Abstract
The red palm weevil (RPW), Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae) is one of the most dangerous pests of major cultivated palms including coconut, oil palm, and sago. The larval stage of the weevil causes the most destruction of the palms as it completely destroys the palm cabbage. In this study, the larvae were given three different diets—coconut cabbage, oil palm cabbage, and sago stem, under laboratory conditions for food consumption and developmental time experiment. The protein profiles of the digestive systems of the larvae fed on these three diets were also determined. Although the coconut diet was the most consumed by RPW larvae compared to oil palm and sago diets, the growth rate of RPW larvae on oil palm diet was however significantly shorter than those on the coconut and sago diets: the RPW only need 1 mo and 9 d to complete the larval duration. Proteins profiling of eight 2-DE gel protein spots that range 50–20 kDa were identified by mass spectrometry sequence analysis. Based on the Matrix Science Software, the most dominant protein was cationic trypsin. However, based on the NCBI BLAST tool, aminopeptidase N was the most dominant enzyme. This finding can lead to the development of pest control strategies based on the antinutritional protease inhibitors as potential biocontrol agents. Urgent action to find effective control methods should be taken seriously as this weevil is presumed to be one of the serious pests of oil palm industry in Malaysia.
Keywords: Red Palm Weevil, Rhynchophorus ferrugineus, protein profile, digestive system, palm pest
Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae), also known as red palm weevil, (RPW) is among the worst insect pests of major cultivated palms in the world. The attack of RPW was first detected in 2007 by the Malaysian Department of Agriculture (DOA) and had spread to 58 localities in all seven districts of the state of Terengganu, East Coast of Peninsula Malaysia. In 2011, the number of infestation sites has increased significantly to 858 localities (Wahizatul et al. 2013). Currently, in 2016, the RPW has been found in other five states of Peninsula Malaysia which have indicated a drastic increase and rapid spread of RPW population in Malaysia. The weevil is believed to have been introduced by date palm trees which were imported from Egypt for landscaping purposes without proper quarantine a few years back (Wahizatul et al. 2013). One of the severe signs of infested coconut palms is the drooping of dried leaves- like those of an umbrella shape, a sign of irreversible damage signaling the eventual death of the palms in 6–8 mo (Murphy and Briscoe 1999). Other symptoms include the presence of holes in petioles and the appearance of chewed up plant tissue with a foul fermented odor (Kaakeh 2005). The damage to the coconut palm is mainly caused by the larvae which can be found within the cabbage of the coconut tree, or in the soft tissue at the base of the petioles and the palm trunk, where the weevils destroy the vascular system. The larvae have the ability to destroy the vascular system because their mouthparts are well-developed and strongly chitinized (Wahizatul et al. 2013).
RPW is widely distributed in many geographical areas, from India and Sri Lanka, eastwards throughout South East Asia, Australia, Middle East, and in Mediterranean countries (Faleiro 2006). This weevil is a lethal, concealed tissue borer that attacks more than 26 palm species worldwide belonging to 16 genera. Due to that, RPW is listed on the A2 list of EPPO (European and Mediterranean Plant Protection Organization) as a significant serious pest (EPPO 2008). Notably, Malaysia houses a broad range of palm species including the coconut, oil palm, sago palm, and several other ornamental palm trees. As such, this weevil has the potential to be one of the aggressive pests that will threaten the oil palm industry, which represents as one of the main sources for Malaysia’s economy. However, to date, there is limited study on the life cycle or feeding preferences of RPW on different varieties of palm trees such as sago, oil palm, and in particular on the coconut. In fact, little is known about the protein study (proteomics) of the RPW as there has yet to be attempted a complete study on the protein profiling of the digestive system of the RPW. No such study has been conducted in Malaysia, particularly on the comparison of protein profiles from the digestive system of RPW larvae reared on different diets.
Currently, pheromone traps are the effective way in controlling RPW, but they are costly. An alternative method to control RPW infestation is biological control because mechanical and chemical control methods are not effective or not sufficient (El-Mergawy and Al-Ajlan 2011). Some of the biocontrol agents are entomopathogenic fungi, entomopathogenic nematode, and cytoplasmic polyhedrosis virus. However, all these methods are still under experimentation and the effectiveness of the biocontrol agents are unclear and inconclusive. Thus, it shows that RPW is one of the pests that is difficult to control using available conventional methods (Manachini et al. 2011). Therefore, another alternative method that can be used to control RPW infestation is through nutrition-based protein study, by identifying the selective inhibitor of the digestive enzymes of RPW larvae which will affect the growth of the larvae leading to their demise (Alarcón et al. 2002). Currently, very limited information on protein inhibitor for the RPW larval digestive process is available.
