Abstract
TNF is a proinflammatory cytokine with established roles in host defense and immune system organogenesis. Here we report a novel physiological function of TNF that extends its effect beyond the host into the developing offspring. A partial/complete maternal TNF-deficit, specifically in hematopoietic cells, resulted in reduced milk levels of chemokines IP-10, MCP-1/−3/−5, and MIP-1β, which in turn, augmented offspring postnatal hippocampal proliferation, leading to improved adult spatial memory in mice. These effects were reproduced by the postpartum administration of a clinically used anti-TNF agent. Chemokines, fed to suckling pups of TNF-deficient mothers, restored both postnatal proliferation and spatial memory to normal levels. This work identifies a TNF-dependent “lactrocrine” pathway that programs offspring hippocampal development and memory. The level of ambient TNF is known to be downregulated by physical activity/exercise and adaptive stress; thus, we propose that the maternal TNF-milk chemokine pathway evolved to promote offspring adaptation to post-weaning environmental challenges/competition.
Tumor necrosis factor-α (TNF) is a cytokine involved in a diverse array of immune functions. It is present in low physiological levels, but is multiplied in various immune and inflammatory conditions. Genetic inactivation of the TNF gene allowed the identification of corresponding functions in host defense and homeostasis1, 2. TNF deficient (TNF−/−) mice succumb to infection with Listeria monocytogenes, and are highly susceptible to Candida albicans and M. tuberculosis1, indicating a significant protective role for the induced levels of TNF in combating pathogens. TNF−/− mice (under unperturbed conditions) also helped identify developmental and homeostatic functions of TNF. These include a role in the organogenesis of Peyer’s patches2, fine organization of secondary lymphoid tissues and maturation of the humoral immune response1. These abnormalities, under normal laboratory conditions, do not cause overt developmental problems and the growth and fertility of TNF deficient mice are normal1.
Besides the immune system, TNF is also produced in the brain. Inflammation and immune activation have been shown to greatly increase the production of TNF by microglia, astrocytes, and neurons, and this mechanism has been implicated in neuronal damage caused by brain insults such as stroke, head trauma, and neurodegenerative disorders3. TNF is also present, although at a very low level, in the normal brain and is constitutively released in both primary neuronal culture and acute hippocampal slices, indicating a physiological role for ambient TNF in the brain. Indeed, glial TNF has been identified as one of the regulators of excitatory synaptic scaling4.
A physiological role of TNF in brain function and/or development is also indicated by the behavioral abnormalities exhibited by TNF null mice. In one study, TNF−/− mice showed improved performance in spatial memory and learning tasks5. However, in another report, TNF−/− mice showed no change in spatial learning6. The use of non-littermate5 and littermate6 controls could explain these discrepancies because in a non-littermate comparison the parental genotype can significantly influence the offspring genotype effect7. Indeed, we found that a deficit in maternal TNF has a more profound effect on offspring episodic memory than a deficit in the offspring’s own TNF. We then found reduced levels of a group of chemokines in the milk of TNF-deficient mothers, which, when given in supplemental doses, normalized cognitive functions. Further data suggested that the maternal TNF-regulated lactocrine pathway is evolutionarily adaptive and that TNF suppressive drugs taken during the postpartum period may alter the functionality of this pathway, and consequently, life-long spatial memory.
RESULTS
Parental TNF deficit increases spatial reference memory
To evaluate the effect of maternal/parental TNF on offspring behavior, wild type (WT) male offspring were generated by either crossing WT or TNF+/− (heterozygote; H) parents1, all on the C57BL/6NTac (B6Tac) genetic background (Supplementary Fig. 1a). While the WT offspring of WT parents (referred to as WToffspring(WTparents/mother); i.e. WT(WT) animals) develop in WT parental environment, the WT offspring of H parents (WT(H)) are exposed to receptor deficient maternal environment both pre- and postnatally. These two groups of offspring were complemented with TNF−/− (KO) offspring obtained by crossing either TNF+/− or TNF−/− parents, referred to as KO(H) and KO(KO) animals.
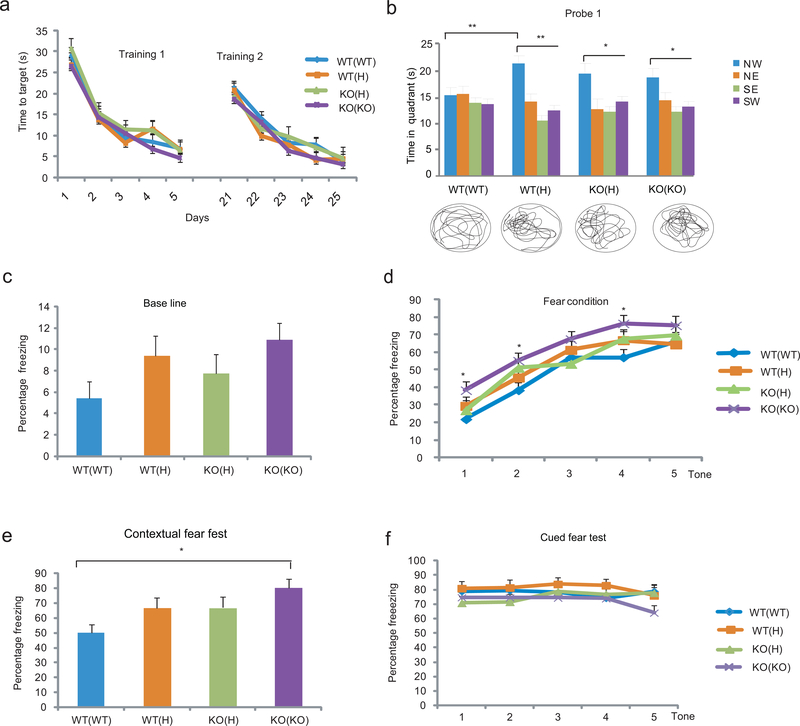
We assessed the spatial learning and memory capabilities of these offspring in the Morris water maze (MWM). While five consecutive training sessions in the MWM (Supplementary Fig. 1b) resulted in the gradual learning of the location of the invisible platform in all groups (Fig. 1a), the probe trial on day 6 (in which the platform was removed) showed a difference between the groups in their ability to recall the location of the platform (Fig. 1b). Specifically, the WT(WT) offspring failed to recall the location, while the WT(H) offspring spent significantly more time in the North-West (NW) quadrant that previously contained the platform. KO(H) and KO(KO) mice were also able to recall the platform location (Fig. 1b). An additional 5 days of training resulted in the recall of the platform location in probe trial 2 in all groups, thus the WT(WT) group is not incapable of learning, but rather requires more training under our experimental conditions in order to recall memories; regardless of maternal genotype, the effect on recall was still present (Supplementary Fig. 1b, c). No group difference was seen in the visible platform task and in total distance traveled in the MWM (not shown).
Figure 1.
Maternal TNF deficit enhances cognitive functions in the offspring. (a) Training in the MWM with hidden platform. Although there was an effect of session in both the first and second training period (Repeated measures ANOVA: F4,752=73.38, P<10−5 and F4,640=45.29, P<10−5, respectively; N= 8,11,12,13/group), there was no genotype or genotype x session effect and consequently no significant difference between the groups at any time point. Data are from two independent experiments Data are shown as means ± SE. (b) In probe trial 1, two way ANOVA showed a significant effect of quadrant (F3,168=11.85, P<10−5) and LSD posthoc. test indicated that only the offspring of H and KO parents (but not those of WT parents) spent more time in the NW quadrant, the location that previously contained the platform (*p<0.05, **<0.005, ***<0.0005, NS=not significant; N= 8,11,12,13/group). To be considered as a significant change, time in NW target quadrant had to be significantly different from the time in all three other quadrants (lowest significance level among the three is displayed). The trace of movements is also displayed at the bottom of the panel. NW, North-West; NE, North-East; SE, South-East; SW, South-West. (c) Contextual and cued fear responses of the offspring of TNF H and KO parents. Baseline freezing (before conditioning) was not significantly different between the groups (ANOVA: F3,47=2.13, P=0.11; N=11,11,14,15/group). Data from three independent experiments (d) There was both a session- and a genotype-effect on freezing during context conditioning (Repeated measures ANOVA: F4.188=64.43, P<10−5 and F3.47=3.70, P=0.018, respectively) with KO(KO) offspring showing increased freezing compared to WT(WT) and WT(H) animals at the indicated time points (LSD posthoc; *p<0.05 vs. WT(WT) and #<0.05 vs. WT(H)). However, at the end of the training, the KO(KO) group was no longer different. (e) KO(KO) offspring showed an increase in contextual fear response (ANOVA: F3,47=4.25, P=0.010. Dunett T3 posthoc test; *p=0.006. When baseline measures were included as a covariate, the increase in contextual fear reaction in KO(KO) mice was still significant P=0.022). (f) The TNF deficient maternal environment has no effect on cued fear conditioning (F3,47:= 1.06, P=0.38).
