Abstract
The neuron is the target of inflammatory demyelinating processes in multiple sclerosis (MS). In progressive MS, however, there is a gathering body of evidence indicating molecular changes within neuronal cell bodies. All of these molecular changes to intrinsic neurons converge on mitochondria, and the most reproduced change relates to mitochondrial respiratory chain complex deficiency. This compromise in the capacity to generate ATP in the neuronal cell body is coupled with an increased demand for energy by the demyelinated axon, which is particularly relevant to the long tracts such as corticospinal tracts with long projection axons. Recent work in our laboratory and that of our collaborators indicate limited reflection of the molecular changes that are intrinsic neurons in the experimental disease models. The mitochondrial changes within neuronal compartments are an under-recognized aspect of progressive MS and likely to offer novel targets for the improvement of neuronal function as well as neuroprotection.
Progressive multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder with ongoing neurodegeneration (Stadelmann 2011). Inflammation is a potent inducer of neuronal damage, particularly to axons and symptoms, whereas lack of restoration of myelin to denuded axons (remyelination) leaves the naked axons much more vulnerable. There is an overall tendency for inflammation to become less prominent with increasing disease duration, although with case-to-case variability, even at the end stage of the disease (Mahad et al. 2015). In contrast to inflammation, the lesion burden or the extent of demyelination gradually increases partly because of the lack of remyelination as well as ongoing and repeated inflammatory demyelination. The cumulative impact of inflammation and chronic demyelination is the profound loss of central nervous system (CNS) tissue reflected by brain and spinal cord atrophy, which expectedly becomes more apparent as the disease progresses. Neuropathological studies indicate extensive loss of axons particularly in the spinal cord long tracts and synapses in the gray matter (>60%–70% loss) (Bjartmar et al. 2003; Jurgens et al. 2016). In contrast, the extent of neuronal cell body loss in the cortical gray matter is marginal, with 15%–20% loss except in cases with so-called follicle-like B cells infiltrated in the meninges (Magliozzi et al. 2010). The existence of neuronal cell bodies at the end stage of progressive MS in autopsy tissue offers the opportunity to study their content and gain insight into how the neurons may play a role in and contribute to the neurodegenerative process of progressive MS.
There is ample evidence implicating an active role for neurons in the pathophysiology of progressive MS (Mahad et al. 2015). We discuss in detail below the molecular, proteomic, biochemical, and dynamic changes that are intrinsic to neurons, and converge on mitochondria.
MOLECULAR CHANGES WITHIN THE NEURONAL CELL BODIES IN PROGRESSIVE MS
It is abundantly clear that the neuronal cell bodies in progressive MS contain a spectrum of molecular changes at autopsy (Fig. 1). All of these molecular changes, discussed in more detail below, converge on mitochondrial function and transport. The most reproduced molecular changes within neurons in progressive MS impact the mitochondrial respiratory chain complexes and hence the ability to produce ATP by oxidative phosphorylation.
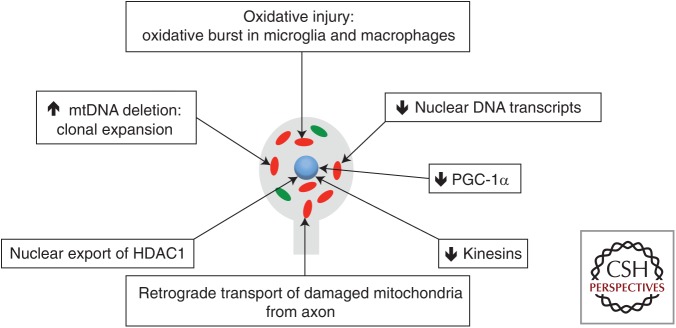
Figure 1.
Molecular changes intrinsic to neuronal cell bodies in multiple sclerosis (MS). A number of studies have now identified molecular changes, all of which converge on mitochondria, in progressive MS. Dutta et al. (2006) described a decrease in nuclear DNA-encoded transcripts of mitochondrial respiratory chain complexes, which was reproduced by others. Witte et al. (2013) proposed a decrease in PGC-1α as a possible cause of the decrease in nuclear-encoded transcripts within neurons. Campbell et al. (2011, 2012, 2013) identified clonally expanded mitochondrial DNA (mtDNA) deletions at high-heteroplasmy levels within single neurons in the progressive MS cortex. Other studies reported molecular changes that impair anterograde transport of mitochondria, for example, a decrease in HDAC1 and kinesins, which are essential for replenishing the axon with healthy mitochondria in physiological conditions. In progressive MS, the respiratory chain complex deficiency means that the neuronal cell body is no longer capable of replenishing the axon with healthy mitochondria, particularly in long projection axons such as those in the corticospinal tracts (see Fig. 2). Red, Respiratory chain complex–deficient mitochondria; green, healthy mitochondria.
