Abstract
Background:
There are multiple multimorbidity measures but little consensus on which measures are most appropriate for different circumstances.
Objective:
To share insights gained from discussions with experts in the fields of ageing research and multimorbidity on key factors to consider when measuring multimorbidity.
Design:
Descriptive study of expert opinions on multimorbidity measures, informed by literature to identify available measures followed by a face-to-face meeting and an online survey.
Results:
The expert group included clinicians, researchers and policymakers in Canada with expertise in the fields of multimorbidity and ageing. Of the 30 experts invited, 15 (50%) attended the in-person meeting and 14 (47%) responded to the subsequent online survey. Experts agreed that there is no single multimorbidity measure that is suitable for all research studies. They cited a number of factors that need to be considered in selecting a measure for use in a research study including: (1) fit with the study purpose; (2) the conditions included in multimorbidity measures; (3) the role of episodic conditions or diseases; and (4) the role of social factors and other concepts missing in existing approaches.
Conclusions:
The suitability of existing multimorbidity measures for use in a specific research study depends on factors such as the purpose of the study, outcomes examined and preferences of the involved stakeholders. The results of this study suggest that there are areas that require further building out in both the conceptualization and measurement of multimorbidity for the benefit of future clinical, research and policy decisions.
Keywords: Multimorbidity, chronic disease, measurement, expert panel
Introduction
As multimorbidity, the coexistence of multiple health conditions within an individual, increases in prevalence, so too does the literature aimed at understanding how best to measure its prevalence and impact.1–9 There is little consensus on how multimorbidity is conceptualized and measured.1 This may be in part because there is little consensus on what is critical to capture when measuring multimorbidity. For example, questions have been raised regarding whether the scope of multimorbidity should go beyond chronic conditions to include associated risk factors and symptoms,10 such as hyperlipidemia and incontinence, and biopsychosocial factors,4 such as a patient’s socioeconomic status and health beliefs and expectations.
Regularly used measures of multimorbidity broadly fall into two categories: simple counts of multiple conditions or diseases or weighted indices that account for conditions as well as their severity and/or number of body systems affected.11 Simple counts are commonly used to measure multimorbidity due to their ease and ready data availability,11 yet considerable heterogeneity exists in the number and type of diagnoses considered to establish the count. A recent systematic review of 39 studies reported that the number of diagnoses included in multimorbidity measures ranged from 4 to 102.2 The majority of studies did not indicate their reasons for including specific diagnoses, thus the authors suggested that conditions may be chosen for pragmatic reasons, such as data availability. Many commonly used comorbidity indices, for example, the Charlson Comorbidity Index,12 were originally designed to measure burden of diseases for use in risk adjustment.11,13,14 More recently, a third type of measure, based on common combinations, or clusters of chronic conditions has also emerged.6,7 The clusters can be based on a number of things including prevalence (i.e. the most common combinations of condition), costs (i.e. the most costly combinations of conditions) or statistical methods, such as factor analysis.
Despite the increase in research activity, there is not sufficient methodologic work to understand how different measures of multimorbidity impact patient-important and policy-relevant outcomes. Our group is undertaking research to compare population-based estimates of multimorbidity derived from different data sources (self-report and health administrative data) and to assess the implications for estimates of health service use outcomes among middle-aged and older adults. As part of this research, we conducted a sub-study in which we convened an expert panel to provide input on which specific multimorbidity measures to include in our project and the strengths and limitations inherent in their use. During this exercise, the expert panel provided feedback on multimorbidity measures specific to our primary study, but also on broader conceptual issues relating to measuring multimorbidity. When reviewing the expert input, the research team was struck by the rich insights expressed by the experts and felt that there would be value in sharing these insights to help guide others focused on multimorbidity research. The purpose of this article is to share the insights from this sub-study with the broader research community to contribute to the discussion of the key elements to consider when selecting a multimorbidity measure in research.
Materials and methods
Participants
The panel was chosen through purposive sampling to include clinicians, researchers and policymakers in Canada with expertise in the fields of multimorbidity and ageing. Through this sampling process, 30 individuals were identified and invited to participate in the expert panel meeting.