Associative memory of WT mice of mutant parents was assessed in contextual fear conditioning (Supplementary Fig. 1d) which, similarly to MWM, requires hippocampal activity8; albeit the two tests differ in sensory and motor demands, as well as motivational aspects. The parental TNF genotype had a significant effect in the contextual, but not cued, fear test (Fig. 1c-f), suggesting a hippocampal, but not amygdala, related change in the offspring. However, the effect was seen only when maternal TNF was completely eliminated (KO). Although the KO(KO) offspring also exhibited increased freezing during fear conditioning (Fig. 1d), this effect disappeared by the end of the training and before the contextual fear testing began.
We concluded that the partial and/or complete genetic inactivation of TNF in the parents, independently of the presence or absence of TNF in the offspring, facilitates the acquisition/recall of hippocampal dependent memory beyond the normal physiological level in the adult offspring.
Maternal hematopoietic TNF is linked to offspring memory
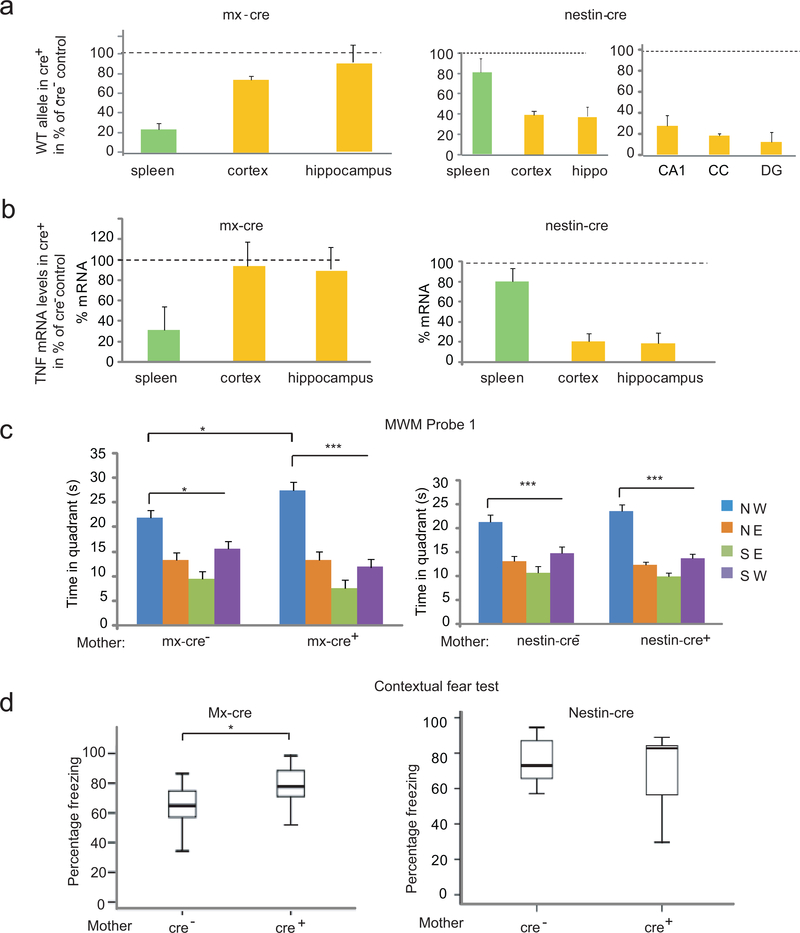
The major source of TNF is the immune system (macrophages/neutrophils and lymphocytes), but TNF is also expressed in the CNS (in glia and neurons)4. We inactivated maternal TNF in the hematopoietic system and brain by using a “floxed” TNF allele in combination with the polyIC inducible mx-cre and the nestin-cre transgenes, respectively (Supplementary Fig. 2a, all C57BL/6J (B6J) mice). Real time PCR based quantification of the TNF allele showed its extensive deletion in the spleen of 9 week old mx-cre females (TNFfloxf/flox; mx-cre+), injected with polyIC three weeks earlier, while the allele was largely intact in the cortex and hippocampus (Fig. 2a). In nestin-cre (TNFflox/flox; nestin-cre+) females on the other hand, TNF was deleted in the cortex, hippocampus, isolated CA1 and dentate gyrus (DG) neurons, and glia rich corpus callosum (CC), while its level in spleen remained close to that of the WT (Fig. 2a). PCR quantification of TNF mRNA levels confirmed the spleen and brain specific gene deletions in the mx-cre+ and nestin-cre+ animals, respectively (Fig. 2b). Consistent with these results, control (mx-cre–) spleen contained 378+50 pg TNF per g protein, as measured by ELISA, while the level in mx-cre+ spleen was undetectable (<70 pg/g), indicating the elimination of at least 80% of the TNF protein. The hematopoietic specificity of mx-cre mediated recombination was further verified by the cre-reporter Gt(ROSA)26Sortm1Sor/J strain9 (Supplementary Fig. 2b). Taken together, mx-cre and nestin-cre resulted in the tissue specific deletion of the TNF allele and reduction of TNF mRNA and protein, beyond 50%, which is the level sufficient to produce the spatial memory phenotype.
Figure 2.
Hematopoietic system specific inactivation of the TNF gene in the mother results in enhanced memory. (a) Inducible mx-cre expression results in recombination at the floxed TNF WT allele seen as a reduction in the overall level of the WT allele in the spleen but not in brain. In contrast, nestin-cre expression results in a substantial reduction of the WT allele in brain (e.g. cortex and hippocampus), in neurons (e.g. CA1 and DG of the hippocampus) and glia (e.g. corpus callosum=CC) but not in spleen. Hip=hippocampus. Data are shown as means ± SE. (b) Reduction of TNF mRNA is limited to the spleen in mx-cre+ animals while it is brain specific in nestin-cre+ mice. (c) Probe trial 1 of the MWM with the offspring of mx-cre+ and nestin-cre+ mothers. There was an effect of the platform location in both groups of animals (ANOVA: F3,56=35.69, P<10−5 and F3,80=42.43, P<10−5, respectively; N=7,9 and N=9,13 /group), but group x location interaction was seen only with the offspring of mx-cre+ mothers (F3,56=3.09, P=0.03) and LSD test showed that offspring of mx-cre+ as compared to the offspring of mx-cre- mothers had an increased memory of the platform location (*p<0.05;*** p< 0.0005). Data from three independent experiments (d) Increased freezing of the offspring of mx-cre+ (N=13,13/group) but not nestin-cre+ mothers (N=7,10/group) during contextual fear testing, t-test *p=0.014 and p=0.42 for mx-cre and nestin-cre, respectively. Box-whisker plots represent the first three quartiles (25%, median and 75%) and values 1.5× the interquartile range below the first quartile (lower horizontal line) and above the third quartile (upper horizontal line).
Male offspring of polyIC mx-cre+ TNFflox/flox mothers, as compared to those of mx-cre– mothers, showed a better than normal performance in the probe trial (Fig. 2c) and training sessions (Supplementary Fig. 3a), while the performance of the offspring of nestin cre+ and nestin cre–mothers was comparable (of note, mice of the B6J substrain, used in these experiments, achieved learning with five training sessions that was suboptimal in B6Tac mice; used in previous experiments; see Fig. 1b). Five days of additional training improved memory in all groups, resulting in no difference between the offspring of mx-cre+ and mx-cre– mothers (Supplementary Fig. 3b). Offspring of polyIC mx-cre+ TNFwt/wt mothers had no enhanced learning/memory, indicating no confounding effects of polyIC and mx-cre on their own (Supplementary Fig. 3c). Offspring of mx-cre+, but not nestin-cre+, mothers also showed increased freezing during contextual fear testing (Fig. 2d) and tone-shock pairings (Supplementary Fig. 3d), but not during cued fear testing (not shown). In summary, a TNF deficit that is limited to the maternal hematopoietic system is sufficient to augment both spatial and associative memory beyond their normal physiological levels in the offspring.