There were many inconclusive results occur in previous studies about the number of instars reared on different diets under laboratory conditions. For example, Nirula (1956) reported that larvae only reached until third instar when rearing RPW on coconut palms under laboratory conditions in India. A study done by Jaya et al. (2000) found that when given the coconut palms as the diet to the larvae, they reached until ninth instar compared to the 7th instar larvae reared on sugarcane. Ju et al. (2011) reared RPW on five host plants, and found that larval period lasted up to 8 instars when fed on Canary island date palm and Washington palm, while feeding on other three palms resulted 9 instars. This showed that the larvae can have fewer instars which shorten developmental time when reared on the suitable host plant. Besides that, the larval instars also can be affected by quality of host plant as well as the environment (Ju et al., 2011).
The type of diets is also believed could influence the type of protein profiles of the digestive system of insects (Macedo et al. 2011, Lazarević and Janković-Tomanić 2015). Thus, this study was conducted to determine food consumption and developmental time of RPW larvae on three different diets (coconut cabbage, oil palm cabbage and sago stem), and to identify protein profiles from the digestive system of RPW larvae reared on these diets. The rationale of this study is to understand the effect of different diets toward the proteomic of RPW digestion system which may be used as a potential new target in RPW eradication.
Materials and Methods
Rearing of RPW
Adults of RPW were collected from highly infested areas of coconut plantations such as Tok Jembal (N 05°23.726′ E 103°05.721′), Teluk Bidara (N 04°77.886′ E 103°43.964′), and Tanjung Jara (N 04°81.039′ E 103°42.134′) in Terengganu, Malaysia using pheromone trappings (Wahizatul et al. 2014). Overall, more than 2,000 RPW adults were successfully collected from October 2012 to January 2014. The captured RPW were cultured in the Biodiversity Laboratory, Universiti Malaysia Terengganu (UMT). RPW adults were allowed to mate in the transparent plastic containers (dimension of 20 cm length × 13 cm width × 12 cm height) filled with sago stem. The sago stem was used as feed as well as for the adults to lay eggs. The containers were kept under ambient temperature conditions of 26 ± 2°C, 70 ± 5% relative humidity (RH) and photoperiod of 12:12 (L:D) h. The sago stem was examined for eggs after the completion of mating process. The inside part of the sago stem was checked daily for eggs which were then transferred using soft forceps individually into small circular plastic containers (5 cm in diameter and 2.5 cm high) for feeding experiments.
Feeding Experiment
The diets used for the feeding experiment were coconut cabbage, oil palm cabbage, and sago stem; the coconut cabbage act as a control in this experiment. Each of the diet ingredients was cut into block shapes each weighing about 1.5 g. The feeding experiment lasted from the time the eggs hatched up until the final instar of the larvae- which were the sixth to seventh instar, before being continued for proteomic analysis. This experiment was done up until the sixth to seventh instar larvae because this stage was the final larval stage before pupation. There were ten replicates for each of the diet and each replicate consists of 20 individuals of RPW larvae. Mortality of the RPW larvae was checked on a daily basis due to fungus attack and stress condition of the larvae. The food that had been infected by the fungus was not counted. The feeding experiments were conducted under laboratory conditions (26 ± 2°C, 70 ± 5% RH, photoperiod of 12 h day: 12 h night. Sago diet was changed every 5 d while the coconut and oil palm diets needed to be replaced every 2 d to avoid fungus infection, as compared to the sago stem. Food consumed by the larvae was recorded and calculated every time the diets were changed. The diets were weighed; X1 (g) before the eggs were placed into the diets. When it was time to replace the old diets, the old diets were weighed again; X2 (g). The procedure was repeated each time new food was given to the larvae, until the larvae reached the final instar which was the sixth to seventh instar. Determination of food consumption by the larvae was calculated as below:
Developmental Time of Larvae
Ten to 15 of deposited eggs were placed and reared on different diets, which were coconut cabbage, oil palm cabbage, and sago stem. Small holes were made on the lid of the containers and wet tissues were used to maintain high humidity of the food. After 4–5 d, the newly hatched eggs were carefully removed using soft forceps and placed individually in small rearing containers equipped with the tested diet for feeding. The widths (mm) of the head capsules of the larvae were measured by Vernier caliper to determine the instar stages of the reared larvae according to the Dyar’s ratio (Mohammadi et al. 2010). The measurements of head capsule width were done every 3 d for first, second, and third instar. For fourth instar and above, the head capsule measurements were carried out on a weekly basis. Once the larvae transformed into coccons, they were placed in another plastic container with lids. The number of days elapsed between the pupation and adult emergence was considered as the pupal period. Cocoons were checked daily for adult emergence.