Mitochondrial Respiratory Chain Complexes in Neuronal Cell Bodies
The first detailed study of upper motor neurons from the nondemyelinated motor cortex was reported by Dutta et al. (2006) just over a decade ago. Dutta et al. subjected their well-preserved gray matter tissue from rapid autopsy cases to microarrays in an unbiased manner and found the majority of the most significant molecular changes to be related to the mitochondrial respiratory chain complex subunits. This study investigated nuclear DNA-encoded transcripts and found 26 transcripts of mitochondrial respiratory chain complexes to have decreased significantly. This decrease in nuclear DNA-encoded mitochondrial transcripts were neuron-specific and affected the majority of upper motor neurons. Microarray findings, which were confirmed using reverse transcriptase polymerase chain reaction (RT-PCR) and western blots, led to the biochemical deficiency of complex I and complex III. Notably, the mitochondrial respiratory chain–deficient neurons were found in the nondemyelinated motor cortex, which raised a number of possibilities apart from the neuronal cell bodies, such as demyelination of the axon in the white matter, as the cause of these molecular changes.
Since the report of the above study in 2006, the decrease in nuclear DNA-encoded mitochondrial respiratory chain transcripts in the nondemyelinated cortex have been reproduced by others (Broadwater et al. 2011; Witte et al. 2013). Both in the cingulate gyrus and frontal cortex, transcripts of mitochondrial respiratory chain complexes were significantly decreased by RT-PCR (Witte et al. 2013). This study extended the transcript data of the mitochondrial respiratory chain complexes by identifying a significant decrease (one-quarter to one-third) in messenger RNA (mRNA) level of peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1-α (PGC-1α), a master regulator of metabolism and mitochondrial function. This finding was confirmed at the protein level and localized to neurons in deeper cortical layers (cortical layers IV–VI). The mitochondrial respiratory chain complex deficiency in the nondemyelinated motor cortex was associated with a decrease in components of GABAergic neurotransmission and loss of inhibitory interneuron processes as well as a decrease in mitochondrial antioxidants (Dutta et al. 2006; Witte et al. 2013). Broadwater and colleagues (2011) undertook a different experimental approach to investigate mitochondria in the nondemyelinated cortex in progressive MS cases. Using mass spectroscopy, they identified proteomic changes relating to the mitochondrial respiratory chain complexes in the nonlesional motor cortex in progressive MS. A significant decrease in mitochondrial respiratory chain complex IV subunit V was confirmed by western blots. Furthermore, another study indicated a number of nuclear DNA-encoded and mitochondrial DNA-encoded transcripts to have decreased in acute white matter MS lesions (Fischer et al. 2012).
Our study undertook a single-cell approach and interrogated mitochondrial DNA and identified neurons in the deeper cortical layers that lacked complex IV and contained complex II, which in the literature are termed as respiratory-deficient neurons (Campbell et al. 2011). Laser microdissection of these respiratory-deficient neurons and detailed analysis of mitochondrial DNA extracted from the captured cell identified clonally expanded mitochondrial DNA deletions at a high heteroplasmy level. Heteroplasmy is when a mitochondrial DNA mutation(s) coexists with wild-type genomes in a single cell (Larsson 2010). The proportion of mitochondrial DNA mutation to total mitochondrial DNA content in a single cell designates the heteroplasmy level (Larsson 2010). The increase in the copy number of mutant mitochondrial genomes in a single cell encompasses the process termed clonal expansion of mitochondrial DNA mutation, which is well recognized in a number of neurodegenerative conditions, although the mechanism is not well understood (Larsson 2010). Judging by the current understanding of clonal expansion of mitochondrial DNA deletion, we expect this process to occur over years or decades in progressive MS rather than in a much shorter time frame. These respiratory-deficient neurons, also reported in a number of classical neurodegenerative disorders, were more prevalent in the nondemyelinated motor cortex and were not directly associated with microglia (Campbell et al. 2011). These observations widen the possible causes and indicate an irreversible respiratory deficiency within neurons in progressive MS.