Maternal TNF regulates hippocampal proliferation
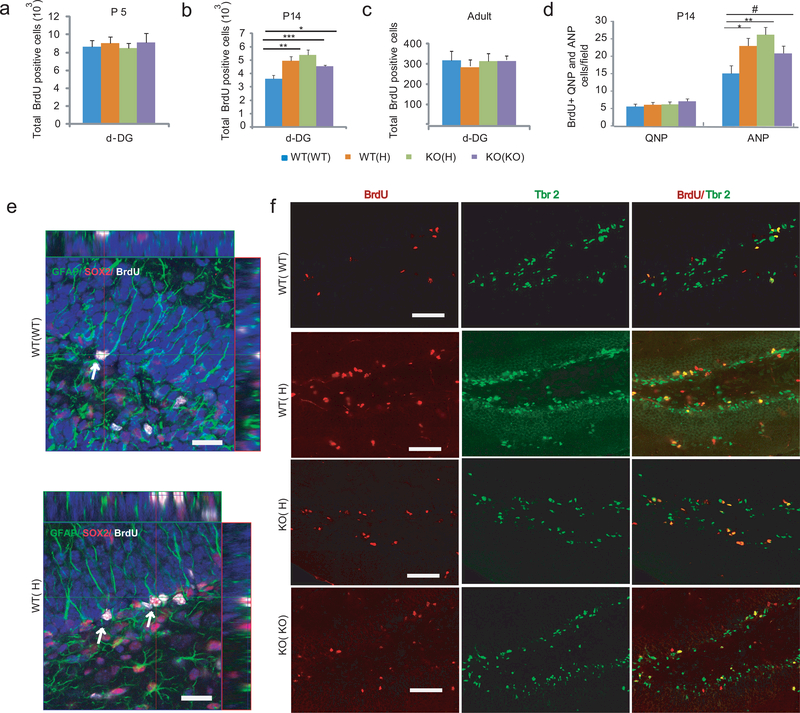
Both hippocampal spatial and associative memory are positively correlated with neuronal proliferation in the adult dentate gyrus DG10. Here we tested if this correlation applies to the offspring of TNF deficient mothers exhibiting higher than normal hippocampal dependent memory. Using BrdU labeling, we found increased proliferation in postnatal day (P) 14 WT(H), as well as KO(H) and KO(KO), offspring, but not at P5, P35, and adult (Fig. 3a-c and not shown). At P14, the total number of granule cells (DAPI+ cells) was also increased slightly (significant in KO(H), but only trend in WT(H) and KO(KO); Supplementary Fig. 4a), proportionately with the increase in the number of BrdU positive cells (Supplementary Fig. 4b); thus, the fraction of BrdU positive cells was comparable across the groups and represented 6.7–7.8% of all neurons in the granule cell layer (GCL)(Supplementary Fig. 4c). However, the total cell number in the GCL was normalized by the time animals reached adulthood, similar to that of the BrdU positive cells. Increased proliferation at P14 was specific for the dorsal DG because other neurogenic areas such as the hilus, the subventricular zone (SVZ), and rostral migratory stream (RSM) showed no maternal genotype dependent change in the offspring of mutant mothers (Supplementary Fig. 4d,e). The number of “surviving” BrdU positive cells in the DG, 3 weeks after labeling at P14 (>95% NeuN+), was not different among the groups (Supplementary Fig. 4f); thus, the early overproduction of cells is later compensated. The volume of the GCL, SVZ and RMS was not changed at any of the time points investigated (Supplementary Fig. 5a-h). The number of GFAP+ astrocytes and Iba+ microglia, in the hippocampus and cortex, was not changed either (Supplementary Fig. 5i-k). The augmented proliferation in the P14 WT(H) DG was due to an increased number of amplifying neuronal progenitors (ANPs; BrdU+, Tbr2+11), representing the majority (~80%) of BrdU+ cells, rather than to an increase in quiescent neuronal progenitors (QNPs; BrdU+/GFAP+/Sox-2+11; ~10% of all BrdU+ cells)(Fig. 3d-f). Overall, these data suggest that the increase in proliferation in the offspring of mutant mothers is transient and specific for the DG.
Figure 3.
Increased proliferation in the developing DG is linked to enhanced adult spatial memory in the offspring of TNF mutant mothers. (a-c) Proliferation in the dorsal (d) subgranular zone (SGZ) at P5, P14 and adult as measured 2h after BrdU labeling. At P14, ANOVA showed a group difference in the number of BrdU positive cells (F3,16=7.38, P=0.003, N=5/group; LSD posthoc. *<0.05, **<0.005, ***<0.0005). Data are shown as means ± SE. (d) Maternal TNF genotype has no effect on QNP proliferation (F3,16=0.69, P=0.57) but increases ANP proliferation (F3,56=7.9, P<0.001; N=5/group; LSD posthoc. *p<0.05, **<0.005, #<0.1) at P14. (e) Confocal micrographs of WT(WT) and WT(H) SGZs with arrows showing QNPs characterized by BrdU and Sox-2 positivity and GFAP positive apical extensions toward the molecular layer of the DG. Bar=20μm. (f) Representative micrographs showing an increased number of BrdU+/Tbr2+ ANPs in the SGZ of WT(H), KO(H) and KO(KO) offspring as compared to the WT(WT) offspring at P14. Bar=50μm.
Hippocampal proliferation is linked to memory
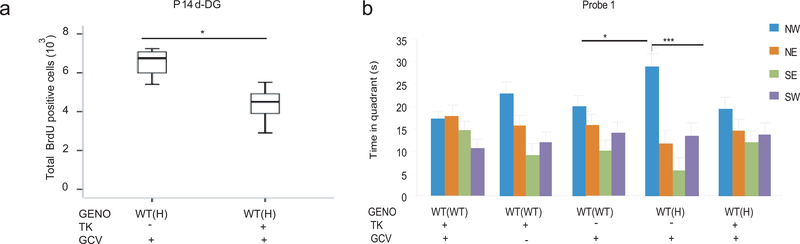
We then tested if reducing the excess ANPs in the WT(H) DG to a normal level during the late postnatal period by gancilovir (GCV) in TK (thymidine kinase, expressed under the control of GFAP promoter in neuronal precursors) transgenic B6Tac mice can normalize the level of spatial memory to the WT level. First, we determined that a partial, approximately 30%, reduction in proliferation, that would be sufficient to compensate the increased proliferation in WT(H) mice (Fig. 3b), can be accomplished by a short, two day (P5+6) treatment, with a low 12.5 mg/kg i.p. dose of GCV (Supplementary Fig. 6a,b). This regimen caused no change in postnatal weight gain and overall development (Supplementary Fig. 6c), reduced the proliferation of ANPs (Tbr2+) but not QNPs (GAFP+,Sox2+)(Supplementary Fig. 6d-g), caused no sustained/adult effect on proliferation (Supplementary Fig. 7a), and did not reduce the number of GFAP positive astrocytes in adult brain (Supplementary Fig. 7b). These data show that GCV and the TNF deficient maternal environment target the same population of neuronal precursors and have the same time course but with opposite effects; thus, GCV can be used to compensate the temporal increase in proliferation caused by the maternal TNF genotype. Indeed, GCV reduced proliferation in WT(H) TK+ P14 pups by approximately 30% (Fig. 4a) and also normalized the level of adult spatial reference memory to the WT level (Figure 4b; WT(H)-GCV vs. WT(H)-TK-GCV and WT(H)-TK-GCV vs. all WT(WT) controls). These data link the temporal increase in postnatal proliferation to enhanced adult spatial reference memory in the offspring of TNF deficient mothers.
Figure 4.