Preparation of Samples and Protein Extraction
Proteomic analyses were carried out according to the methodologies described by Alarcón et al. (2002), Nguyen et al. (2007) and Yapi et al. (2009). Larvae of RPW of sixth to seventh instar of each diet treatment were dissected following the procedure described by Broadway and Duffey (1985). Larvae at this stage were used for protein extraction because the digestive system is well-developed and it is easier to dissect compared to the other stages (Alarcón et al. 2002). A total of 20 larvae from each diet treatment were used for the protein extraction. Larvae were rinsed in cold water individually and blotted with filter paper. Digestive system was dissected in cold 0.15 M NaCl at 4°C for protein analysis. The digestive system samples were homogenized in 0.15 M NaCl by sonication. Protease inhibitors were put into the sample to avoid denaturing or degradation of the protein and active extracts were obtained after centrifugation of 12,500 g for 20 min at 4°C of the homogenates. The collected supernatant constituted the crude extract was then stored at −20°C prior to analysis.
Protein Concentration Determination and Acetone Precipitation
Protein concentration was determined according to Bradford (1976), with 1 mg/ml bovine serum albumin (BSA) used as a standard protein. The measurement of absorbance was carried out using spectrophotometer at wavelength of 595 nm (Biospectrometer Series, Eppendorf). Relative measurement of protein concentration was obtained by comparing with the BSA standard curve constructed earlier. The protein sample was placed inside an acetone-compatible tube. Then, a volume of four times of protein sample of cold acetone (−20°C) was added to the tube. The tube was vortexed and incubated for 1 h at −20°C. After that, it was centrifuged at 13,000 to 15,000 ×g for 10 min. The supernatant was carefully disposed and not to dislodge the protein pellet. The acetone was allowed to evaporate from the uncapped tube at room temperature for about 30 min (avoid letting it from being overdried). Finally, distilled water was added in an appropriate amount for the downstream process and vortexed thoroughly to dissolve the protein pellet (Pierce Biotechnology 2004).
Two-dimensional Electrophoresis (2-DE)
The ReadyPrep 2-D Clean-up Kit which contains precipitation agent 1 and 2, wash reagent 1 and 2, and wash 2 additive are from Bio-Rad Laboratories (USA), was used to remove the salts and other contaminants that can disrupt the protein sample. It was used in accordance to the manufacturer’s instructions in order to get better results. A total 125 µL of crude extract with rehydration buffer was loaded on immobiline IPG strips (7 cm) with nonlinear pH 3–10 gradients and resolved using the Protean i12 IEF System (Bio-Rad Laboratories, USA). After active rehydration for 12 h, isoelectric focusing (IEF) was performed in 6 h using Bio-Rad IEF protocols for the IPG strip that was used (250 V for 15 min, 4,000 V for 1 h, and 4,000 V to reach 15,000 V-h). Before the second step (SDS-PAGE), the IPG strips were first equilibrated for 15 min in an equilibration buffer I containing fresh dithiotreitol (DTT) and then another 15 min in the equilibration buffer II containing fresh iodoacetamide (IAA). The second dimension was carried out in 4–20% Mini-PROTEAN TGX precast polyacrylamide gels from Bio-Rad Laboratories (USA). Gels were running at 400 mA of a constant current until the bromophenol blue dye front migrated 2 cm from the bottom. The gels were stained with colloidal Coomassie brilliant blue G-250 overnight and destained with distilled water.
Image Analysis and Protein Identification
2-D gels were scanned by Gel Doc XR+ System with PDQuest software version 8.0.1 (Bio-Rad Laboratories, USA). Protein spots were quantified using the PDQuest software and comparison were made between gel images of coconut, oil palm and sago diets. The different spots that were present between the diets were later excised from gels for identification. The spots were cut out from gels manually, and put in the microcentrifuge tubes. The microcentrifuge tubes containing the spots were dried in the laminar flow and were sent to the Proteomics International Pty Ltd (Broadway, Nedlands, Western Australia) for liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis. The peptide sequences obtained from this analysis were compared with Ludwig NR database by using Mascot sequence matching software (Matrix Science). The acquired sequences were also searched for identification from the database at the National Centre for Biotechnology Information (NCBI) by using Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical Analysis
One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used to determine the differences of food consumption between instar levels and type of diets, as well as to evaluate the differences of developmental growth of RPW larval stage on different diets. Prior to analysis, data were log(x+1) transformed to ensure normality in calculations of means and ANOVAs. All statistic analyses were conducted using statistical software analysis of SPSS version 20.0.