Sub-study phases
The sub-study was conducted in three phases. In phase 1, a literature review was undertaken in the fall of 2014. This was not intended to be an exhaustive review of multimorbidity measures, rather its purpose was to identify a list of those most commonly used to focus the discussion in the expert panel meeting (phase 2) and to include in an online survey on measures that had potential for use in our primary study (phase 3). For example, medications-based multimorbidity measures were not included in our list because these data were not available in our primary study. From the literature review, 13 commonly used multimorbidity measures were identified and grouped into three broad categories: (1) counts (a simple count of chronic conditions from a specified set); (2) indices (a weighted combination of a specified set of chronic conditions); and (3) combinations or clusters (specific subsets of chronic conditions that coexist, usually defined by the most prevalent chronic conditions; Online Supplemental Appendix 1).
In phase 2, a half-day in-person meeting (with teleconference option for those who could not travel) of an expert panel was held to discuss in more detail the multimorbidity measures identified in our literature review, the broader practical and conceptual issues related to the current ways of measuring multimorbidity and to finalize the measures and associated questions for a follow-up online survey. Prior to the meeting, attendees were provided with a detailed overview of the primary study, its purpose and the available data. They were also presented with the 13 multimorbidity measures from our review. During this meeting, the 13 measures were used to generate a broader discussion on what the different kinds of measures represented and peoples’ experience in using them. The feedback solicited was less focused on the 13 specific measures but more broadly the kinds of conditions common to these measures and the types of conditions typically overlooked. Attendees were then split into four working groups of equal size and representing a diversity of disciplines. Each in-person working group was facilitated by one of the study team leads (LG, AG and KF); all experts participating by phone were included in a single group which was led by a trained facilitator. The experts were asked to consider the following questions focused on broader issues relating to measuring multimorbidity: (1) what important concepts should be included in measures of multimorbidity; (2) what key factors should be considered when creating measures of multimorbidity; and (3) what underlying concepts are currently missing in measures of multimorbidity. The facilitators took notes during the small group discussions. Each working group facilitator then reported back to all attendees for further discussion and clarification of the input received. Feedback from the in-person meeting was used to finalize the list of measures and questions included in a subsequent online survey which was used in phase 3.
In phase 3, approximately 2 months after the expert panel meeting, an online survey was circulated to the full group of 30 experts (Online Supplemental Appendix 2). Respondents were asked to consider each of 13 specific measures of multimorbidity and indicate whether or not they believed each measure would be relevant to our primary research study. Respondents could endorse more than one measure that was identified in the survey or choose not to endorse any if they felt that all measures were inadequate for use in our study. The survey also allowed respondents to provide free-text to describe areas of concern with each specific measure and to identify additional multimorbidity measures that we should consider. Based on the discussion from the in-person meeting, we included open-ended questions about how to address episodic or recurring conditions and what other conditions should be incorporated in our measures of multimorbidity.
Finally, the responses to the online survey regarding the relevance of each of the 13 multimorbidity measures to our primary research were summarized and the notes from the expert panel meeting and the free-text responses submitted in the surveys were consolidated using a qualitative descriptive approach.15 The team leads (LG, AG and KF) independently reviewed this material and grouped them into themes. They then met to review their analyses, reconcile differences and arrive at consensus on the themes. In this article, we share the themes generated from the meeting discussion and survey free-text responses.
Ethical considerations
This study was approved by the Hamilton Integrated Research Ethics Boards at McMaster University (13-590). Participants of the in-person meeting provided written informed consent.
Results
Expert panel and online survey
The expert panel meeting took place in January 2015. Of the 30 experts invited, 15 (50%) attended the meeting. Six of the attendees were primarily clinicians, seven were primarily researchers and two were policymakers. The majority of attendees were from Ontario; one was from Alberta and one from Quebec. Of the 30 experts approached, 14 (47%) responded to the subsequent online survey. Because the survey was anonymous, we were not able to identify how many experts participated in both the expert panel and online survey. Among the 14 respondents, 71.4% (10/14) were self-identified as senior researchers (including 6 respondents who identified themselves as clinician-researchers), 1 respondent was a non-physician clinician, 1 respondent was a policymaker and 2 were junior researchers. Most respondents indicated that their research and clinical work either involved multimorbidity ‘All of the Time’ (7/14) or ‘Often’ (5/14).