Genetic compensation of increased proliferation in the WT(H) offspring normalizes spatial memory. (a) GCV administered to neonates at P5 and P6 (12.5 mg/kg s.c.) resulted in an approximately 30% reduction in proliferation in P14 WT(H) offspring (F1,9=13.8, P=0.008; N=5,12/group). Box-whisker plots represent the first three quartiles (25%, median and 75%) and values 1.5× the interquartile range below the first quartile (lower horizontal line) and above the third quartile (upper horizontal line). Data are from two independent experiments. (b) In probe trial 1, control WT(WT) offspring with or without TK and in the presence or absence of GCV failed to learn the platform location. WT(H) offspring with no TK but injected with GCV could recall the platform location (two way ANOVA: quadrant F3,148=21.27, P<0.0001; group x quadrant, F12,148=1.9, P=0.04; N=6,7,8,10,11/group; LSD posthoc, *p<0.05, ***<0.0005). However, these offspring, if also harboring the TK gene, showed no learning. Data are shown as means ± SE.
Maternal TNF deficit causes adult hippocampal changes
Next we tested if there are persistent functional and/or structural changes in DG granule cells as a result of the transient maternal effect and which can potentially explain the enhanced memory of adult WT(H) mice. RNA-Seq based expression profiling of WT(H) vs. WT(WT) adult granule cells identified 121 upregulated and also 121 downregulated genes (>2.5 fold; Q<0.01). Gene ontology analysis showed that the functional category most significantly enriched in differentially expressed genes was “Neurotransmission at synapses” (p=6.48E-6; Ingenuity Pathway Analysis), consisting of 6 genes that were all substantially upregulated in WT(H) granule cells (Supplementary Table 1). We were surprised to find that five of these genes are linked to acetylcholine (ACh), an excitatory neurotransmitter with a role in encoding new episodic memories in the hippocampus12. Specifically, the genes encode the nicotinic cholinergic receptor α3, β3, and β4 subunits (Chrna3, 7.2-fold increase; Chrnb3, 14.8-fold; Chrnb4, 16.4-fold) that can combine and form pentameric ACh receptors13, a solute carrier family 5 protein that transports choline (ChT) from outside to inside of the cell (Slc5a7, 7.7-fold), and choline O-acetyltransferase (Chat, 14.6-fold), the ACh synthetic enzyme. An additional gene encodes α-synuclein (Sncg, 2.9-fold), a presynaptic vesicular membrane protein with a physiological role in neurotransmitter release. Although the cholinergic genes are either lowly expressed or not present in adult granule cells, in situ hybridization data demonstrate their robust expression in postnatal DG, at P4 and P14 and, at reduced levels, at P28, with no or very low expression in P56 adult DG (Allen Developing Mouse Brain Atlas; http://developingmouse.brain-map.org). Similarly, α-synuclein is expressed in young, but not mature, granule cells in the DG14. It has been proposed that ACh receptors in young granule neurons respond to cholinergic inputs from the forebrain that densely innervate the subgranule zone and could contribute to their high excitability15. Retention of Ach receptor expression in adult WT(H) neurons suggests their extended excitability that could contribute to enhanced spatial memory12. The increased expression of Slc5a7 and Chat in WT(H) granule cells, by producing ACh, could also contribute to the increased hippocampal memory. It is of note that acetylcholinesterase inhibitors, by preventing the degradation of ACh, are used in the management of Alzheimer’s disease. Overall, these data indicate that the developmentally programmed transcriptional silencing of some early synaptic genes, in particular cholinergic genes, fails in WT(H) granule neurons during their maturation, which may have a long term effect on neuronal network function and encoding new episodic memories in the hippocampus.
A broader functional category of “Neurotransmission”, which includes non-synaptic transmission as well, was also enriched in differentially expressed genes (although less significantly; p=1.91E-4)(Supplementary Table 1). Besides of the cholinergic genes listed above, this category contained almost exclusively genes encoding neuropeptides and their receptors, including an opioid receptor (Npbwr1), galanin (Gal), hypocretine (orexin)( Hcrt1), somatostatin (Sst) and its receptor (Sstr). In contrast to the cholinergic genes, the neuropeptide related genes were all downregulated in WT(H) neurons (2.5–8.0-fold). Since the effects of neuropeptides on excitatory transmission are modulatory in general, with inhibition on glutamate release and excitation16; their downregulation in WT(H) granule cells could add to the excitatory effect of the upregulated cholinergic genes.
A similar expression profiling of CA1 pyramidal neurons from adult WT(H) vs. WT(WT) mice yielded 27 up- and 121 downregulated genes. Functional analysis returned no highly significant clustering of genes (p<E-5), and the overlap in differentially expressed genes between the DG granule and CA1 pyramidal neuron was only 17%. Finally, only the cholinergic Chrna4 gene, first identified as upregulated in WT(H) granule cells, showed differential expression in WT(H) CA1 pyramidal cells, but the direction of change was opposite. These data suggest that CA1 pyramidal neurons are not affected the way the DG granule cells are impacted by the maternal TNF deficit.
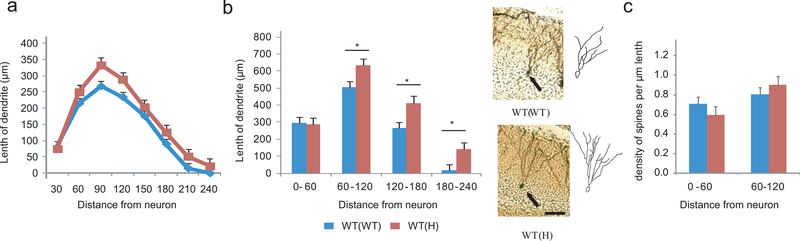
Another neuronal characteristic that is modulated by early life environment and which can also be correlated with cognitive performance is dendritic morphology/complexity17. Golgi-Cox staining and Sholl analysis of the dendritic length of DG granule cells of adult WT(H) and WT(WT) offspring showed a maternal genotype effect (Figure 5a). While the proximal one fourth of the arbor (between 0 and 60 μm from the neuron) were similar, WT(H) neurons exhibited longer dendritic length in more distal areas (60–240 μm)(Figure 5b). Although spine density was not significantly changed either in the proximal or at more distal areas in the arbor (Figure 5c), the longer dendritic length indicates an increased number of spines, and presumably synaptic contacts, per neuron. These data suggest that the TNF deficient maternal environment leads to permanent morphological changes in DG granule cells that could contribute to the enhanced cognitive performance of WT(H) mice (see discussion).
Figure 5.
Dendritic morphology-changes in adult WT(H) granule cells. (a) Dendritic length as a function of distance from the neuron in adult WT(WT) and WT(H) neurons. Two way ANOVA: group F1,288=10.94, P=0.0001. Neuron number=16,23 per genotype. Data are shown as means ± SE. (b) Increased dendritic length at distal areas in adult WT(H) granule cells. Two way ANOVA: group F1,148=13.42, P=0.001; LSD posthoc, *p<0.05. Representative images and corresponding tracings of a WT(WT) and a WT(H) neuron. Scale bar=20μm. (c) Spine density in adult WT(H) and WT(WT) granule cells. Two way ANOVA: group F1,66=0.13, P=0.91; distance from neuron F1,66=6.62, P=0.012. Neuron number=14,21 per genotype.
The maternal TNF genotype-effect is postnatal
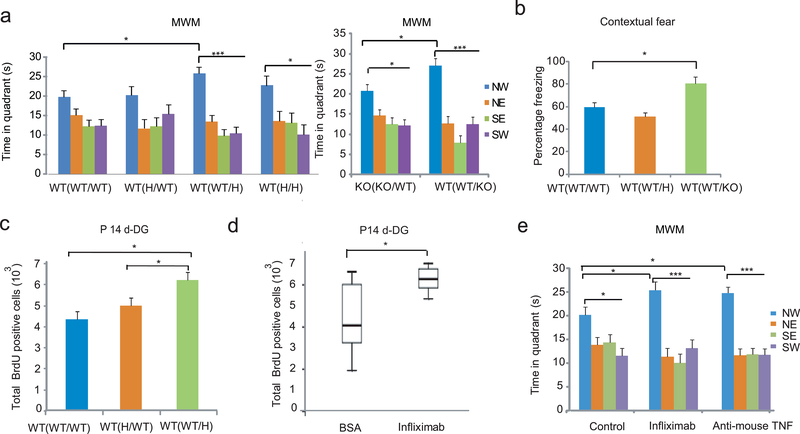
Crossfostering B6Tac WT(WT) pups to TNF+/− or TNF−/− mothers (WToffspring(WTpre/Hpostnatal) and WT(WT/KO)) within 24hr of birth resulted in the recall of the platform location in probe trial 1 in MWM, while offspring crossfostered to WT mothers (WT(WT/WT)), as expected, failed to identify the platform location (Figure 6a). WT mice exposed to prenatal TNF+/− maternal environment (WT(H/WT) also failed to recall the platform location. Although TNF−/− pups crossfostered to WT mothers (KO/KO/WT) recalled the platform location, the level of memory was significantly lower than that of WT pups crossfostered to TNF−/− mothers (WT(WT/KO)). Overall, these data indicate that the postnatal TNF deficient maternal environment is required for the high level of adult spatial memory, observed in WT(H) mice when limited training is used (in probe trial 1).