We then investigated the choroid plexus and skeletal muscle, which contain metabolically highly active postmitotic cells, to extend the study of respiratory deficiency and mitochondrial DNA deletions in progressive MS to nonneuronal cells. Respiratory-deficient choroid plexus epithelial cells were much more prominent in progressive MS cases compared with controls, Alzheimer’s disease, and Parkinson’s disease (Campbell et al. 2012). Again, we detected high-heteroplasmy levels of clonally expanded mitochondrial DNA deletions in the respiratory chain–deficient cells. In contrast, we did not detect a significant change in the extent of respiratory-deficient muscle fibers in progressive MS compared with age-matched controls (Campbell et al. 2013). These findings suggest that the mitochondrial changes observed within neuronal cell bodies in progressive MS are likely to have been induced within the CNS, rather than representative of a multiorgan disorder with an inherited etiology.
The cause(s) of these molecular changes intrinsic to neurons in progressive MS is not known, that is, whether this is a primary affect or a secondary phenomenon following the loss-of-functional connectivity caused by the loss of synapses and transection of the long projecting axon. In terms of the mitochondrial DNA deletions, the molecular events encompass a likely active or a positive selection phenomenon, and the resulting mitochondrial respiratory chain complex deficiency is irreversible. Further studies are needed to understand both the cause of the neuronal molecular changes and the consequences of the neuronal mitochondrial respiratory chain deficiency, particularly of those that are irreversible, for the survival of the demyelinated long projection axon, synapses as well as at the level of the clinical presentation of progressive MS.
Mitochondrial Transport Machinery within Neurons
Beside the studies that found consistent molecular changes relating to the mitochondrial respiratory chain complexes, other independent groups identified molecular changes intrinsic to neurons that also impact on mitochondria, in particular the anterograde transport of these organelles from cell body to the axon and further along the axon.
A study that investigated histone deacetylase 1 (HDAC1), which is an enzyme that is found within the nucleus and represses nuclear DNA transcription, detected aberrantly located enzyme in the cytoplasm and degenerated axons in MS autopsy tissue as well as in an in vivo experimental system with cuprizone-induced demyelination (Kim et al. 2010). This nuclear export of HDAC1 was neuron specific, related to the increase in calcium entry into the neuron, and occurred before the beading of neuritis. The aberrantly placed enzyme formed protein complexes with kinesins (KIF2A and KIF5), which are motor proteins involved in anterograde transport of protein complexes, mRNA, and membranous organelles, such as mitochondria, in axons. This interaction between aberrantly placed HDAC1 and kinesins, following exposure of neuron to glutamate and tumor necrosis factor α (TNF-α), meant that the anterograde transport of mitochondria in the axon was impaired. The inhibition of the nuclear export of HDAC1 using pharmacological agents prevented the damage to neuritis. The impaired mitochondrial transport within axons in the context of inflammation was confirmed by another group using acute experimental autoimmune encephalomyelitis (EAE) and chronic EAE and live imaging (Sorbara et al. 2014). Both anterograde and retrograde transport of mitochondria within axons was significantly decreased by a slower speed and more frequent stops. These mitochondrial transport deficits preceded structural alterations of axons as well as the morphological changes in their cargo, including mitochondria. The transport deficits in EAE were rescued by methylprednisolone and redox scavengers. Further evidence of impaired anterograde transport of mitochondria in progressive MS is indicated by the significant decrease in kinesin mRNA for KIF5A, KIF21B, and KIF1B within neurons (Hares et al. 2013). These changes, again evident in the nondemyelinated cortex in progressive MS, were associated at the protein level by a decrease in KIF5A.
Impaired anterograde transport of mitochondria in neuronal cells will have a clear impact on the ability of the neuronal cell body to replenish the population of mitochondria in the axon and result in an energy failure state through the depletion of mitochondria in the axon. Whether agents such as glucocorticoids, redox scavengers, and anti-inflammatory drugs can reverse these molecular changes (aberrant location of HDAC1 and decrease in KIF5A) in progressive MS and restoring mitochondrial transport in neurons with irreversible respiratory chain complex deficiency is beneficial or harmful are uncertain. These fundamental aspects require detailed study and proof-of-concept in future experimental disease models with chronic white matter demyelination, irreversible respiratory deficiency, and gray matter inflammation, which are likely to have greater relevance to progressive MS.
