01), serous (6.01, P <0.001) and mucinous (9.23) ovarian cancer. Endometrioid ovarian cancer risk was associated with family history of the concordant histological type of ovarian cancer (3.59); increased risk of this histological type was also observed in family of patients affected by undifferentiated (9.27, P <0.01) and serous (2.26) ovarian cancer. With the exception of clear cell type, serous ovarian cancer risk was associated with family history of all the other histological types. Mucinous ovarian cancer risk was associated with the concordant histological type (6.91, P <0.001) and undifferentiated type (7.08). Risk of sex cord-stromal type increased in families of clear cell ovarian cancer patients (9.70). A striking finding was that concordant familial risks were highest only for mucinous cancer, for all others some discordant associations showed the highest RRs. For example, the endometrioid-undifferentiated RR was 9.27 and the serous-undifferentiated RR was 4.80. This suggests that histology in familial ovarian cancer is not specific, and if genes or polygenes contribute to familial clustering they may not define histology or that they are influenced by hormonal and environmental factors to a variable degree.
Table 4. Familial associations among different histological types of invasive ovarian cancers.
| Histological types | Cases without family history | Cases with family history | ||||
|---|---|---|---|---|---|---|
| Offspring | First-degree relative | N | RR | 95%CI | ||
| Lower | Upper | |||||
| Undifferentiated | Clear cell | 175 | 1 | 15.44 | 2.16 | 110.37 |
| Serous | 175 | 4 | 6.01 | 2.23 | 16.2 | |
| Mucinous | 175 | 1 | 9.23 | 1.29 | 65.89 | |
| Endometrioid | Undifferentiated | 951 | 2 | 9.27 | 2.31 | 37.12 |
| Endometrioid | 951 | 3 | 3.59 | 1.15 | 11.14 | |
| Serous | 951 | 8 | 2.26 | 1.13 | 4.53 | |
| Serous | Undifferentiated | 3934 | 4 | 4.80 | 1.80 | 12.8 |
| Clear cell | 3934 | 3 | 2.08 | 0.67 | 6.45 | |
| Endometrioid | 3934 | 12 | 3.50 | 1.99 | 6.17 | |
| Serous | 3934 | 36 | 2.47 | 1.78 | 3.43 | |
| Mucinous | 3934 | 6 | 2.44 | 1.09 | 5.43 | |
| Sex cord-stromal | 3934 | 3 | 4.62 | 1.49 | 14.33 | |
| Mucinous | Undifferentiated | 708 | 1 | 7.08 | 1.00 | 50.33 |
| Endometrioid | 708 | 2 | 3.26 | 0.81 | 13.05 | |
| Serous | 708 | 4 | 1.56 | 0.58 | 4.17 | |
| Mucinous | 708 | 3 | 6.91 | 2.22 | 21.49 | |
| Sex cord-stromal | Clear cell | 295 | 1 | 9.70 | 1.36 | 69.12 |
| Serous | 295 | 2 | 1.97 | 0.49 | 7.93 | |
Only items with at least two cases with family history, or with significant results are displayed in Table 4. No such items were found for clear cell type of ovarian cancer.
Bolding, italic and underlining indicate that the 95% CI, 99% CI and 99.9% CI did not overlap with 1.00 respectively.
The present study is by far the largest family study of its kind in the world published and one of the few studies reporting familial risks by specific histology in cases and probands. The main limitation of this study is that the cases with identifiable histology were diagnosed only after 1993 since the application of ICD-O/2 in the cancer registry. This affects familial risk estimates because 22 years of follow-up is short for intergenerational studies considering risks of both the parental and offspring generations. Furthermore, histological classification has not been updated to meet the current guidelines. For example, serous histology is now considered to be either low-grade or high-grade with different prognoses and molecular events/etiologies. Moreover, compared to low-grade serous ovarian cancer, high-grade serous, endometrioid, clear cell, mucinous types are considered to evolve from different pathways and originate outside of ovary: high-grade serous type may evolve from fallopian tube while endometroid and clear cell may originate from endometrial tissue passing through the fallopian tube resulting in endometriosis [3]. Insufficient clinico-behavioral information, such as smoking, is also a caveat in the analysis since they can be construed as potential confounders. However, as we adjusted the data for socioeconomic factors, this reduces greatly the possible confounding by smoking [34].
Conclusions
In summary, by using the latest Swedish Family-Cancer Database, we found that each histological type of ovarian cancer was associated with at least two other histological types and was associated with the overall ovarian cancer, suggesting that the causes for familial clustering do not define a specific histology. Sharing of different histological types of ovarian cancer is likely an indication of the complexity of the underlying mechanisms. Our results provide useful information for genetic counseling; familial risks are high, particularly, for early-onset patients and for those with multiple affected relatives.