Expert panel and online survey: Emerging themes
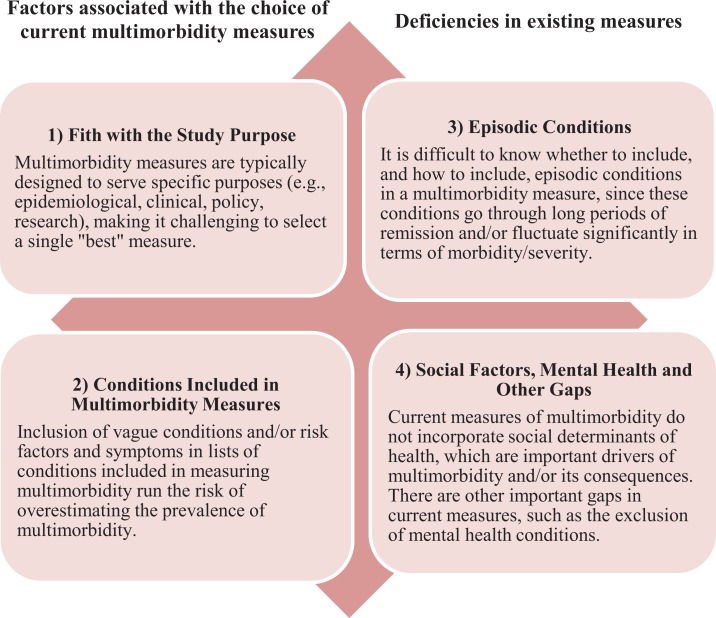
The respondents’ endorsement of each of the 13 measures is shown in Table 1. In this section, we focus on the themes that emerged from the expert panel meeting and the open-text segments of the online survey. Four main themes emerged from these two sources. These themes fell into two general categories: factors associated with the choice of current multimorbidity measures and deficiencies in existing measures. The two themes regarding the choice among current multimorbidity measures were: (1) fit with the study purpose and (2) the conditions included in multimorbidity measures. The two themes around the deficiencies of all current multimorbidity measures were: (3) accounting for episodic conditions or disease and (4) the role of social factors, mental health and other concepts missing in existing measures (Figure 1).
Table 1.
Details of selected measures of multimorbidity and percent endorsement by expert panel.
| Measure | Chronic condition list/description | Percent endorsement |
|---|---|---|
| Count measures | ||
| Canadian Institutes of Health Research (CIHR) List27,a | 1. Hypertension, 2. depression/anxiety, 3. chronic musuloskeletal conditions causing pain or limitation, 4. osteoarthritis and other arthritis, 5. osteoporosis, 6. asthma, chronic obstructive pulmonary disease or chronic bronchitis, 7. cardiovascular disease, 8. heart failure, 9. stroke and transient ischemic attack (TIA), 10. stomach problem, 11. colon problem, 12. chronic liver disease, 13. diabetes, 14. thyroid disorder, 15. any cancer within the last 5 years, 16. chronic kidney disease or failure, 17. chronic urinary problem, 18. dementia, 19. hyperlipidemia, 20. human immunodeficiency virus. | 78.6 |
| Centers for Disease Control and Prevention (CDC) List28,29 | 1. Hypertension, 2. congestive heart failure, 3. coronary artery disease (e.g., ischemic heart disease, coronary heart disease, etc.), 4. cardiac arrhythmias, 5. hyperlipidemia, 6. stroke or transient ischemic attack, 7. arthritis, 8. asthma, 9. autism spectrum disorder, 10. cancer (all but skin), 11. chronic kidney disease, 12. chronic obstructive pulmonary disease, 13. dementia, 14. depression, 15. diabetes, 16. hepatitis, 17. HIV, 18. osteoporosis, 19. schizophrenia, 20. substance abuse (drug or alcohol). | 71.4 |
| Health Systems Performance Research Network (HSPRN) List30 | 1. Acute myocardial infarction, 2. rheumatoid arthritis, 3. osteoarthritis arthritis, 4. asthma, 5. cancer, 6. cardiac arrythmia, 7. coronary heart failure, 8. chronic obstructive pulmonary disease, 9. dementia, 10. depression, 11. diabetes, 12. hypertension, 13. osteoporosis, 14. renal failure, 15. stroke, 16. coronary syndrome (excluding myocardial infarction). | 42.9 |
| Index measures | ||
| Charlson Comorbidity Index31 | The original index recognizes 19 conditions identified using International Classification of Diseases (ICD) 9/10 codes, which are weighted to reflect severity and then summed to create a total score. The index uses medical and self-report electronic records and has been extensively validated in hospital and specialist settings. A number of variations exist (including adaptations using administrative data) that appear to perform equally well in predicting a range of outcomes (mortality, healthcare costs, hospital length of stay). | 92.9 |
| Elixhauser Comorbidity Index (EI)32 | The original index recognizes 30 conditions identified using ICD 9 codes. The index uses administrative data and predicts a range of health-related outcomes (mortality, length of stay, cost). The index has been extensively validated for use with administrative data. | 71.4 |
| Adjusted Clinical Groups (ACG) System33 | This index uses a case-mix adjustment system that groups patients into clinically-cogent groups using age, sex and diagnosis codes (ICD 9/10 or Read Code). It uses medical records or insurance claims data, has been validated in multiple settings, and predicts a range of outcomes including morbidity burden and health service use and costs. | 71.4 |
| Cumulative Index Illness Rating Scale (CIRS)34 | This index classifies conditions into 1 of 14 organ domains, which are each rated (0–4) for severity and summed to obtain a total score (0–56). The index uses medical records data, has been validated in family practice, and is used to predict medical burden. | 71.4 |
| Cluster measures | ||
| Prevalence-based approach | Identify the most prevalent combination of conditions (e.g., dyads, triads) stratified by age and gender | 92.9 |