MITOCHONDRIAL CHANGES WITHIN DEMYELINATED AXONS IN MS
The Energy Demands and the Distribution of Mitochondria in the Myelinated Axon
The last decade has seen significant evidence of mitochondrial modifications within the demyelinated axon. To understand the significance of these changes, it is important to recognize the role mitochondria have to play in the myelinated axon within the CNS. Directed by the requirement for energy, the precise location of mitochondria within the axoplasm is crucial to axonal function.
The major advantage of myelination of axons is the saltatory conduction of action potentials that result in their fast propagation along the nerve. Recent evidence also suggests that myelinating cells of the CNS, the oligodendrocytes, supply axonal mitochondria with the metabolites required for them to efficiently perform oxidative phosphorylation to produce ATP, the cellular energy currency (Funfschilling et al. 2012). Within the myelinated axoplasm, mitochondria are far from uniform, differing in morphology and velocity most often depending on their location, whether at the node of Ranvier, paranode, juxtanode, or internode where changes in calcium levels and energy demand are different. It has been reported that the highly energy demanding Na+/K+ ATPase is richly distributed along the internode and juxtaparanode axonal membrane while absent from the nodal space and paranode (Young et al. 2008). These changes are reflected in the distribution of mitochondria, which varies dramatically along the axon. A third of nodes of Ranvier in mouse myelinated axons contain no mitochondria while the majority of the remaining nodes contain only a single mitochondria (Ohno et al. 2011). Both the length and volume of mitochondria within the node and paranode are significantly decreased compared with their counterparts in the juxtaparanode and internode. In these regions, where large stationary mitochondrial networks are found, the ratio of mitochondrial to axonal volume is significantly increased. The variable distribution of the mitochondria is therefore likely to result from the changeable energy demands along the axon. It has also been hypothesized that axonal Ca2+ is responsible for the distribution of mitochondria along the axon. Peripheral nervous system (PNS) axons show a marked increase in calcium signaling at the node of Ranvier (Zhang et al. 2010), where mitochondria are highly enriched, although in vitro evidence also suggests a similar role for axoplasmic Ca2+ in the CNS (Ohno et al. 2011). The mechanism for this may be an interaction of Ca2+ with the microtubule motor kinesin-1 responsible for anterograde transport of mitochondria along the axon (Saotome et al. 2008). Although only shown in the PNS to date, artificial stimulation of the Na+/K+ ATPase has also been shown to enhance retardation of mitochondrial velocity (Zhang et al. 2010). Experimentally, blocking action potentials results in a decrease in mitochondrial movement, while the reverse is also true, suggesting a likely role for axonal electrical conductivity in mitochondrial distribution.
Many of these factors become increasingly important as dramatic axonal metabolic changes in the event of demyelination lead to important modifications in mitochondrial dynamics.
The Changing Energy Demands of the Demyelinated Axon
Unmyelinated axons provide a useful guide as to the prediction of mitochondrial changes one might observe in the demyelinated axon (Bristow et al. 2002). Studies of the unmyelinated axons of the lamina cribrosa have provided such information in which complex IV activity has been investigated. Complex IV is the terminal subunit in the electron transport chain and consumes 90% of cellular oxygen. The unmyelinated segment of the lamina cribrosa was found to have increased complex IV activity compared with its myelinated counterpart, an indicator of increased energy demand, highlighted by the uniform distribution of certain isoforms of Na channels (Nav 1.1 and Nav1.6) along the unmyelinated segment (Bristow et al. 2002; Balaratnasingam et al. 2009). This is contrast to myelinated axons where Na channels are specifically localized to the nodes of Ranvier within the CNS. The early redistribution of Na channels along the denuded axonal segment has been shown to be a consistent feature of demyelinated axons, which may allow the continuation of action potentials and, in the context of MS, recovery of clinical function (Craner et al. 2004b). The increase of complex IV activity has also been noted in animal models of hypomyelination and dysmyelination resulting from the loss of myelin basic protein and overexpression of proteolipid protein, respectively (Andrews et al. 2006; Hogan et al. 2009). In both models, an increase of axonal mitochondrial content was observed. It may be that the increase in axonal complex IV plays an important role highlighted by the fact that in an animal model of only partial demyelination, caused by the hemizygous overexpression of PLP, an increase of mitochondrial content without complex IV activity was associated with axonal degeneration.