Results
Food Consumption and Developmental Time of RPW Larvae
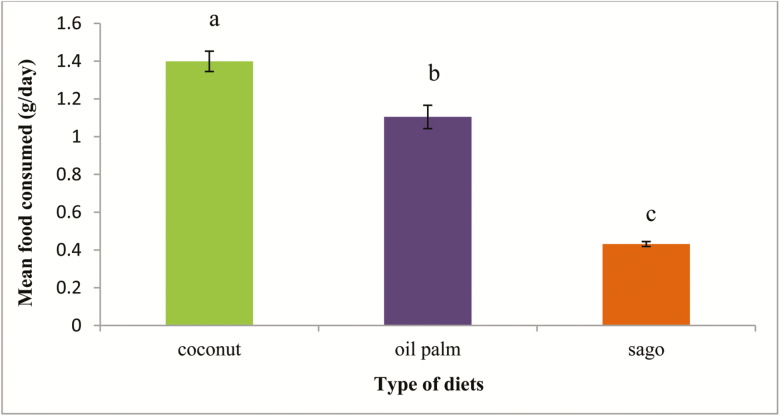
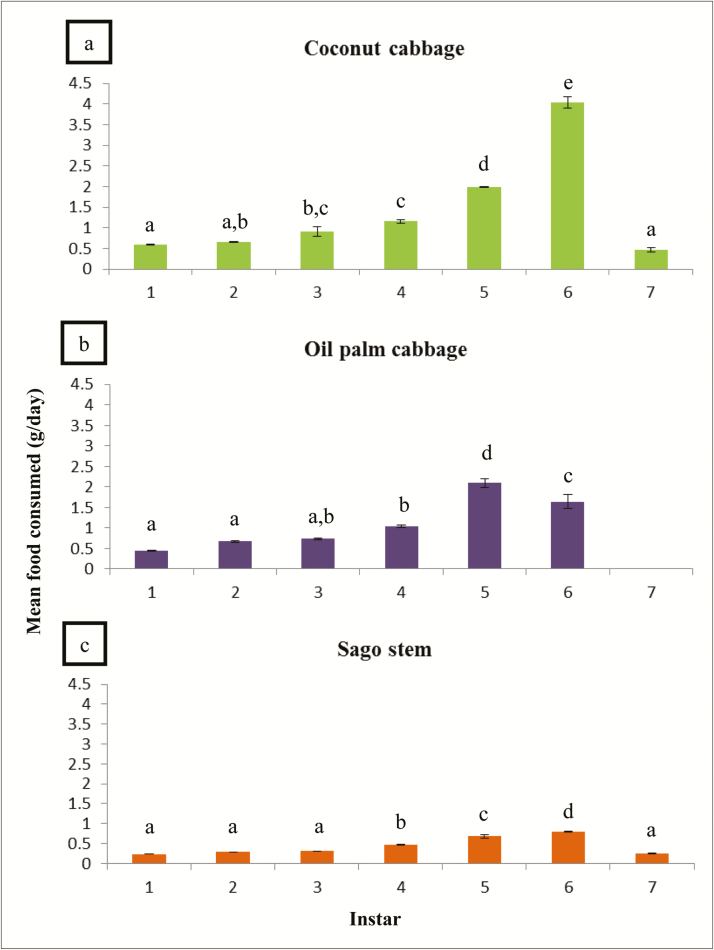
Coconut cabbage was the most consumed (1.4 ± 0.25 g/day) and preferred food by the RPW larvae, followed by oil palm (1.1 ± 0.17 g/day) and sago was the least consumed (0.4 ± 0.01 g/day) by the larvae (F2,29 = 177.18; P < 0.001) (Fig. 1). The consumption of three different diets showed similar trends, which increased gradually from the early instar (first to third instar), but then decreased drastically in the late instar (sixth to seventh instar) (Fig. 2). The life cycle of RPW larvae reared on oil palm diet was the shortest compared to coconut and sago diets (F2,29 = 110.90; P < 0.001). The larvae reared on oil palm diet reached up to the sixth instar before turning into pupae, but for coconut and sago diets at least seventh instar was needed. Both the coconut cabbage (4.04 ± 0.13 g) and sago stem (0.80 ± 0.01 g) were highly consumed by the RPW larvae of sixth instar stage as compared to first, second, third, fourth, and seventh instar larvae. However, the oil palm cabbage was highly consumed by fifth instar larvae (2.09 ± 0.11 g) compared to the other stages.
Fig. 1.
Comparison between different diets consumed by the RPW larvae. Means with different letters indicate significantly different (Tukey’s honest significant difference test, P < 0.05); N = 60.
Fig. 2.
Food consumption by the larvae based on different instar levels. Means with different letters indicate significantly different (Tukey’s HSD, P < 0.05); N = 60.
In terms of the developmental growth of RPW reared on different diets under laboratory conditions, there was a significant difference in the duration of developmental growth of the larval period among the three diets (F2,29 = 19.50; P < 0.001) (Fig. 2). The growth of RPW larvae reared on oil palm was significantly shorter as compared to the other diets, it only took about 39.8 ± 0.47 d or 1 mo 9 d to complete the larval stage. The larvae reared on coconut diet took 86.4 ± 0.37 d or 2 mo and 26 d, while the longest time to complete the larval developmental growth was from the diet of sago which took about 111.8 ± 0.39 d or 3 mo 21 d.