Figure 6.
A. The maternal effect on offspring phenotypes is postnatal. (a,b) Association of the postnatal TNF deficient maternal environment with enhanced cognitive performance in MWM (Two way ANOVA; platform locationleft: F3,124=21.70, P<10−5, N= 12,6,12,5/group; platform locationright: F3,48=26.050, P=0.0001 and location x group F3,38=3.78, P=0.016, N=8,6; LSD posthoc. *p<0.05; ***<0.0005) and fear conditioning (ANOVA: F2, 21=4.32, P=0.03; LSD posthoc *p<0.05; N= 6,7,10). Data are shown as means ± SE. (c) Association of the postnatal TNF deficient maternal environment with increased proliferation in P14 DG (ANOVA: F2,16=6.3, P=0.01; N= 6,7,6; LSD posthoc. *p<0.05). (d) Postpartum administration of infliximab increases P14 DG proliferation in the offspring (t-test, T=2.709 p=0.014, N=14,6). Data are from two independent experiments. Box-whisker plots represent the first three quartiles (25%, median and 75%) and values 1.5× the interquartile range below the first quartile (lower horizontal line) and above the third quartile (upper horizontal line). (e) Offspring of infliximab and anti-mouse TNF antibody treated mothers had a higher level of recall of the platform location in probe trial 1 than that of the control offspring (Two way ANOVA; quadrant: F3,132=35.93, P<10−5; group x quadrant: F6,132=2.14, P=0.05; N=16,9,11/group; LSD posthoc. *p<0.05; ***p<0.0005). Controls, derived from mothers injected postnatally with either BSA or IgG1 isotype control antibodies, were pooled as their behavior did not differ.
A postnatal exposure was also sufficient to enhance fear contextual memory, but only when the offspring were crossfostered to TNF−/− mothers (Figure 6b); an effect which is in agreement with previous data (Figure 1a). Finally, proliferation in DG was increased at P14 when exposure to the TNF+/− maternal environment was limited to the postnatal period (Figure 6c), consistent with a causative link between postnatal proliferation and adult memory shown in Figure 4b. These postnatal effects could be due to altered maternal care/behavior18, but TNF deficient mothers exhibited no apparent changes in any of the established “maternal care behaviors” including arched-back nursing and licking/grooming of the pups during the light and dark periods, latency to retrieve pups, and nest building behavior (Supplementary Fig. 8a-f), suggesting a non-behavioral transmission.
Postpartum anti-TNF elicits the offspring phenotypes
Next we tested if infliximab, a chimeric mouse-human TNF antibody19 that is widely used in the treatment of autoimmune and chronic inflammatory diseases, can reproduce the offspring effects seen with the genetic deletion of maternal TNF, when administered to lactating mothers. In contrast to receptor based anti-TNF agents, infliximab is selective to TNF as it does not neutralize the similar cytokine lymphotoxin alpha20. Infliximab neutralizes mouse TNF21 and it has been used to prevent TNF-induced inflammatory responses in numerous acute and chronic rodent models22,23. Due to its long half life, infliximab is typically administered once a week at 10μg/g concentration for chronic effect22,23. Administration of the drug at postpartum days 1 and 7 to B6Tac mothers increased DG proliferation in the P14 offspring (Figure 6d). Furthermore, adult offspring of mothers treated at postpartum days 1, 7 and 14 with infliximab or anti-mouse TNF antibodies exhibited a higher level of spatial memory in the MWM than the control offspring (Figure 6e). Controls were pooled from offspring born to mothers injected postnatally with either BSA or IgG1 isotype control antibodies because their behavior did not differ statistically. There was no drug effect in offspring fear responses (not shown) presumably because this effect requires a complete or nearly complete inactivation of maternal TNF (Figure 1e). These data indicate that non-genetically induced maternal TNF deficit during the postpartum period is sufficient to elicit the hippocampal and spatial memory changes, previously seen in the offspring crossfostered to constitutive TNF deficient mothers.
TNF deficit alters the chemokine composition of milk
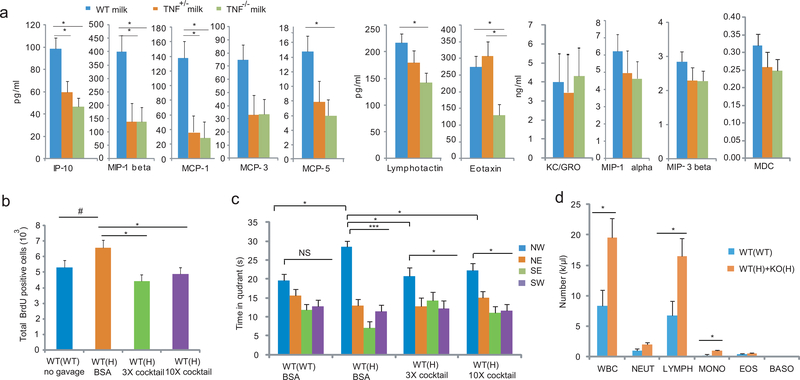
A conceivable postnatal non-behavioral mechanism of the maternal TNF genotype-effect may involve milk. As reported24, TNF was below the detectable levels in postpartum day 2 mouse milk of WT mothers (<30 pg/ml, ELISA). However, we detected significantly reduced levels of IP-10, MIP-1β, MCP-1, and MCP-5 (MCP-3 was significantly reduced only when uncorrected for multiple testing) in both TNF+/− and TNF−/− milk; a maternal genotype dependent pattern, similar to that was seen with increased proliferation and enhanced MWM cognitive performance in the offspring (Figure 7a). In contrast, the levels of lymphotactin and eotaxin were reduced only in TNF−/− milk. Levels of KC/GRO, MIP-1α, MIP-3β, and MDC were unchanged in TNF+/−/TNF−/− milk, while another 24 cytokines/growth factors were undetectable (multiplex immuoassay Luminex; Myriad RBM, Mouse Cytokine MAP A,B,C). These data suggest that TNF, produced by milk macrophages or other immune cells, acts locally on immune and epithelial cells in the mammary gland to modulate the expression of milk chemokines. These data are consistent with reports showing that IP-10 and MCP-1 are TNF inducible25, 26. Because there is delayed production of gastric acid and pancreatic proteases in neonates27, these chemokines could reach the offspring gut in biologically relevant concentrations and modulate the enteric immune and/or nervous system and consecutively brain development and behavior28, 29.
Figure 7.
Reduced milk chemokine levels in TNF deficient mothers are responsible for the WT(H) phenotypes. (a) Chemokine levels in postpartum day2 milk of TNF TNF+/− and TNF−/− mothers (two way ANOVA; genotype effect: F2,110=26.40, P<0.0001; group x chemokine: F20,110=4.31, P<0.0001; Tukey HSD posthoc test *p<0.05; N=5,3,5/group). Data are shown as means ± SE. (b) A cocktail of 5 recombinant cytokines at 3x and 10x doses given by gavage daily (1x: IP-10, 4 pg/g mouse weight; MIP-1β, 25pg/g; MCP-1, 10pg/g; MCP-3, 4 pg/g, and MCP-5, 0.7 pg/g) between P1 and P14 reduced proliferation in P14 DG (ANOVA; F3,118=4.14, P=0.021; N=5,6,6,5/group, LSD posthoc. *p<0.05, #<0.1). (c) The cytokine cocktails given between P1 and P21 reduced adult MWM memory in probe trial 1 at the 3x and 10x doses (Two way ANOVA; platform location: F3,75=24.98, P<10−5; group x platform location: F6,75=2.60, P=0.024; N=8,5,7,9/group, LSD posthoc. *p<0.05; ***<0.0005). (d) Increased WBC counts (t-test, T=2.678, p=0.019), due to elevated levels of lymphocyte and monocyte numbers in P10 pup of TNF+/− mothers as compared to pups of WT mothers (Manual count; Two way ANOVA, group: F1,65=9.37, P=0.0003; N=6,9/group, LSD posthoc. *p<0.05; **<0.005). Because the WT and KO pups of H mothers were not different, data were combined. NEUT, neutrophil; LYMPH, lymphocyte; MONO, monocyte; EOS, eosinophil; BASO, basophil.