| Cost-based approach | Identify the most costly combination of conditions (e.g. dyads and triads) stratified by age and gender | 64.3 |
| Cluster-based approach | Identify the clusters of conditions using statistical methods such as factor analysis, cluster analysis or latent class analysis | 64.3 |
a The CIHR list included in the survey was further adapted to the final list presented in the cited reference
Figure 1.
Key themes identified by expert panel on considerations required in the measurement and operationalization of multimorbidity.
Theme 1: Fit with the study purpose
The expert panel feedback indicated that different multimorbidity measures serve different purposes and research teams need to consider both the purpose of their current project and the different stakeholders (e.g. patients/clients, researchers, clinicians, policymakers) who will use the information when choosing a measure. For example, clinicians and patients may be focused on patient-important outcomes while policymakers may need to consider population prevalence and costs. One expert suggested that ‘lived experience’ and the perspective of the patient/client, particularly how they cope/manage and prioritize, were important to capture in a multimorbidity measure. Another expert noted that population prevalence and healthcare system cost are important criteria for identifying conditions to be included in a multimorbidity measure. These criteria are typically of great importance to policymakers, whereas patients/clients are concerned with their conditions regardless of population prevalence or system cost. From a clinician perspective, a condition like dementia may not be prevalent in most community-living populations, but it is a clinically dominant condition that can greatly increase patient complexity, especially in the context of multimorbidity. This highlights the potential tension between various perspectives within a research study, that is, what is it that shapes the clinical care of a patient with multimorbidity versus what should be measured when attempting to describe multimorbidity in a population. It is clear that stakeholder’s may differ in their perspectives on multimorbidity and how to best measure it to achieve the goals of a study, thus considering the study’s purpose and differing stakeholder perspectives at the outset of the study is critical to selecting an appropriate measure and facilitating meaningful interpretation of study results.
Theme 2: Conditions included in multimorbidity measures
The experts noted that some of the simple count measures included a broad variety of conditions, not just diseases. Embedded in their comments was the debate over what should be considered a ‘condition’ in multimorbidity measures. The experts expressed concern about overestimating multimorbidity due to the inclusion of less specific conditions or diseases, for example, stomach problems (which almost anyone could report) and risk factors (which may overlap with other chronic conditions). One expert indicated that double counting could apply to concordant conditions such as osteoporosis and hip fractures or hypertension and myocardial infarction in which we would count both as a risk factor for a condition and the condition itself. Yet other experts suggested items were missing and should be included in lists of chronic conditions, such as symptoms (e.g. pain and incontinence) and functional limitations. Although it was noted that this could increase the estimated prevalence of multimorbidity, these non-disease ‘conditions’ were seen to be very relevant in terms of patient, caregiver and health systems burden. One expert noted that one of the measures of multimorbidity ‘blends specificity (e.g. hypertension) with vagueness (e.g. stomach problem) and blends potentially limiting and symptomatic conditions (e.g. arthritis, depression, heart failure) with risk factors (e.g. hyperlipidemia, hypertension and osteoporosis)’. The experts did not come to consensus on whether or not these non-disease conditions should be included or excluded from multimorbidity measures. They noted that many different types of conditions get rolled into these measures, but at the same time, there are other non-disease conditions often missing. It is clear that arguments can be made for or against the inclusion of these non-disease conditions as they can have implications for patient treatment and health system burden. It follows, however, that the inclusion of risk factors and symptoms should be evaluated based on the research purpose and/or use of the measure.