An increase in axonal mitochondrial content is a consistent feature of animal models of demyelination. Our recent studies (unpubl.) show that this encompasses the classic modes of demyelination, including lysolecithin, lipopolysaccharide, cuprizone, and various forms of EAE. It is now established that this is a feature of morphological intact, large-caliber axons in demyelinated lesions of MS (Fig. 2). An increase in the axon-specific mitochondrial docking protein, syntaphilin, suggests that these mitochondria are likely to be stationary (Mahad et al. 2009). In vitro evidence has provided further details of these changes, including an increased size of stationary mitochondria in demyelinated axons induced by lysolecithin and velocity of motile mitochondria (Kiryu-Seo et al. 2010). It is perhaps surprising to note that the actual number of stationary sites did not change, rather it is hypothesized that newly formed mitochondria fuse with those already at stationary sites along the axon. All of these changes were found to be reversed, in vitro, on remyelination. This may not be entirely the case in vivo in which axonal mitochondrial content does not return to baseline, intriguingly suggesting that remyelinated axons have different energy demands compared with myelinated axons (Zambonin et al. 2011).
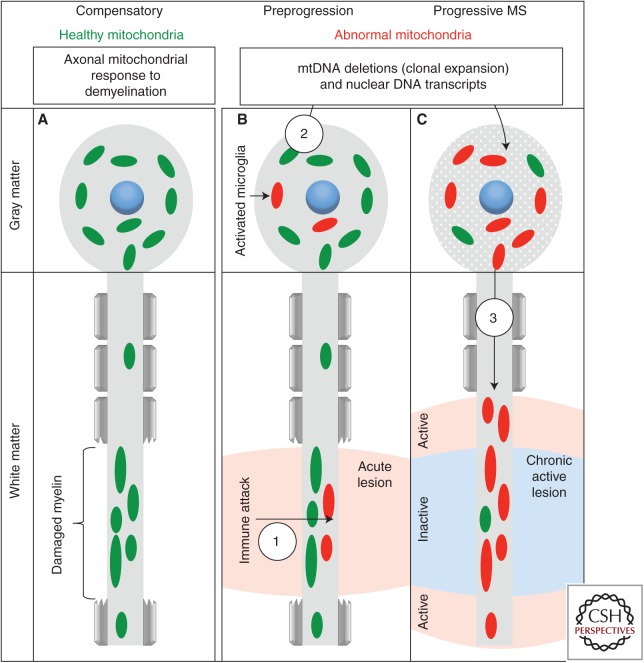
Figure 2.
How neuronal mitochondria play a role in axon degeneration in progressive multiple sclerosis (MS). (A) We reported the gathering of functional mitochondria in the demyelinated axon in MS and on nonautoimmune demyelination of wild-type neurons in vivo (Mahad et al. 2015). These mitochondrial changes (increased number, size, activity, and speed of movement of mitochondria reported as “axonal mitochondrial response to demyelination”) protected the demyelinated axon because the inability to mount the axonal mitochondrial response to demyelination in mice lacking syntaphilin, an axon-specific mitochondria docking protein, led to an excess of axon ovoids on demyelination. (B) During the preprogressive stage of MS, inflammatory products injure mitochondria in multiple cell types, including neurons, and the oxidative injury to DNA leads to the formation of mitochondrial DNA (mtDNA) deletions in both the white matter (1) and gray matter. Over time and with age, abnormal mitochondria are amplified in neuronal cell bodies (2), for example, through clonal expansion of the inflammation-induced mtDNA deletions and depleted nuclear DNA-encoded mitochondrial transcripts. (C) The resulting biochemical deficiency of the mitochondrial respiratory chain complexes or enzymes in neuronal cell bodies then act as a reservoir of abnormal mitochondria, which then undergo aberrant placement to the demyelinated axon and cause energy failure and increased reactive oxygen species production in the axon (3). In addition, any residual or active inflammation in the progressive MS brain would further impair mitochondrial function particularly at the active edge of chronic active MS lesions. This forms a three-step hypothesis (formation, amplification, and displacement) for the role of mitochondria in axon degeneration in progressive MS.