Supporting information
(TIF)
Risk of histology with grey background was significant within concordant histology of ovarian cancer. Risk of the two histologies between full line was significant in the two-way comparison. Risk of the two histologies between imaginary line was significant in one way and the histology the arrow points to is from offspring.
(TIF)
(DOCX)
Acknowledgments
We thank Patrick Reilly for language editing.
Data Availability
The use of these data is governed by an agreement with the Swedish National Board of Health and Welfare with Jan Sundquist, which does not allow redistribution of original data. Anyone who is interested in the dataset should contact the Swedish National Board of Health and Welfare and apply for the access to the dataset (https://www.socialstyrelsen.se/statistics). If anyone gets the approval, they can access to the database in the same manner as the authors.
Funding Statement
This work was supported by the German Cancer Aid; and the Swedish Research Council for Health, Working Life and Welfare (in Swedish: FORTE; Reg. no. 2013-1836), and FORTE (Reg. no. 2014-0804) and the Swedish Research Council (2012-2378 and 2014-10134); and ALF funding from Region Skåne. This research was also supported by the China Scholarship Council (201606100057 to GZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. AACR; 2017. [DOI] [PubMed] [Google Scholar]
- 2.CentreforEpidemiology. Cancer incidence in Sweden 2012. Stockholm: The National Board of Health and Welfare, 2013. [Google Scholar]
- 3.Kurman RJ, Shih I-M. The Origin and pathogenesis of epithelial ovarian cancer-a proposed unifying theory. The American journal of surgical pathology. 2010;34(3):433 10.1097/PAS.0b013e3181cf3d79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glance A. Ovarian cancer: an overview. Am Fam Physician. 2009;80(6):609–16. [PubMed] [Google Scholar]
- 5.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23257 women with ovarian cancer and 87303 controls. The Lancet. 2008;371(9609):303–14. 10.1016/S0140-6736(08)60167-1 [DOI] [PubMed] [Google Scholar]
- 6.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. American journal of epidemiology. 2002;156(4):363–73. [DOI] [PubMed] [Google Scholar]
- 7.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. Journal of Clinical Oncology. 2016;34(24):2888–98. 10.1200/JCO.2016.66.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soegaard M, Jensen A, Høgdall E, Christensen L, Høgdall C, Blaakær J, et al. Different Risk Factor Profiles for Mucinous and Nonmucinous Ovarian Cancer: Results from the Danish MALOVA Study. Cancer Epidemiology Biomarkers & Prevention. 2007;16(6):1160. [DOI] [PubMed] [Google Scholar]
- 9.Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. Plos Med. 2012;9(4):e1001200 Epub 2012/05/19. 10.1371/journal.pmed.1001200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010;171(1):45–53. Epub 2009/11/17. 10.1093/aje/kwp314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank C, Sundquist J, Yu H, Hemminki A, Hemminki K. Concordant and discordant familial cancer: Familial risks, proportions and population impact. International journal of cancer. 2017;140(7):1510–6. 10.1002/ijc.30583 [DOI] [PubMed] [Google Scholar]
- 12.Hemminki K, Granström C. Familial invasive and borderline ovarian tumors by proband status, age and histology. International journal of cancer. 2003;105(5):701–5. 10.1002/ijc.11151 [DOI] [PubMed] [Google Scholar]
- 13.Hemminki K, Sundquist J, Brandt A. Incidence and mortality in epithelial ovarian cancer by family history of any cancer. Cancer. 2011;117(17):3972–80. 10.1002/cncr.26016 [DOI] [PubMed] [Google Scholar]
- 14.Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genetics in Medicine. 2012;14(1):107–14. 10.1038/gim.2011.2 [DOI] [PubMed] [Google Scholar]
- 15.Jervis S, Song H, Lee A, Dicks E, Tyrer J, Harrington P, et al. Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. Journal of medical genetics. 2013:jmedgenet-2013-102015. [DOI] [PubMed] [Google Scholar]
- 16.Hemminki K, Li XJ. Familial risks of cancer as a guide to gene identification and mode of inheritance. International Journal of Cancer. 2004;110(2):291–4. 10.1002/ijc.20107 [DOI] [PubMed] [Google Scholar]
- 17.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer Risks for BRCA1 and BRCA2 Mutation Carriers: Results From Prospective Analysis of EMBRACE. JNCI: Journal of the National Cancer Institute. 2013;105(11):812–22. 10.1093/jnci/djt095 [DOI] [PubMed] [Google Scholar]