Theme 3: Accounting for episodic conditions or diseases
Experts noted that some of the conditions or diseases included in existing multimorbidity measures fluctuate between ‘active’ and ‘inactive’ diagnoses. On a conceptual level, there was concern as to whether or not conditions that are episodic in nature should be included as chronic conditions. It was noted as well that it may be difficult from some data sources to differentiate ‘active’ from ‘inactive’ disease, but the majority of experts agreed that conditions should be ‘counted’ if they are under current treatment or had been treated in the past 12 months. Some experts distinguished between conditions like depression that can recur even after very long periods of remission compared to conditions like cancer which one expert suggested ‘should be considered chronic until it is declared cured or inactive for at least 5 years’. Yet others saw the episodic nature of chronic conditions as a fundamental property of all chronic conditions and critical to our understanding of multimorbidity. For example, an online survey respondent indicated, ‘if we really want to understand multimorbidity, we need to look at chronic conditions as subject to temporal fluctuations (that is they do not ever go away, they wax and wane, so like a signal, they have varied intensity)’.
Theme 4: Social factors, mental health conditions, and other gaps
Finally, there was discussion about how to integrate other important factors or conditions currently absent in all multimorbidity measures. The role of social factors including social determinants of health was a significant focus of this discussion. More specifically, there was debate around whether social factors should be included as part of a multimorbidity measure or considered as potential correlates and/or effect modifiers of the impact of multimorbidity. Some experts considered social factors as ‘conditions’ to include in a multimorbidity measure. For example, one expert indicated that, ‘thus far many of the important drivers such as adequacy of caregivers, social determinants such as food security, income, and housing have not been considered, poverty and loneliness are chronic conditions associated with adverse health outcomes’. The role of social factors was often linked to complexity, which was recognized as challenging to operationalize yet important to be considered as part of (or along with) multimorbidity measures. One expert noted, for example, that ‘complexity is common in home care patients, because of involvement of multiple physicians, multiple care plans, challenge in delivering treatment’. It was further noted that ‘considering social diagnoses moves toward capturing the complexity of multimorbidity, but it may be difficult to put into balance with other physical diagnoses’. One expert suggested that multi-domain measures of multimorbidity that include disease burden, functional status, and social determinants of health could be reported separately or possibly combined.
Even if restricting multimorbidity to broadly recognized health conditions, a number of experts noted that some conditions are characteristically omitted. Many experts identified an under-representation of mental health conditions in multimorbidity measures. Additionally, multimorbidity measures that included mental health conditions most often restricted the conditions to a select few. One expert noted that we should consider other mental health conditions because ‘right now it is very limited to depression and anxiety’. Another gap identified by the group is the need for a more consistent inclusion of chronic pain as it ‘has an impact on treatment and treatment decisions, a person’s ability to follow-through on recommendations made in relation to other chronic conditions, and the person’s quality of life’.
Discussion
This research originated from a need to select measures of multimorbidity to be used in a larger study to assess the effect of different data sources on multimorbidity prevalence and health service use outcomes. The panel discussions and online survey responses generated common themes and considerations for how to address the challenge of selecting a measure of multimorbidity more generally that have implications for the broader multimorbidity research community.