Feeding chemokines normalize offspring phenotypes
To test if the reduced levels of milk chemokines can be directly linked to the hippocampal and behavioral changes in the WT(H) offspring, a cocktail of five chemokines that were downregulated in TNF+/− milk (Figure 7a) was administered to WT pups by daily gavage from P1 to P21 while being nursed by TNF deficient mothers. Both a 3x and a 10x cocktail (3/10x times the amount present in WT milk consumed daily; ~0.1ml milk/g pup weight) reduced proliferation in P14 DG (Figure 7b). In addition, both the 3x and 10x cocktails in WT(H) offspring reduced reference memory in the MWM, as compared to control WT(H) mice (Figure 7c).
Next, we measured cytokine levels in the blood of P10 pups of WT and mutant mothers, but found no significant differences in any of the four chemokines that showed reduced levels in TNF+/− milk, neither in another twelve cytokines that were also detectable (Supplementary Table 2). Levels of another 19 of the total of 35 tested cytokines were undetectable. However, we found a significant increase in the number of circulating white blood cells (WBCs) in P10 pups of TNF+/−, as compared to WT, mothers (Figure 7d). Both flow cytometry based and manual counting of WBCs showed a significant increase in the number of lymphocytes and monocytes of pups from TNF+/− mothers (Figure 7d). This increase in circulating lymphocytes and monocytes could be due to their reduced retention in the gut30 (which normally has more immune cells than the rest of the body), as a result of lower than normal levels of IP-10 (a chemokine that attracts lymphocytes), MCP-1 and −5 (attract monocytes as well as lymphocytes), and MIP-1β (attracts lymphocytes) in TNF+/− milk. Alternatively, the production and differentiation of lymphocytes and monocytes could be altered because chemokines are also involved in the maturation of immune cells31; especially during the neonatal period characterized by a significant expansion of the immune system. In turn, these immunological changes could elicit increased proliferation in the hippocampus as immune cells can communicate, via cytokines, with the brain32 and because cytokines have been shown to modulate neuronal progenitor proliferation 28, 29.
DISCUSSION
In this report, a novel function of the proinflammatory cytokine TNF is identified. We found that the partial or complete inactivation of the TNF gene in the maternal hematopoietic system results in a life-long enhancement of episodic memory in the offspring; specifically, of spatial reference memory in the MWM and contextual fear response. Interestingly, performance in these behavioral tasks was not influenced by the offspring’s own TNF level. While a partial inactivation of maternal TNF (in TNF+/− mothers) was sufficient to elicit the improved MWM performance in the offspring, its complete (in TNF−/−) and near complete (in mx-cre+) elimination was required to increase fear memories, indicating a difference in TNF dosage dependence between the two hippocampal processes that are both spatial learning/memory related, but differ in sensory and motor demands and in motivational aspects. Finally, crossfostering studies showed that the maternal effect was a result of the postnatal environment. Since newborn mice are “underdeveloped” relative to human infants, it is possible that a similar maternal programming in human would begin earlier, during fetal life.
The identified function of TNF differs from its known immunological functions in several ways. First, in this new paradigm, the effect of TNF is manifested across a generation, while known functions of TNF have been described in the host itself. Second, the new function is related to behavior, while known functions are associated with either host defense or organ development. Third, this function is related to physiological levels of TNF, while most known functions have been associated with excessive/pathological levels of TNF (e.g. in host defense and inflammation).
The maternal TNF deficit-related increase in memory recall was not only strongly correlated with, but also directly linked to, the rate of postnatal ANP proliferation in the offspring DG. Specifically, the increased performance of WT(H) offspring could be brought back to the level characteristic of the WT(WT) offspring by normalizing the otherwise enhanced DG proliferation. Correlation between increased granule cell precursor proliferation and enhanced spatial memory has previously been described in adult rodents as a result of voluntary running and exposure to enriched environment10, 33. These adult effects are temporal and eventually both the proliferation rate and memory return to their normal levels. In contrast, increased proliferation during the late postnatal period in the offspring of TNF mutant mothers, albeit temporal in its own, caused a permanent increase in spatial memory.
Since the maternal effect is concluded at weaning (at P21), and the increase in granule cell proliferation was normalized soon after weaning, persistent functional and/or structural changes must be present in the WT(H) offspring that underlie their adult cognitive phenotype. We found evidence for the dysregulation of the developmental program in WT(H) neurons; namely, cholinergic genes that are active in young neurons, but silenced during maturation, were upregulated in adult WT(H) granule cells, suggesting that they were still active in mature neurons. Opposite changes occurred in a number of neuropeptide related genes. Cholinergic neurotransmission promotes, while neuropeptides in general suppress, neuronal activity12, 16, suggesting that the net effect in WT(H) neurons is increased neuronal activity. We also found structural changes, specifically increased dendritic length in WT(H) neurons, as a result of the maternal TNF deficient environment, that may be the direct consequence of increased neuronal activity because branching and dendritic complexity are readily increased by neuronal activity34. Finally, both increased neuronal activity and dendritic complexity are consistent with the enhanced hippocampal memory of WT(H) animals as numerous groups reported that enriched environment increases neuronal activity, dendritic complexity, and spatial learning35.
Maternal TNF does not interact directly with neuronal precursors, but rather via the regulation of a group of milk chemokines. Because of the delayed production of gastric acid and pancreatic proteases in neonates27, these chemokines can reach the offspring gut intact and modulate the enteric immune system. Indeed we found evidence for significant changes in the number of circulating WBCs, specifically lymphocytes and monocytes, in pups of TNF+/− mothers. Immune cells, homed in the meningeal space or perivascular areas in the brain, can have significant effects on brain function and behavior32. Indeed, depletion of lymphocytes reduces spatial memory and replenishment with T cells normalizes cognitive performance36. However, further studies will be necessary to determine if the increased number of circulating lymphocytes and monocytes in the pups of TNF+/− mothers are indeed linked to the increased hippocampal proliferation and enhanced spatial memory of these animals.
Although most TNF related studies during pregnancy/postpartum are focused on diseases with pathologically high TNF levels37, 38, there are conditions associated with reduced ambient TNF. The level of ambient TNF is known to be downregulated by physical activity/exercise and adaptive stress39–41 via glucocorticoids42 and low TNF levels reflect low inflammatory physiology39. Drugs can also reduce TNF levels. The level of TNF is reduced by TNF antibody products19 such as infliximab and adalimumab, in order to achieve therapeutic benefit in rheumatoid arthritis (RA) and other inflammatory diseases43. Since RA often has its onset at reproductive age, these medications are also used during pregnancy and the postpartum period. Other drugs that have potent anti-TNF activity and which are used during pregnancy and postpartum include the antidepressant compound buproprion44 and some 5-HT2A receptor antagonists45.