Two-dimensional Electrophoresis (2-DE) Analysis
A total of 143 spots appeared on the gels which referred to the three different diets given to the larvae. The total spots present on the gel for coconut diet was 58, whereas 42 and 43 spots were from the oil palm and sago diets, respectively. The molecular mass of the spots ranging from 10 to 75 kDa and isoelectric point was between 3 and 10 (pI 3–10).
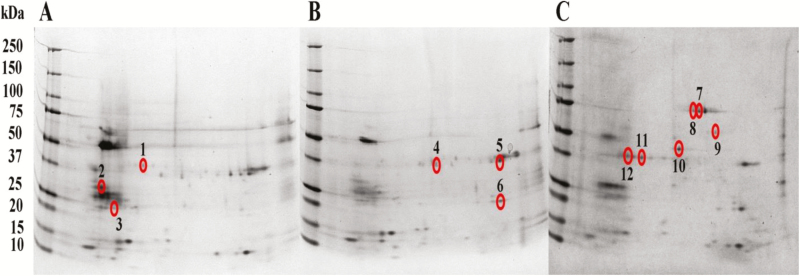
Out of the 143 spots, only 12 spots were different amongst the three diets as analysed by the PDQuest software (Bio-Rad Laboratories, USA). These spots were, three from coconut diet, three from oil palm diet and six from sago diet (Fig. 3). The 12 spots were sent for identification using LC-MS/MS and the results were tabulated in Table 1. The molecular mass (kDa) for each spot of the food treatments was estimated based on the molecular mass of the marker used and the position of the spots. For the coconut diet treatment, the spots were estimated to be protein with molecular weight of 40 kDa (spot 1), 32 kDa (spot 2), and 24 kDa (spot 3). Spots for 2-DE gel result from oil palm treatment were 38 kDa (spots 4 and 5) and 23 kDa (spot 6). As spots for sago 2D gel were protein with molecular weight of 80 kDa (spots 7 and 8), 58 kDa (spot 9), 43 kDa (spot 10), and 37 kDa (spots 11 and 12).
Fig. 3.
Two-DE gel of the protein content of the digestive system of RPW larvae reared on (A) coconut; (B) oil palm; and (C) sago diets. Spots circled and numbered are spots that are different among the diets, coconut (spots 1–3), oil palm (spots 4–6), and sago (spots 7–12).
Table 1.
List of all spots for protein identification from digestive system of RPW larvae reared on different diets as determined by LCMS/MS
| Spot number/ Diet | Accession numbera | Peptide sequence | Protein identified (using Matrix Science) | Score (%) | Protein identified (using NCBI BLAST tool) | Score (%) |
|---|---|---|---|---|---|---|
| Coconut | ||||||
| 1 | P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
56 | Enzyme: Aminopeptidase N Source: Sitophilus oryzae |
76 |
| 2 | G3QLP7 | 256- NMQDM VEDYR -265 | Protein: Uncharacterized protein Source: Gorilla gorilla gorilla |
90 | Protein: CAZy families GH58 protein Source: Uncultured Eschericia sp |
90 |
| H9Z8R8 | i)73- ISISTSGG SFR -83 ii)364- YEELQVT AGR -373 |
Protein: Keratin type II cytoskeletal 5 Source: Macaca mulata |
64 | (shown too small score in protein identification) | ||
| 3 | P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
70 | Protein: Bgal_small_N Source: Uncultured Arthrobacter sp |
70 |
| Oil palm | ||||||
| 5 | P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
57 | Enzyme: Aminopeptidase N Source: Sitophilus oryzae |
76 |
| 6 | P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
70 | Enzyme: Aminopeptidase N Source: Sitophilus oryzae |
76 |
| Sago 7 |
P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
62 | Enzyme: Aminopeptidase N Source: Sitophilus oryzae |
76 |
| 10 | P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
56 | Enzyme: Aminopeptidase N Source: Sitophilus oryzae |
76 |
| 11 | P00760 | 149- SS -150 151- GTSYPDVLK -159 |
Enzyme: Cationic trypsin Source: Bos taurus |
69 | Enzyme: Aminopeptidase N Source: Sitophilus oryzae |
76 |
Table 1 shows the list of all the selective spots that were sent for protein identification using LC-MS/MS and the database matching with the LudwigNR database. However, there were four spots which showed no significant hit due to insufficient amount of protein, they were the three spots from sago diet and one spot from the oil palm diet.
The dominant protein identified using the Matrix Science was cationic trypsin enzyme from the source of Bos taurus with different scores of spots. Interestingly, all the spots from the sago diet were identified as cationic trypsin with scores of 62% (spot 7), 56% (spot 10), and 69% (spot 11). On the other hand, spots from coconut diet (spot 2) showed two identified protein, the uncharacterized protein from the Gorilla gorilla gorilla and keratin type II cytoskeletal from Macaca mulata. Besides that, the spots were also identified using the NCBI BLAST tool, which showed different proteins or enzymes. The aminopeptidase N enzyme was the most dominant enzyme for most of the spots. Correspondingly, the sago spots showed the same enzyme—aminopeptidase N with the score of 76%, similarly spots from the oil palm diet also showed that the dominant enzyme was aminopeptidase N. However, spot from the coconut diet (spot 2) showed another identified protein, known as CAZy families GH58 protein with 90% score. Bgal_small_N from the uncultured Arthrobacter sp. was identified from the spot of the coconut diet (spot 3).