The expert panel discussion highlighted that there does not appear to be a single ‘best’ measure of multimorbidity. Multimorbidity measures vary in their suitability for specific study purposes and stakeholders involved, and there are advantages and disadvantages associated with each measure currently in use. The experts underscored the importance of considering the research purpose, outcome(s) of interest, stakeholders involved, study population and data availability in choosing a multimorbidity measure. In terms of the multimorbidity measurement trade-offs, the experts recommended that the decisions made by the research team in their choice of measure should be explicitly stated, a point that has been emphasized in the literature. For example, Stewart et al. recommends that researchers publish the list of health conditions considered in the determination of multimorbidity, and the potential implications of utilizing a health system- or patient-centred measurement.16
The theme of double counting has also been highlighted in previous literature, most recently in a systematic review conducted by Willadsen et al.10 The authors noted that risk factors are often included in the definition of multimorbidity, while symptoms and severity of the diagnoses are less frequently included based on previous literature. This systematic review called for a concept of multimorbidity relevant to clinical work that distinguishes between each of these interrelated concepts. For example, focusing just on the disease alone does not necessarily explain the experience of the patient/client; to do so requires inclusion of symptoms/severity, goals and needs. From a public health perspective, the burden of disease and associated treatments (medical and social) are critical to inform resource planning. However, from the perspective of an individual with the condition, their experience is much broader than the identification of diagnosed conditions. These varying perspectives relate back to the importance of considering how suitable a multimorbidity measure is for the studies in which it is being used. If the outcome of interest in a study is disease prevalence, including risk factors will likely result in higher prevalence estimates. This raises concerns about comparability with studies that exclude risk factors. This was reflected in one expert’s comment regarding the exclusion of hyperlipidemia as it can be seen as an asymptomatic risk factor rather than a ‘felt’ condition or disease. On the other hand, if the outcome of interest is cost, hyperlipidemia may be important to consider because of high prevalence and high drug costs. Of interest, the expert panel did not discuss other methods that may reduce the issue of ‘double counting’ of conditions, such as focusing on body systems instead of individual chronic condtions.17 This may be because our discussions were focused on ‘measures’ and not ‘definitions’ of multimorbidity. However, it should be noted that the experts expressed that complexity was not well captured in existing measures and indicated the need for more detail and consideration of a larger set of conditions in multimorbidity measures. Focusing on body systems could obscure that even further.
One of the deficiencies of current multimorbidity measures identified by the experts was the treatment of episodic or cyclic diagnoses. For conditions or diseases with varying symptomatology, researchers must decide on which ‘clinical phase’ should be captured in the research (e.g. asymptomatic but diagnosed, early phase of disease, late phase of disease or relapse) and the spectrum of morbidity that should be included.18,19 This suggests the need for a dynamic measure that can change over time, increasing or decreasing based on a person’s health status, for example, hypertension and diabetes reversed by lifestyle changes. Some limitations to having dynamic measures lie in the data sources used to measure multimorbidity. In the future, electronic health record data in combination with natural language processing may enable more robust measures.
Finally, the importance of the conceptual placement of social factors has been recognized in previous multimorbidity literature20–22 and was recently raised as an issue in the definition of multimorbidity by the European General Practice Research Network.4 In our discussions, it was clear, especially from the front-line healthcare practitioners, that social factors are important to understanding patient complexity and are relevant in the care for those living with multimorbidity. Because of this, many experts saw the social factors as part of the definition, but there was not consensus on how it should be incorporated. In the development of guiding principles for decision-making about multimorbidity, Muth et al.23 identified that the balance between resources and burden, as well as self-care and need for assistance, can be disrupted by contextual circumstances, including poor living conditions, lack of social support and financial constraints. Schaink et al.,24 Grembowski et al.25 also identified the importance of social determinants of health in capturing complexity and service gaps in caring for those living with multimorbidity. Contextual factors were linked to health service gaps in turn affecting the access to service, quality of care and care experiences of those patients with multimorbidity. There is a clear understanding that patient-centred care requires a healthcare practitioner to create a ‘portrait’ of their patient to understand the totality of the individual’s life experiences and impact, successes and challenges. When describing the future of multimorbidity research, Boyd and Fortin underscored that ‘multimorbidity needs to be considered within the context of a person, or patient’.26 This more comprehensive understanding of the experience of multimorbidity differs, however, from more parsimonious measures of multimorbidity selected for research purposes. The definition of multimorbidity proposed by the European General Practice Research Network explicitly states that ‘any bio-psychosocial factor, any somatic risk factor, the social network…and the patient’s coping strategies may function as modifiers (of the effect of multimorbidity)’.4 This suggests that social determinants of health may represent factors that can modify or mediate the relationship between multimorbidity and select outcomes (e.g. health service use, further morbidity or mortality) and seemingly etiology as well.
Limitations of our work include the small sample size of participants involved in the expert panel meeting (phase 2) and the online survey (phase 3). Although the measures of multimorbidity we identified were from the international literature, all of the experts were Canadian or working in the Canadian context at the time of the meeting. As well, we did not recruit participants who were identified as patients or caregivers living with multimorbidity. However, the participating experts represented individuals who were regular generators and users of multimorbidity-related research in their daily work. They had an advanced understanding of the current strengths and limitations of existing measures commonly encountered in the literature. As well, because the online survey was anonymous, we could not tell how much overlap there was in terms of the participants across the phases. The main purpose of this article, however, is to share the discussion generated from the experts. In this context, we were interested in identifying all themes that arose across the two phases. Finally, our work focused on those measures that were considered ‘common’ in the literature. This ultimately included an assessment of 13 measures categorized into three broad categories. Although these 13 do not reflect all existing multimorbidity measures, the discourse that arose from the expert panels uncovered broader conceptual insights that can benefit all those involved in multimorbidity research.