It has been a matter of debate as to whether these mitochondrial changes in response to demyelination represent a compensatory or pathological phenomenon. Evidence does suggest, at least in the short term, that these changes are essential for the survival of the axon. Histological evidence from MS lesions indicates that axons that have morphological signs of degeneration and transport block do not show any increase in mitochondrial content (Mahad et al. 2009). When this response is blocked, evidence for increased axon stress is observed and the experimental loss of syntaphilin results in an increase of axonal degeneration (Kiryu-Seo et al. 2010; Ohno et al. 2014). In the long term, however, recent evidence points toward a potential detrimental outcome. When syntaphilin was knocked out in the Shiverer dysmyelinated model, an improved clinical outcome was noted, speculated to arise from the now proper degradation of unhealthy mitochondria, which can produce harmful reactive oxygen species (ROS) over a prolonged period of time (Joshi et al. 2015).
Axon Degeneration in Progressive MS—A Specific Role for Complex IV Activity?
Axon degeneration is a significant pathological hallmark of MS, and its importance highlighted by the hypothesis that axon loss is the crucial factor in the development of progressive MS from relapsing remitting disease (Bjartmar et al. 2003). Inflammation is a key cause of axon degeneration particularly during the initial stages of disease. Anti-inflammatory treatments, effective during the acute stage of disease, are rendered redundant in the progressive stage of disease, the reasons for which remain unresolved. Chronic demyelinated lesions persist for decades and slow burning axonal degeneration is significant to CNS plasticity. One hypothesis for inflammation-independent axon degeneration results from mitochondrial dysfunction leading to loss of Na+/K+ ATPase activity, coupled with persistent sodium influx via Nav1.6 channels and reversal of the Na+/Ca2+ exchanger (Waxman 2006). An increase of axonal calcium, exacerbated by mitochondrial dysfunction, can activate a number of degenerative pathways. Evidence from MS postmortem tissue supports this mechanism. Degenerative axons are associated with colocalization of Nav1.6 channels and the Na+/Ca2+ exchanger (Craner et al. 2004a).
Histological studies of human MS and animal model tissue also highlight the potential importance of complex IV activity over mitochondrial content. Degenerating axons in MS do not show the compensatory increase of axonal mitochondrial content nor complex IV activity but further studies suggest it is complex IV activity rather than mitochondrial content, which is the more important factor (Mahad et al. 2009). In the hemizygous PLP overexpression mouse model, axon degeneration was apparent in the light of an increase in mitochondrial content but no concurrent increase of complex IV activity (Hogan et al. 2009). Our recent studies (unpubl.) show that despite the fact that all classic demyelination animal models show an increase of axonal mitochondrial content on demyelination, this is not always the case for complex IV activity. Following lysolecithin-induced demyelination, both mitochondrial content and complex IV activity increase within demyelinated axons. In contrast, we did not find a corresponding increase in complex IV activity within demyelinated axons in a number of EAE in mouse, rat, and marmoset species, despite an increase in axonal mitochondrial content. Interestingly, the level of axonal of complex IV activity was significantly correlated with the amount of axonal damage within the demyelinated lesion, which was not the case for the amount of axonal mitochondrial content. Where complex IV was observed in transected axons, this may suggest a role of CD8 direct transection of axons and replenishing of the axon with functional mitochondria. Future studies should focus on the timing of complex IV loss and its relation to changes in axonal calcium level, mitochondrial ROS production, as well as mitochondrial membrane potential. Evidence so far suggests that the loss of axonal complex IV activity is an early phenomenon, particularly in EAE. Complex IV activity is lost in elongated mitochondria in EAE axons, which may precede axonal mitochondrial morphological changes during the reversible form of axon damage and clinical symptoms (Nikic et al. 2011). The mechanism of complex IV activity loss may be nitration of complex IV subunit IV that occurs in EAE even before the arrival of inflammatory cells (Qi et al. 2006).
The increased energy demand of demyelinated axons, highlighted by changes in the mitochondrial network, must be met by the neuronal cell body, which is the site of mitochondria generation (Fig. 2). Any dysfunction to the neuronal soma, as increasing evidence suggests occurs in MS as detailed above, would therefore put the axon at risk of degeneration. Hence, the neuronal cell body changes that converge on mitochondrial respiratory chain and mitochondrial anterograde movement impairment are a molecular process that is intrinsic to neurons and will contribute to axon energy failure in progressive MS.
ARE THE MOLECULAR CHANGES INTRINSIC TO NEURONS IN PROGRESSIVE MS PRIMARY OR SECONDARY?