Discussion
The outcome of this study indicates that the RPW larvae highly prefer coconut cabbage, followed by oil palm and sago diets. Thus, it shows that coconut would be the most preferred and would be a better food source than oil palm and sago palm. We deduce that the coconut diet is more attractive as they supply additional nutrients such as fiber, protein and vitamins (USDA 2014); and presumably contain more nutrients than oil palm and sago palm. It has been reported that the composition of fiber in raw coconut meat is approximately 29% (USDA 2014). Chitin, one of the type of fibers is used as a basic material for exoskeleton development in adult insects (Fabritious et al. 2011). Besides, high fiber content is required for the late instar larvae to build the cocoon during the pupal stage (Wahizatul et al. 2013), which might explain why coconut diet was the most consumed rather than the other diets.
Higher protein food source which functions as a main insect body builder in the coconut diet might also be one of the reasons for the RPW larvae to consume more on the coconut instead of the oil palm and sago diets (McClure et al. 2012). Protein has also been reported to influence and improve the development of the larvae by synthesizing body tissues and hormones (Fagbohun et al. 2012). These hormones and enzymes are important during the insects’ growth as they involve the regulatory system of the digestion and maturation. According to Bong et al. (2008), red-stripe larvae (Rhynchophorus vulneratus) fed on coconut copra cake had better growth rate as the copra cake contained important protein that can improve the larvae’s development. Based on nutritional facts, the coconut also contained more vitamins and minerals (USDA 2014) which are very significant in the larvae’s development and energy production during the pupal stage (Cohen 2004). In this study, we noticed that the larvae preference was to consume on the freshly prepared food and on the softest part of a plant (coconut and oil palm cabbages). In contrast, the sago stem was the least preferred as the texture of the sago stem was quite tough and rigid compared to the other diets.
Generally, a complete life cycle of RPW from eggs to adults takes around 6–8 mo and RPW need the first to seventh instar to complete its larval development (Murphy and Briscoe 1999). In some cases, RPW might reach up to the ninth instar depending on the food sources of their host plant (Jaya et al. 2000, Ju et al. 2011). In Malaysia, a study by Yong (2015) showed that the eggs took 4–5 d to hatch, larvae duration took about 120 d, pupal duration took 21–25 d, and the complete life cycle of the RPW took approximately 4 to 5 mo.
In this study, the RPW larvae fed on oil palm cabbage reached as early as the sixth instar and took only 1 mo 9 d (39.8 ± 0.47 d) for the larval developmental time before they turned into pupa. This result suggests that oil palm could be served as a good food source besides coconut and sago palm. Ju et al. (2011) stated that the larvae may have fewer instars and the developmental time can be shortened when reared on suitable host plants. According to USDA (2014), vitamins E (alpha-tocopherol) and K are highly available in oil palm compared to the coconut even though coconut composed all types of vitamins. Vitamin E and K content in the diet can enhance the insects’ growth and development which will affect the reproductive performance of the insects such as in producing viable offspring (Parra 2012).
In contrast, the larval period reared on coconut cabbage and sago stem required a duration of up to the seventh instar. These findings were different from the previous studies. Jaya et al. (2000) found that the larval period required the duration of the seventh instar when fed on sugarcane and artificial diet, while RPW took ninth instar when reared on coconut palm. Besides, a study done by Prabhu and Patil (2009) showed that the RPW larvae reached up to the eighth instar when reared with sugarcane. Ju et al. (2011) who reared RPW on five host plants found that the larval period took eighth instar for Canary island date palm and Washington palm, while another three palms required a period of ninth instar larvae. Thus, it shows that different diets have a significant effect on the longevity and instar period of RPW. Rapid and specific target to control is required due to the fast breeding nature of RPW larvae.
The presence of certain digestive enzymes will ensure that the RPW could consume and digest these palms. The relationship between enzyme and food will determine the degree of food preference as they might choose food which could be easily digested (Lazarević and Janković-Tomanić 2015). The results from 2-DE gel indicated a total of 12 different spots that were present among the coconut, oil palm and sago diets. The different position of enzyme spots on 2-DE gel among these three diets might be due to their very specific actions against specific amino acid (substrate) presence in each food (Lazarević and Janković-Tomanić 2015). However, LC-MS/MS results based on Ludwig NR and NCBI protein database showed that the major enzyme were trypsin (cationic trypsin) and aminopeptidase, respectively. Thus, it shows that trypsin and aminopeptidase generally could digest all types of dietary ingredients tested in this study. This result was parallel to the previous studies which revealed that trypsin and aminopeptidase were the major digestive enzymes in living things including insects, and commonly play vital roles in the gut regions (Lwalaba et al. 2010, Macedo et al. 2011). Based on trypsin responsibilities in the digestion system, this enzyme or proteases could be considered as a potential candidate for the development of pest control strategies based on the antinutritional protease inhibitors (Gatehouse 2011).