There clearly remains a need to establish approaches to measure multimorbidity that balances comprehensiveness (e.g. including all important conditions or diseases from the clinical- and patient-perspectives), feasibility (e.g. availability of data sources and algorithms) and efficiency (e.g. particularly for use in large secondary databases and to compare among study settings), and no single multimorbidity measure is suitable for all research studies. There are instead important advantages and disadvantages associated with each existing measure and key factors that must be considered by a research team before a specific measure of multimorbidity is selected and implemented. Based on the discussion among our group of clinicians, researchers and policymakers in multimorbidity and ageing, all current approaches to measure multimorbidity are challenged in addressing the issues of clinical/research/policy trade-offs, episodic diagnoses, the inclusion of risk factors and symptoms and the incorporation of the social determinants of health and other factors related to patient complexity. These are areas that require further consideration in both the conceptualization and measurement of multimorbidity for the benefit of future clinical, research and policy decisions.
Supplemental Material
Supplemental Material, JOC_Expert_Panel_Journal_of_Comorbidity_Supplemental_Appedix_1 for Key factors to consider when measuring multimorbidity: Results from an expert panel and online survey by Lauren E Griffith, Andrea Gruneir, Kathryn A Fisher, Kathryn Nicholson, Dilzayn Panjwani, Christopher Patterson, Maureen Markle-Reid, Jenny Ploeg, Arlene S Bierman, David B Hogan, and Ross Upshur in Journal of Comorbidity
Supplemental Material
Supplemental Material, JOC_Expert_Panel_Journal_of_Comorbidity_Supplemental_Appedix_2 for Key factors to consider when measuring multimorbidity: Results from an expert panel and online survey by Lauren E Griffith, Andrea Gruneir, Kathryn A Fisher, Kathryn Nicholson, Dilzayn Panjwani, Christopher Patterson, Maureen Markle-Reid, Jenny Ploeg, Arlene S Bierman, David B Hogan, and Ross Upshur in Journal of Comorbidity
Acknowledgements
The authors would like to thank the participants of the in-person meeting: Dr Arlene Bierman, Dr Lisa Dolovich, Dr Martin Fortin, Dr Anne Gilsing, Ms Dilys Haughton, Dr David B Hogan, Mr David Kanters, Dr Maureen Markle-Reid, Ms Melissa Northwood, Dr Doug Oliver, Dr Christopher Patterson, Mr Justin Peffer, Dr Jenny Ploeg, Dr Ross Upshur and Ms Jennifer Soyoung Youn. The authors would also like to thank the respondents to the online survey.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This project was funded through an operating grant from the Canadian Institutes of Health Research (CIHR; funding reference number: FRN 130546). Dr Griffith and Dr Gruneir are supported by CIHR New Investigator Awards. Dr Griffith is also supported by the McLaughlin Foundation Professorship in Population and Public Health. Dr Markle-Reid is funded through the Canada Research Chairs program. The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. The views expressed by Dr Arlene Bierman do not necessarily reflect those of the Agency for Healthcare Research and Quality, the US Department of Health and Human Services or the Federal government.