The nature of the autopsy tissue available in progressive MS makes the delineation of the time course of the neuron-specific irreversible mitochondrial injury challenging. Although biopsy material from early stage of MS has been used to define inflammation in the cortex, such material is limited for more detailed analysis of neuronal cell bodies (Lucchinetti et al. 2011). Our current view is that at least part of the molecular changes within neuronal cell bodies that compromise the mitochondrial respiratory chain are induced within the CNS and are irreversible. The clonal expansion of mitochondrial DNA deletions, for example within neurons, may take several years or a decade or so for the biochemistry of the mitochondrial respiratory chain to be affected. Iron accumulation and increase in oxidative injury are likely to further amplify the mitochondrial damage over time. Chronic demyelination, as mentioned above, will be another factor that will trigger axonal mitochondrial injury independent of inflammation, as shown in Shiverer mice (Joshi et al. 2015). Nevertheless, when the subclinical phase of MS shown by a radiologically isolated syndrome and the early relapsing phase of the disease, which precedes secondary progressive MS, are considered, it is likely that the neuronal compartment compromise occurs early in progressive MS and is likely to contribute to the inflammation-induced neurodegeneration.
THERAPEUTIC IMPLICATION OF THE NEURONAL MITOCHONDRIAL RESPIRATORY CHAIN COMPLEX DEFICIENCY
Immunomodulation has clearly been a very successful strategy in relapsing MS and increasingly, at least in a subset of patients, with progressive MS and active disease on radiology. Administration of effective disease-modifying therapy will decrease the accumulation of disability, at least that which follows incompletely recovered relapses. The second strategy that targets remyelination is currently being tested in clinical trials and is likely to offer benefit to patients with MS in the future (Franklin et al. 2012). We see the improvement of the neuronal bioenergetics by targeting mitochondrial function to be the third therapeutic strategy for progressive MS, which needs to be combined with the two other strategies for an optimal outcome. In this respect, experimental disease models that reflect the inflammatory component, as well as chronic demyelination and neuron-specific mitochondrial injury, are much needed to both increase the understanding of the pathogenesis of progressive MS and to develop novel therapeutic strategies and agents.
ACKNOWLEDGMENTS
We thank the Multiple Sclerosis Society in the United Kingdom, the National Multiple Sclerosis Society in the United States, and a Wellcome Trust Institutional Strategic Support Fund awarded to D.M. for their support of the work in our laboratory.
Footnotes
Editors: Howard L. Weiner and Vijay K. Kuchroo
Additional Perspectives on Multiple Sclerosis available at www.perspectivesinmedicine.org
REFERENCES
- Andrews H, White K, Thomson C, Edgar J, Bates D, Griffiths I, Turnbull D, Nichols P. 2006. Increased axonal mitochondrial activity as an adaptation to myelin deficiency in the Shiverer mouse. J Neurosci Res 83: 1533–1539. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Johnstone V, Cringle SJ, Yu DY. 2009. Heterogeneous distribution of axonal cytoskeleton proteins in the human optic nerve. Invest Ophthalmol Vis Sci 50: 2824–2838. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. 2003. Axonal loss in the pathology of MS: Consequences for understanding the progressive phase of the disease. J Neurol Sci 206: 165–171. [DOI] [PubMed] [Google Scholar]
- Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. 2002. The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol 120: 791–796. [DOI] [PubMed] [Google Scholar]
- Broadwater L, Pandit A, Clements R, Azzam S, Vadnal J, Sulak M, Yong VW, Freeman EJ, Gregory RB, McDonough J. 2011. Analysis of the mitochondrial proteome in multiple sclerosis cortex. Biochim Biophys Acta 1812: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, Lassmann H, Turnbull DM, Mahad DJ. 2011. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol 69: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Kraytsberg Y, Krishnan KJ, Ohno N, Ziabreva I, Reeve A, Trapp BD, Newcombe J, Reynolds R, Lassmann H, et al. 2012. Clonally expanded mitochondrial DNA deletions within the choroid plexus in multiple sclerosis. Acta Neuropathol 124: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Reeve AK, Ziabreva I, Reynolds R, Turnbull DM, Mahad DJ. 2013. No excess of mitochondrial DNA deletions within muscle in progressive multiple sclerosis. Mult Scler 19: 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. 2004a. Co-localization of sodium channel Nav1.6 and the sodium–calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127: 294–303. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. 2004b. Molecular changes in neurons in multiple sclerosis: Altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci 101: 8168–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, et al. 2006. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59: 478–489. [DOI] [PubMed] [Google Scholar]
- Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H. 2012. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135: 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, ffrench-Constant C, Edgar JM, Smith KJ. 2012. Neuroprotection and repair in multiple sclerosis. Nat Rev Neurol 8: 624–634. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, et al. 2012. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hares K, Kemp K, Rice C, Gray E, Scolding N, Wilkins A. 2013. Reduced axonal motor protein expression in non-lesional grey matter in multiple sclerosis. Mult Scler 20: 812–821. [DOI] [PubMed] [Google Scholar]
- Hogan V, White K, Edgar J, McGill A, Karim S, McLaughlin M, Griffiths I, Turnbull D, Nichols P. 2009. Increase in mitochondrial density within axons and supporting cells in response to demyelination in the Plp1 mouse model. J Neurosci Res 87: 452–459. [DOI] [PubMed] [Google Scholar]
- Joshi DC, Zhang CL, Lin TM, Gusain A, Harris MG, Tree E, Yin Y, Wu C, Sheng ZH, Dempsey RJ, et al. 2015. Deletion of mitochondrial anchoring protects dysmyelinating shiverer: Implications for progressive MS. J Neurosci 35: 5293–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens T, Jafari M, Kreutzfeldt M, Bahn E, Bruck W, Kerschensteiner M, Merkler D. 2016. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 139: 39–46. [DOI] [PubMed] [Google Scholar]
- Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, Casaccia P. 2010. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci 13: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryu-Seo S, Ohno N, Kidd GJ, Komuro H, Trapp BD. 2010. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J Neurosci 30: 6658–6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson NG. 2010. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem 79: 683–706. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, et al. 2011. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365: 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R. 2010. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68: 477–493. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS, Lassmann H, Turnbull DM. 2009. Mitochondrial changes within axons in multiple sclerosis. Brain 132: 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DH, Trapp BD, Lassmann H. 2015. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14: 183–193. [DOI] [PubMed] [Google Scholar]
- Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D, Misgeld T, Kerschensteiner M. 2011. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17: 495–499. [DOI] [PubMed] [Google Scholar]
- Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S, Avishai A, Komuro H, Trapp BD. 2011. Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J Neurosci 31: 7249–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Chiang H, Mahad DJ, Kidd G, Liu L, Ransohoff RM, Sheng Z, Komoro H, Trapp BD. 2014. Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc Natl Acad Sci 111: 9953–9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. 2006. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem 281: 31950–31962. [DOI] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G. 2008. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci 105: 20728–20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara CD, Wagner NE, Ladwig A, Nikic I, Merkler D, Kleele T, Marinkovic P, Naumann R, Godinho L, Bareyre FM, et al. 2014. Pervasive axonal transport deficits in multiple sclerosis models. Neuron 84: 1183–1190. [DOI] [PubMed] [Google Scholar]
- Stadelmann C. 2011. Multiple sclerosis as a neurodegenerative disease: Pathology, mechanisms and therapeutic implications. Curr Opin Neurol 24: 224–229. [DOI] [PubMed] [Google Scholar]
- Waxman SG. 2006. Ions, energy and axonal injury: Towards a molecular neurology of multiple sclerosis. Trends Mol Med 12: 192–195. [DOI] [PubMed] [Google Scholar]
- Witte ME, Nijland PG, Drexhage JA, Gerritsen W, Geerts D, van Het Hof B, Reijerkerk A, de Vries HE, van der Valk P, van Horssen J. 2013. Reduced expression of PGC-1α partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol 125: 231–243. [DOI] [PubMed] [Google Scholar]
- Young EA, Fowler CD, Kidd GJ, Chang A, Rudick R, Fisher E, Trapp BD. 2008. Imaging correlates of decreased axonal Na+/K+ ATPase in chronic multiple sclerosis lesions. Ann Neurol 63: 428–435. [DOI] [PubMed] [Google Scholar]
- Zambonin JL, Zhao C, Ohno N, Campbell GR, Engeham S, Ziabreva I, Schwarz N, Lee SE, Frischer JM, Turnbull DM, et al. 2011. Increased mitochondrial content in remyelinated axons: Implications for multiple sclerosis. Brain 134: 1901–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Ho PL, Kintner DB, Sun D, Chiu SY. 2010. Activity-dependent regulation of mitochondrial motility by calcium and Na/K-ATPase at nodes of Ranvier of myelinated nerves. J Neurosci 30: 3555–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]