Besides its role in the digestive system, aminopeptidase serves as a direct defence in the insect midgut and is known to be a crucial enzyme in plant–insect interactions (Lomate et al. 2013). In turn, it keeps RPW staying healthy and protected from any hazardous material or substance as a consequence of their feeding habitat. As these enzymes play various roles such as in digestion and other physiological reactions, there must be an effective regulatory system. This system will determine whether some reactions should be turned off while others keep running in an organized way as is necessarily needed. In this case, the enzymatic activity of the digestive enzyme can later be turned off by an enzyme inhibitor which could stop the reaction between the enzyme and the substrates (Popova et al. 2015). Thus, it is hoped that protein profiling from the digestive system of RPW will lead to the study of biological control by introducing specific inhibitor against trypsin and peptidase growth, which in turn leads to digestion failure and fatality of this coconut pest weevil.
Acknowledgments
We would like to thank the Ministry of Higher Education (MOHE) for the Exploratory Research Grant Scheme Fund (ERGS) (Vot No. 55066), School of Fundamental Science and School of Marine & Environmental Sciences at UMT for the laboratory facilities. We would also like to extend our thanks to the Plant Biosecurity Unit, Department of Agriculture of Terengganu for their excellent co-operation and supports during this study.
References Cited
- Alarcón F. J., T. F. Martínez P. Barranco T. Cabello M. Díaz, and Moyano F. J.. 2002. Digestive proteases during development of larvae of red palm weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae). Insect Biochem. Mol. Biol. 32: 265–274. [DOI] [PubMed] [Google Scholar]
- Bong C. F. J., Er C.C., Yiu P. H., and Rajan A.. 2008. Growth performance of the Red-Stripe Weevil, Rhynchophorus schach Oliv. (Insecta: Coleoptera: Curculionidae) on meridic diets. American J. Agric. Biol. Sci. 3: 403–409. [Google Scholar]
- Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Broadway R. M., and Duffey S. S.. 1985. The effect of dietary protein on the growth and the digestive physiology of larval Helios zea and Spodoptera exigua. J. Insect Physiol. 32: 673−680. [Google Scholar]
- Cohen A. C. 2004. Insects-Feeding and feeds. In A. C., Cohen (ed.), Insect diets science and technology. CRC Press, Boca Raton, Florida, USA. [Google Scholar]
- El-Mergawy A. A. M. R., and Al-Ajlan M. A.. 2011. Red palm weevil, Rhynchophorus ferrugineus (Olivier): economic importance, biology, biogeography and integrated pest management. J. Agric. Sci. Tech. 1: 1−23. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization).. 2008. Rhynchophorus ferrugineus.Bull. OEPP. 38: 55−59. [Google Scholar]
- Fabritious H., Sachs C., Raabe D., Nikolov S., Friak M., and Neugebauer J.. 2011. Chitin in the exoskeleton of arthropoda: from ancient design to novel material science. In N. S., Gupta (ed.), Chitin. Springer, Netherlands. [Google Scholar]
- Fagbohun E. D., Egbebi A. O., and Lawal O. U.. 2012. Phytochemical screening, proximate analysis and in-vitro antimicrobial activities of methanolic extract of Cnidoscorus Aconitifolius leaves. Int. J. Pharm. Sci. Rev. Res. 13: 28−33. [Google Scholar]
- Faleiro J. R. 2006. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Ins. Sci. 26: 135−154. [Google Scholar]
- Gatehouse J. A. 2011. Prospects for using proteinase inhibitors to protect transgenic plants against attack by herbivorous insects. Curr. Protein Pept. Sci. 12: 409–416. [DOI] [PubMed] [Google Scholar]
- Jaya S., Suresh T., Sobhita R. R. S., and Sreekumar S.. 2000. Evidence of seven larval instars in the red palm weevil, Rhynchophorus ferrugineus Oliv. reared on sugarcane. J. Entomol. Res. 24: 27−31. [Google Scholar]
- Ju R. T., Wang F., and Wan F. H.. 2011. Effect of host plants on development and reproduction of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). J. Pest Sci. 84: 33−39. [Google Scholar]
- Kaakeh W. 2005. Longevity, fecundity, and fertility of the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) on natural and artificial diets. Emirates J. Agric. Sci. 17: 22−23. [Google Scholar]