ORCID iD: Jenny Ploeg  http://orcid.org/0000-0001-8168-8449
http://orcid.org/0000-0001-8168-8449
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 10(2): 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 2011; 66(3): 301–311. [DOI] [PubMed] [Google Scholar]
- 3. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10(4): 430–439. [DOI] [PubMed] [Google Scholar]
- 4. Le Reste JY, Nabbe P, Manceau B, et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J Am Med Dir Assoc 2013; 14(5): 319–325. [DOI] [PubMed] [Google Scholar]
- 5. Salive ME. Multimorbidity in older adults. Epidemiol Rev 2013; 35: 75–83. [DOI] [PubMed] [Google Scholar]
- 6. Sinnige J, Braspenning J, Schellevis F, et al. The prevalence of disease clusters in older adults with multiple chronic diseases—a systematic literature review. PLoS One 2013; 8(11): e79641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, et al. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014; 67(3): 254–266. [DOI] [PubMed] [Google Scholar]
- 8. Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One 2014; 9(7): e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu X, Mishra GD, Jones M. Evidence on multimorbidity from definition to intervention: an overview of systematic reviews. Ageing Res Rev 2017; 37: 53–68. [DOI] [PubMed] [Google Scholar]
- 10. Willadsen TG, Bebe A, Koster-Rasmussen R, et al. The role of diseases, risk factors and symptoms in the definition of multimorbidity—a systematic review. Scand J Prim Health Care 2016; 34(2): 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huntley AL, Johnson R, Purdy S, et al. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med 2012; 10(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. Am J Manag Care 2006; 12(2): 110–117. [PubMed] [Google Scholar]
- 13. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173(6): 676–682. [DOI] [PubMed] [Google Scholar]
- 14. Brilleman SL, Salisbury C. Comparing measures of multimorbidity to predict outcomes in primary care: a cross sectional study. Fam Pract 2013; 30(2): 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandelowski M. What’s in a name? Qualitative description revisited. Res Nurs Health 2010; 33(1): 77–84. [DOI] [PubMed] [Google Scholar]
- 16. Stewart M, Fortin M, Britt HC, et al. Comparisons of multi-morbidity in family practice—issues and biases. Fam Pract 2013; 30(4): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrison C, Britt H, Miller G, et al. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open 2014; 4(7): e004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract 1996; 2: 65–70. [Google Scholar]
- 19. McWhinney IR, Freeman T. Textbook of family medicine. 3rd ed Oxford, England, UK: Oxford University Press, 2009. [Google Scholar]
- 20. Northwood M, Ploeg J, Markle-Reid M, et al. Integrative review of the social determinants of health in older adults with multimorbidity [Review]. J Adv Nurs 2018; 74(1): 45–60. [DOI] [PubMed] [Google Scholar]
- 21. Agborsangaya CB, Lau D, Lahtinen M, et al. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health 2012; 12: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380(9836): 37–43. [DOI] [PubMed] [Google Scholar]
- 23. Muth C, Beyer M, Fortin M, et al. Multimorbidity’s research challenges and priorities from a clinical perspective: the case of ‘Mr Curran’. Eur J Gen Pract 2014; 20(2): 139–147. [DOI] [PubMed] [Google Scholar]
- 24. Schaink AK, Kuluski K, Lyons RF, et al. A scoping review and thematic classification of patient complexity: offering a unifying framework. J Comorb 2012; 2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grembowski D, Schaefer J, Johnson KE, et al. AHRQ MCC research network. A conceptual model of the role of complexity in the care of patients with multiple chronic conditions. Med Care 2014; 52(suppl 3): S7–S14. [DOI] [PubMed] [Google Scholar]
- 26. Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Rev 2010; 32: 451–474. [Google Scholar]
- 27. Fortin M, Almirall J, Nicholson K. Development of a research tool to document self-reported chronic conditions in primary care. J Comorb 2017; 7(1): 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodman RA, Posner SF, Huang ES, et al. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis 2013; 10: E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider KM, O’Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ medicare population. Health Qual Life Outcomes 2009; 7: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pefoyo AJ, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health 2015; 15: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 32. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998; 36(1): 8–27. [DOI] [PubMed] [Google Scholar]
- 33. Starfield B, Weiner J, Mumford L, et al. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res 1991; 26(1): 53–74. [PMC free article] [PubMed] [Google Scholar]
- 34. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968; 16(5): 622–626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, JOC_Expert_Panel_Journal_of_Comorbidity_Supplemental_Appedix_1 for Key factors to consider when measuring multimorbidity: Results from an expert panel and online survey by Lauren E Griffith, Andrea Gruneir, Kathryn A Fisher, Kathryn Nicholson, Dilzayn Panjwani, Christopher Patterson, Maureen Markle-Reid, Jenny Ploeg, Arlene S Bierman, David B Hogan, and Ross Upshur in Journal of Comorbidity
Supplemental Material, JOC_Expert_Panel_Journal_of_Comorbidity_Supplemental_Appedix_2 for Key factors to consider when measuring multimorbidity: Results from an expert panel and online survey by Lauren E Griffith, Andrea Gruneir, Kathryn A Fisher, Kathryn Nicholson, Dilzayn Panjwani, Christopher Patterson, Maureen Markle-Reid, Jenny Ploeg, Arlene S Bierman, David B Hogan, and Ross Upshur in Journal of Comorbidity