What is the evolutionary benefit for the offspring to respond to maternal TNF and consequently adjust their spatial reference memory capacity? Since TNF production is suppressed by glucocorticoids in the range induced by adaptive stress and physical activity/exercise39–41, TNF levels could be a measure of challenges/competition in a natural environment that is reported, via a lactocrine pathway, to the developing offspring. It is important to point out that only moderate and acute increases in maternal glucocorticoids lead to adaptive adjustments in the offspring46, while large and sustained increases have detrimental effects47. Consistent with the idea of the maternal environment in offspring adaptation, voluntary wheel running of female mice during pregnancy and lactation increased postnatal hippocampal proliferation transiently in their offspring48, a pattern reminiscent of that seen in the offspring of TNF deficient mothers. Also, low dose glucocorticoids, administered through the drinking water provided during lactation, was shown to permanently increase offspring spatial memory49. Taken together, we hypothesize that a more challenging/competitive environment programs a higher level of spatial reference memory during the postnatal period in the offspring because of its benefit to the survival of the individual. Because of the cost associated with maintaining a higher level of cognitive performance, less challenging/competitive environment would program a lower level of cognitive performance that is still appropriate for survival under these conditions. Alternatively, the negative effect of maternal chemokines on offspring cognition could be a trade-off of their beneficial effect on the maturation of the neonatal immune system50. However, this scenario does not explain why a mechanism has not been developed that would protect the brain against this undesirable peripheral effect. Therefore, we propose that the maternal TNF dependent mechanism of programming adult spatial reference memory is adaptive in nature.
METHODS
Animals.
Animal experiments were carried out in accordance with the Weill Cornell Medical College IACUC guidelines. All animals were group housed up to 5 per cage with 12 hr light/dark cycle with lights on at 6 am. Food and water were available ad libitum. TNF−/− N1F2 hybrid (B6;129S-Tnftm1Gkl/J) mice and their appropriate WT controls (B6129SF2/J) were obtained from The Jackson Laboratory, Bar Harbor, ME. They were backcrossed onto the B6/Tac background 4 times. Nestin-cre mice51 (B6.Cg-Tg(Nes-cre)1kln/J) and mx1-cre mice52 (B6.Cg-Tg(Mx1-cre)1Cgn/J) were purchased from The Jackson Laboratory. The generation of TNFflox/flox mice on the B6J background was previously described53. Mx1-cre recombinase expression was induced in females by the intraperitoneal injection of polyIC (250 μg Sigma-Aldrich) at the age of 6 weeks as described previously52. Three weeks later, females were bred to generate conditional TNF mutants. Mice expressing R26R-LacZ as well as GFAP-TK mice54 (B6.Cg-Tg(Gfap-TK)7.1Mvs/J) were obtained from the Jackson Laboratory. Crossfostering was performed within 24hrs of birth. Male TK+ pups received 12.5 mg/kg Ganciclovir (GCV) by i.p. injection (Cytovene-IV, Roche Lab Inc, NDC 0004–6940), 1–6 times between P5 and P10. Lactating WT B6/Tac mice received 10μg/g Remicade (infliximab, Janssen Biologics BV, Leiden, The Netherlands) in 0.1 ml i.p. at postpartum day 1, 7 and 14. Another group of mice received purified NA/LE hamster anti-mouse/rat TNF (BD Pharmingen 557370), 300μg/mice, at postpartum day 1, 7 and 14. Control groups received either 10μg/g BSA or purified NA/LE hamster IgG1 isotype control (BD Pharmingen 554709), 300μg/mice, at postpartum day 1, 7 and 14. Milk was collected at postpartum day 2–3 from mammary glands by a vacuum operated system (10–50 μl per animal), one min after the administration of 2 IU oxytocin in 0.1ml 55. Dams and her litter were separated for 2 h prior to milking. The cocktail of chemokines (dissolved in 1mg/ml BSA solution) was administered by daily oral gavage to pups with animal feeding needles (24 gauge, Harvard Apparatus) from birth until P14 or P21. Based on similar prior behavioral experiments, not less than 5–6 animals per group were used, but when difficult to obtain mother/offspring genotype combinations were not a limiting factor, the number of animals were at least 8 but typically more than 10. At least 3 litters per group were used. Because of the low frequency of appropriate genotypes in the conditional KO experiments, the number of litters was 5 to 6. The same or similar number of animals were selected from individual litters randomly and assigned to the groups. During all behavioral tests the investigators who performed the tests were blind to the genotype and treatment of the animals. Moreover, all behavioral tests were fully automatized with no human input on data collection.
Immunohistochemistry.
At P5, P14, and at adult age (4–6 months), mice were given a single 100 mg/kg s.c. injection of BrdU (Sigma, B5002) followed 2 h later by transcardial perfusion with 4% paraformaldehyde. Immunohistochemistry was performed as previously described56 by using mouse monoclonal anti-BrdU (1:500; Novocastra, Newcastle upon Tyne, UK, MCA2060) and biotinylated horse anti-mouse antibody (Vector Laboratories, Burlingame, CA, BA-2000). For double- or triple-immunofluorescence labeling, sections were incubated in 1 mM EDTA, 5 mM Tris, pH 8.0 at 95–100°C for 15–20 min for antigen recovery, blocked in 10% normal donkey serum, and incubated at room temperature overnight with primary antibodies against BrdU (1:50, Mouse monoclonal, DSHB, G3G4), Tbr2 (1:500, Rabbit polyclonal, Abcam, ab115986), Sox2 (Y 17) (1:500, Goat polyclonal, Santa Cruz, sc-17320), GFAP (1:500, Rabbit polyclonal, DAKO, IS52430–2), and/or β-Galactosidase (1:500, mouse monoclonal, Promega, Z3781), then with donkey anti-rabbit IgG–Alexa-Fluor-488 (A-21206), anti-goat IgG–Alexa-Fluor-555 (A-21432), and/or anti-mouse Alexa-Fluor 647 (A-31571) secondary antibodies (Jackson ImmunoResearch, West Grove, PA). The dorsal hippocampus was defined as the anterior 50% of the structure. The total dorsal BrdU+ cell number, GCL volume, and cell density were measured by using the StereoInvestigator software (MBF Bioscience, Williston, VT).
Golgi impregnation and tracing.
We impregnated fresh brains in Golgi-cox using the FD Rapid GolgiStain Kit (Neurodigitech, San Diego, CA) solution for 14 d at 25°C in the dark and then transferred them to 30% sucrose at 4°C for 72 h. We prepared 150-μm coronal serial sections with a vibrotome; slides were soaked in 50% sucrose and air-dried for 72 h in the dark. Quantitative microscopy was performed on a Microbrightfield imaging system (MBF Bioscience). The granular neurons in the DG were chosen by systemic random sampling, and 14–23 ‘traceable’ neurons for each genotype were reconstructed three dimensionally with the Neurolucida system (MBF Bioscience). The partial spines of each granular neuron were also reconstructed at proximal (30–60 um) and distal (60–240um) separately. The morphological traits of cells and spines number were analyzed with NeuroExplorer (Next Technologies, Madison, AL).
Quantification of DNA and mRNA levels.
Quantification of genomic DNA levels and mRNA expression was performed as described in previous reports 57. Primers for the WT and floxed TNF alleles were as described previously 53.
RNASeq.
Total RNA was isolated from the microdissected granule cell layer and CA1 pyramidal layer of 5–6 WT(WT) and WT(H) male mice of 12–14 weeks of age from 3 or more litters. RNA-seq libraries were prepared using the Illumina HiSeq2000 sequencing platform, generating approximately 45 million 51 nt reads per pool. Reads were processed and aligned to the mm9 reference genome (NCBI v37 build) using TopHat v1. 2.0, which incorporates the Bowtie algorithm to perform splice junction mapping of RNA-seq transcripts. The aligned reads were then processed by Cufflinks v1.3.0. Reads were assembled into transcripts, their abundance estimated, and tests for differential expression and regulation between the tissue samples were performed. We used the “-G” argument, which calculates the expression levels for all known/annotated transcripts using the NCBI build 37 GTF file downloaded from ftp://ftp.ccb.jhu.edu/pub/data/bowtie_indexes/m_musculus_ncbi37.ebwt.zip. The Cuffdiff program in the Cufflinks software was used to estimate the abundance of transcripts from the reference transcriptome using the Tophat alignments and concurrently test for differential expression. The unit of measurement for the relative abundances of transcripts is Fragments per Kilobase of exon per Million fragments mapped (FPKM), and the measure for differential expression is the log2 fold change of the treatment FPKM divided by the control FPKM.
Milk cytokine levels.
Milk samples from individual mice were diluted 1:1 in ice-cold Protease buffer (0.15 mM spermine, 0.5 mM spermidine, 1mM PMSF, 1x complete protease inhibitor in PBS) and were centrifuged for 10 min (5000 rpm, 4°C). Supernatant was analyzed for cytokines/chemokines by multiplex Luminex immunoassay by Myriad RBM (A, B, and C panels).