- Lazarević J., and Janković-Tomanić M.. 2015. Dietary and phylogenetic correlates of digestive trypsin activity in insect pests. The Netherlands Entomological Society. 157: 123–151. [Google Scholar]
- Lomate P. R., B. R. Jadhav A. P. Giri, and Hivrale V. K.. 2013. Alterations in the Helicoverpa armigera midgut digestive physiology after ingestion of pigeon pea inducible leucine aminopeptidase. Plos One. 8: e74889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwalaba D., K. H. Hoffmann, and Woodring J.. 2010. Control of the release of digestive enzymes in the larvae of the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 73: 14–29. [DOI] [PubMed] [Google Scholar]
- Macedo M. L. R., Filho E. B. S. D., Freire M. G. M., Oliva M. L. V., Sumikawa J. T., Toyama M. H., and Marangoni S.. 2011. A trypsin inhibitor from Sapindus saponaria L. seeds: purification, characterization and activity towards pest insect digestive enzyme. Protein Journal. 30: 9−19. [DOI] [PubMed] [Google Scholar]
- Manachini B., V. Arizza D. Parrinello, and Parrinello N.. 2011. Hemocytes of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) and their response to Saccharomyces cerevisiae and Bacillus thuringiensis. J. Invertebr. Pathol. 106: 360–365. [DOI] [PubMed] [Google Scholar]
- McClure M., Morcos L., and Despland E.. 2012. Collective choice of a higher-protein food source by gregarious caterpillars occurs through differences in exploration. International Soc. Behav. Ecol. 24: 113−118. [Google Scholar]
- Mohammadi D., Pour Abad R. F., Rashidi M. R., and Mohammadi S. A.. 2010. Study of cotton bollworm, Helicoverpa armigera hubner (Lepidoptera: Noctuidae) using Dyar’s rule. Munis Entomol. Zool. 5: 216−224. [Google Scholar]
- Murphy S. T., and Briscoe B. R.. 1999. The red palm weevil as an alien invasive: biology and the prospects for biological control as a component of IPM. Biocontrol News and Information. 20: 35−46. [Google Scholar]
- Nguyen T. T., Michaaud D., and Cloutier C.. 2007. Proteomic profiling of aphid, Macrosiphum euphorbiae responses to host-plant mediated stress induced by defoliation and water deficit. J. Ins. Physiol. 53: 601−611. [DOI] [PubMed] [Google Scholar]
- Nirula K. K. 1956. Investigations on the pests of coconut palm, Part IV Rhynchophorus ferrugineus.Indian Coconut Journal. 9: 229–247. [Google Scholar]
- Parra J. R. P. 2012. The evolution of artificial diets and their interactions in science & technology. InPanizzi A. R. and J. R. P. Parra (eds.), Insect bioecology and nutrition for integrated pest management. CRC Press, Boca Raton, Florida, USA. [Google Scholar]
- Popova V. V., Dunaevsky Y. E., Domash V. I., Semenova T. A., Beliakova G. A., and Belozersky M. A.. 2015. Some properties and possible biological role of peptidase inhibitors from the entomopathogenic fungus Tolypocladium cylindrosporum. Arch. Microbiol. 1: 1−10. [DOI] [PubMed] [Google Scholar]
- Prabhu S. T., and Patil R. S.. 2009. Studies on the biological aspects of red palm weevil, Rhynchophorus ferrugineus (Oliv.). Karnataka J. Agric. Sci. 22: 732−733. [Google Scholar]
- USDA (United States Department of Agriculture) .2014. https://ndb.nal.usda.gov/ndb/foods/show/3656?fgcd=&manu=&lfacet=&format=&count=&max=35&offset=&sort=&qlookup=coconut National nutrient database for standard reference release 28. California, United States of America.
- Wahizatul A. A., Zazali C., Abdul Rahman A. R., and Nurul ‘Izzah A. G.. 2013. A New invasive coconut pest in Malaysia: the red palm weevil (Curculionidae: Rhynchophorus ferrugineus). The Planter. 89: 97−110. [Google Scholar]
- Wahizatul A. A., Shahrol N. D., Haris M. H., Yong K. W., Zazali C., and Ahmad S. S.. 2014. Field trapping of adult red palm weevil, Rhynchophorus ferrugineus olivier (Coleoptera: Curculionidae) with kairomone-releasing food baits and synthetic pheromone lure in a coconut plantation. Philip. Agric. Sci. 97: 342−348. [Google Scholar]
- Yapi Assoi Yapi D., D. Gnakri S. Lamine Niamke, and Patrice Kouame L.. 2009. Purification and biochemical characterization of a specific beta-glucosidase from the digestive fluid of larvae of the palm weevil, Rhynchophorus palmarum. J. Insect Sci. 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K. W., Aisyah A. B., and Wahizatul A. A.. 2015. Fecundity, fertility & survival of Red Palm Weevil (Rhynchophorus ferrugineus) larvae reared on sago palm. Sains Malaysiana. 44: 1371−1375. [Google Scholar]