- 18.Geary J, Sasieni P, Houlston R, Izatt L, Eeles R, Payne SJ, et al. Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC). Familial cancer. 2008;7(2):163–72. 10.1007/s10689-007-9164-6 [DOI] [PubMed] [Google Scholar]
- 19.Pal T, Akbari M, Sun P, Lee J, Fulp J, Thompson Z, et al. Frequency of mutations in mismatch repair genes in a population-based study of women with ovarian cancer. Brit J Cancer. 2012;107(10):1783 10.1038/bjc.2012.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA oncology. 2016;2(4):482–90. 10.1001/jamaoncol.2015.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakhani SR, Manek S, Penault-Llorca F, Flanagan A, Arnout L, Merrett S, et al. Pathology of Ovarian Cancers in BRCA1 and BRCA2 Carriers. Clinical Cancer Research. 2004;10(7):2473–81. 10.1158/1078-0432.ccr-1029-3 [DOI] [PubMed] [Google Scholar]
- 22.Pal T, Permuth‐Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. 10.1002/cncr.21536 [DOI] [PubMed] [Google Scholar]
- 23.Watson P, Bützow R, Lynch HT, Mecklin J-P, Järvinen HJ, Vasen HF, et al. The clinical features of ovarian cancer in hereditary nonpolyposis colorectal cancer. Gynecologic oncology. 2001;82(2):223–8. 10.1006/gyno.2001.6279 [DOI] [PubMed] [Google Scholar]
- 24.Chui MH, Gilks CB, Cooper K, Clarke BA. Identifying Lynch syndrome in patients with ovarian carcinoma: the significance of tumor subtype. Advances in anatomic pathology. 2013;20(6):378–86. 10.1097/PAP.0b013e3182a92cf8 [DOI] [PubMed] [Google Scholar]
- 25.Hemminki K, Ji J, Brandt A, Mousavi SM, Sundquist J. The Swedish Family-Cancer Database 2009: Prospects for histology-specific and immigrant studies. Int J Cancer. 2010;126:2259–67. Epub 2009/07/31. 10.1002/ijc.24795 . [DOI] [PubMed] [Google Scholar]
- 26.Doll R, Cancer IUa. Cancer Incidence in Five Continents: A Technical Report: International Union Against Cancer; 1966.
- 27.Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. International journal of cancer. 2008;123(8):1897–901. 10.1002/ijc.23724 [DOI] [PubMed] [Google Scholar]
- 28.Kjærbye-Thygesen A, Huusom LD, Frederiksen K, Kjær SK. Trends in the incidence and mortality of ovarian cancer in Denmark 1978–2002. Comparison with other Nordic countries. Acta Obstetricia et Gynecologica Scandinavica. 2005;84(10):1006–12. 10.1111/j.0001-6349.2005.00878.x [DOI] [PubMed] [Google Scholar]
- 29.Sopik V, Iqbal J, Rosen B, Narod SA. Why have ovarian cancer mortality rates declined? Part I. Incidence. Gynecol Oncol. 2015;138(3):741–9. Epub 2015/06/17. 10.1016/j.ygyno.2015.06.017 . [DOI] [PubMed] [Google Scholar]
- 30.Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. Journal of gynecologic oncology. 2016;27(1):e5 Epub 2015/10/16. 10.3802/jgo.2016.27.e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji J, Försti A, Sundquist J, Lenner P, Hemminki K. Survival in ovarian cancer patients by histology and family history. Acta oncologica. 2008;47(6):1133–9. 10.1080/02841860701784544 [DOI] [PubMed] [Google Scholar]
- 32.Conklin CM, Gilks CB, Group IMW, Sankaranarayanan, Anttila, Prat, et al. Differential diagnosis and clinical relevance of ovarian carcinoma subtypes. Expert Review of Obstetrics & Gynecology. 2013;8(1):67–82. [Google Scholar]
- 33.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. Jama. 2004;292(12):1480–9. Epub 2004/09/24. 10.1001/jama.292.12.1480 . [DOI] [PubMed] [Google Scholar]
- 34.Hemminki K, Zhang H, Czene K. Socioeconomic factors in cancer in Sweden. Int J Cancer. 2003;105:692–700. 10.1002/ijc.11150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Risk of histology with grey background was significant within concordant histology of ovarian cancer. Risk of the two histologies between full line was significant in the two-way comparison. Risk of the two histologies between imaginary line was significant in one way and the histology the arrow points to is from offspring.
(TIF)
(DOCX)
Data Availability Statement
The use of these data is governed by an agreement with the Swedish National Board of Health and Welfare with Jan Sundquist, which does not allow redistribution of original data. Anyone who is interested in the dataset should contact the Swedish National Board of Health and Welfare and apply for the access to the dataset (https://www.socialstyrelsen.se/statistics). If anyone gets the approval, they can access to the database in the same manner as the authors.