Behavioral Procedures.
The cognitive tests were conducted using male offspring aged 8–16 weeks. First animals were tested in open field for overall activity followed in two days by the Morris water maze test as described by Dumont et al. 58. One week after the completion of this test, animals were tested for fear conditioning according to the protocol of Pattwell et al. 59. All the water maze and fear condition tests run between 1–5 pm (i.e. during the light cycle). Maternal care behavior was assessed both during the light and dark phases, as we have described 60.
Statistical analysis.
Data are shown as means ± SE. No data or animals were excluded. One or two way ANOVA or t-test was used in the analyses to compare groups. Homogeneity of variance was assessed by Levene’s test. LSD or Tukey HSD posthoc analyses were used to assess statistical significance. If the assumption for equal variances and normality was not met, the Dunnett’s T3 posthoc test algorithm was used. Differences between groups were considered to be significant when P<0.05.
Supplementary Material
Acknowledgements.
We thank Sergei Nedospasov for providing us the floxed TNF mouse strain, and for discussions on inducible knockout and TNF neutralization strategies. We thank Deqiang Jing from the Department of Psychiatry, Weill Cornell Medical College, for providing Golgi staining reagents and technical support. We would like to thank Dr. Concetta Dipace and Kara Rosania for the ELISA based assays of TNF and Chirlien Pang for counting BrdU positive cells. This work was supported by US National Institute of Mental Health grant 1RO1MH080194 to MT.
Footnotes
Database accession. RNA expression data have been deposited to GenBank. Accession number pending.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Pasparakis M, Alexopoulou L, Episkopou V & Kollias G Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. The Journal of experimental medicine 184, 1397–1411 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuprash DV, et al. Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. European journal of immunology 35, 1592–1600 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Muller N & Ackenheil M Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 22, 1–33 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Stellwagen D & Malenka RC Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Golan H, Levav T, Mendelsohn A & Huleihel M Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex 14, 97–105 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Yamada K, et al. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. Journal of neuroimmunology 111, 131–138 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Gleason G, et al. The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc Natl Acad Sci U S A 107, 7592–7597 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neves G, Cooke SF & Bliss TV Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nature reviews 9, 65–75 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Soriano P Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics 21, 70–71 (1999). [DOI] [PubMed] [Google Scholar]
- 10.van Praag H, Kempermann G & Gage FH Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2, 266–270 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS & Alvarez-Buylla A Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478, 359–378 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Hasselmo ME The role of acetylcholine in learning and memory. Current opinion in neurobiology 16, 710–715 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry DC, et al. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem 82, 468–481 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Winner B, et al. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci 32, 16906–16916 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko N, Okano H & Sawamoto K Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 11, 1145–1159 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Baraban SC & Tallent MK Interneuron Diversity series: Interneuronal neuropeptides--endogenous regulators of neuronal excitability. Trends in neurosciences 27, 135–142 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Fiala BA, Joyce JN & Greenough WT Environmental complexity modulates growth of granule cell dendrites in developing but not adult hippocampus of rats. Exp Neurol 59, 372–383 (1978). [DOI] [PubMed] [Google Scholar]
- 18.Francis DD & Meaney MJ Maternal care and the development of stress responses. Curr Opin Neurobiol 9, 128–134 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Knight DM, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Molecular immunology 30, 1443–1453 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Mohler KM, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 151, 1548–1561 (1993). [PubMed] [Google Scholar]
- 21.Zalevsky J, et al. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol 179, 1872–1883 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Triantafillidis JK, et al. Favorable response to subcutaneous administration of infliximab in rats with experimental colitis. World J Gastroenterol 11, 6843–6847 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grounds MD, et al. Silencing TNFalpha activity by using Remicade or Enbrel blocks inflammation in whole muscle grafts: an in vivo bioassay to assess the efficacy of anti-cytokine drugs in mice. Cell and tissue research 320, 509–515 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Garrett WS, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell host & microbe 8, 292–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumder S, et al. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol 161, 4736–4744 (1998). [PubMed] [Google Scholar]
- 26.Murao K, et al. TNF-alpha stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochemical and biophysical research communications 276, 791–796 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Blais DR, Harrold J & Altosaar I Killing the messenger in the nick of time: persistence of breast milk sCD14 in the neonatal gastrointestinal tract. Pediatric research 59, 371–376 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Krathwohl MD & Kaiser JL Chemokines promote quiescence and survival of human neural progenitor cells. Stem cells (Dayton, Ohio) 22, 109–118 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser B, Wolf M, Walz A & Loetscher P Chemokines: multiple levels of leukocyte migration control. Trends Immunol 25, 75–84 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Luther SA & Cyster JG Chemokines as regulators of T cell differentiation. Nat Immunol 2, 102–107 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Kipnis J, Gadani S & Derecki NC Pro-cognitive properties of T cells. Nat Rev Immunol 12, 663–669 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempermann G, Kuhn HG & Gage FH More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Chen Y & Ghosh A Regulation of dendritic development by neuronal activity. Journal of neurobiology 64, 4–10 (2005). [DOI] [PubMed] [Google Scholar]
- 35.van Praag H, Kempermann G & Gage FH Neural consequences of environmental enrichment. Nature reviews 1, 191–198 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Kipnis J, Cohen H, Cardon M, Ziv Y & Schwartz M T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A 101, 8180–8185 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tosun M, et al. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med 23, 880–886 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Buka SL, et al. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun 15, 411–420 (2001). [DOI] [PubMed] [Google Scholar]
- 39.DeRijk R, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. The Journal of clinical endocrinology and metabolism 82, 2182–2191 (1997). [DOI] [PubMed] [Google Scholar]
- 40.O’Connor TM, O’Halloran DJ & Shanahan F The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. Qjm 93, 323–333 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Sellers TL, Jaussi AW, Yang HT, Heninger RW & Winder WW Effect of the exercise-induced increase in glucocorticoids on endurance in the rat. J Appl Physiol 65, 173–178 (1988). [DOI] [PubMed] [Google Scholar]
- 42.Steer JH, Kroeger KM, Abraham LJ & Joyce DA Glucocorticoids suppress tumor necrosis factor-alpha expression by human monocytic THP-1 cells by suppressing transactivation through adjacent NF-kappa B and c-Jun-activating transcription factor-2 binding sites in the promoter. The Journal of biological chemistry 275, 18432–18440 (2000). [DOI] [PubMed] [Google Scholar]
- 43.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP & Hazes JM Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis and rheumatism 59, 1241–1248 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL & Soares MB A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. International immunopharmacology 6, 903–907 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Yu B, et al. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. The Journal of pharmacology and experimental therapeutics 327, 316–323 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Love OP & Williams TD The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am Nat 172, E135–149 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Harris A & Seckl J Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59, 279–289 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Bick-Sander A, Steiner B, Wolf SA, Babu H & Kempermann G Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proceedings of the National Academy of Sciences of the United States of America 103, 3852–3857 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catalani A, et al. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain research 624, 209–215 (1993). [DOI] [PubMed] [Google Scholar]
- 50.Garofalo R Cytokines in human milk. J Pediatr 156, S36–40 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23, 99–103 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Kuhn R, Schwenk F, Aguet M & Rajewsky K Inducible gene targeting in mice. Science 269, 1427–1429 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Grivennikov SI, et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A 103, 17501–17506 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DePeters EJ & Hovey RC Methods for collecting milk from mice. Journal of mammary gland biology and neoplasia 14, 397–400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatapudy S, Bruening S, Gleason G & Toth M Validation and use of a computer-assisted counting procedure to quantify BrdU-labeled proliferating cells in the early postnatal mouse hippocampus. J Neurosci Methods 172, 173–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey SJ & Toth M Variability in the benzodiazepine response of serotonin 5-HT1A receptor null mice displaying anxiety-like phenotype: evidence for genetic modifiers in the 5-HT-mediated regulation of GABA(A) receptors. J Neurosci 24, 6343–6351 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dumont M, et al. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. Faseb J 23, 2459–2466 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pattwell SS, Bath KG, Casey BJ, Ninan I & Lee FS Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci U S A 108, 1182–1187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Velzen A & Toth M Role of maternal 5-HT(1A) receptor in programming offspring emotional and physical development. Genes Brain Behav 9, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.