Abstract
Patients with heart failure (HF) frequently struggle to adhere to health behaviors, and psychological factors may contribute to nonadherence. We examined the feasibility and acceptability of a 10-week, positive psychology-(PP-) based intervention to promote health behavior adherence in patients (N=10) with mild to moderate HF and suboptimal health behavior adherence. Participants engaged in weekly phone sessions, completed PP exercises (e.g., writing a gratitude letter, using a personal strength), and set goals related to diet, medication adherence, and physical activity. Feasibility was assessed by the number of sessions completed, and acceptability by participant ratings of ease and utility. Preliminary efficacy was measured by changes in psychological and adherence outcomes. The intervention was feasible (87% of exercises completed) and acceptable. Furthermore, in exploratory analyses, the intervention was associated with improvements in psychological and health behavior adherence outcomes. Larger, randomized trials are needed to further investigate the utility of this intervention.
Keywords: positive psychology, heart failure, health behaviors, physical activity, adherence
Introduction
Heart failure (HF) is a chronic and debilitating illness that affects nearly 6 million Americans and is associated with poor functioning, reduced quality of life (QoL), and high rates of mortality (Celano et al, 2011; Roger et al, 2011). Adherence to important health behaviors, such as physical activity, a low-sodium diet, and medications has been associated with better outcomes for patients with HF, including reduced hospital readmissions and lower rates of mortality (Arcand et al, 2011; Belardinelli et al, 1999; O’Connor et al, 2009; Pina et al, 2003; Wright et al, 2003; Wu et al, 2008). However, most people with HF struggle to adhere to these health behaviors (Corotto et al, 2013; Lemon et al, 2010).
Both educational and psychological factors may help to explain impaired health behavior adherence among patients with HF. Many HF patients lack specific knowledge about how best to care for their HF symptoms, which can significantly hinder their abilities to engage in self-care behaviors (Hope et al, 2004; Lainscak et al, 2007). Providing specific treatment recommendations and information to adhere to these recommendations is an important way to improve health behavior adherence; however, interventions that have focused on HF education alone have had limited success (Corotto et al, 2013).
Psychological well-being, including both subjective (e.g., life satisfaction, positive affect) and psychological (e.g., meaning or purpose in life) components (Keyes et al, 2002), also may play a role in adherence to health behaviors and subsequent cardiovascular outcomes in HF. Psychiatric disorders characterized by negative emotions and psychological ill-being, such as depression, are associated with both reduced adherence to health behaviors in patients with heart disease (Ziegelstein et al, 2000) and with poor health outcomes—including mortality—in patients with HF (Rutledge et al, 2006). In contrast, psychological well-being is associated with improved cardiovascular health and adherence to health behaviors in both healthy and medically ill populations, including those with heart disease, oftentimes independent of negative emotions (Chida et al, 2008; DuBois et al, 2015; Giltay et al, 2004; Kim et al, 2014; Rasmussen et al, 2009; Tindle et al, 2009). Psychological well-being may therefore be an important treatment target to improve health behavior adherence in patients with HF.
Positive psychology (PP) interventions, which consist of systematic exercises (e.g., writing a letter of gratitude, performing acts of kindness) that target the enhancement of positive feelings (Seligman et al, 2006; Seligman et al, 2005), may be useful to promote psychological health and adherence to health behaviors in HF. PP interventions are effective at improving well-being in healthy individuals (Bolier et al, 2013; Sin et al, 2009), and preliminary studies suggest that they may be effective in medically ill populations as well (DuBois et al, 2016; Moskowitz et al, 2017; Nikrahan et al, 2016). Furthermore, similar interventions have improved physical activity and medication adherence in patients with coronary artery disease and hypertension, respectively (Ogedegbe et al, 2012; Peterson et al, 2012). However, aside from our group’s pre-pilot work (Huffman et al., 2011a), no studies have utilized formal PP interventions to promote health behaviors in patients with HF, despite the need for novel approaches to promote such behaviors in this population.
To address this gap, we developed a PP-based intervention that included both PP exercises and HF-specific health behavior education to promote physical activity and other important health behaviors. We then examined the feasibility and acceptability of this 10-week, telephone delivered, PP-based intervention in a small (N=10) single-arm, proof-of-concept trial. We hypothesized that a majority of participants would complete most PP exercises, that individual PP exercises would be associated with significant improvements in happiness and optimism, and that participants would rate individual PP exercises as easy to complete and subjectively helpful. Furthermore, in exploratory analyses we examined the preliminary efficacy of the intervention on key psychological and health behavior outcomes, though we did not expect the impact of the intervention on those outcomes to be significant given the study’s sample size.
Methods
This was a single-arm, proof-of-concept trial of a 10-week, telephone-delivered, PP-based health behavior intervention for outpatients with mild to moderate HF. Participants were recruited between December 2016 and May 2017 through contact by mail and subsequent phone calls. All participants provided written informed consent prior to study procedures, and approval was obtained from (the Partners Healthcare Institutional Review Board.). The trial was registered on clinicaltrials.gov (NCT # NCT02938052).
Participants
Participants were adults with New York Heart Association (NYHA) (The Criteria Committee of the New York Heart Association, 1994) class I, II, or III HF, with suboptimal adherence to health behaviors, as measured by the Medical Outcomes Study Specific Adherence Scale (MOS-SAS) (DiMatteo et al, 1992). HF diagnosis was confirmed via participant interview and medical record review. NYHA class was evaluated via patient interview at the time of eligibility screening and confirmed with the participant prior to enrollment (The Criteria Committee of the New York Heart Association, 1994). The NYHA classification system classifies patients based on the functional impact of their HF symptoms, ranging from I (no symptoms) to IV (severe, with HF symptoms present at rest) (The Criteria Committee of the New York Heart Association, 1994). We chose to include participants with NYHA class I-III symptoms given that they would be able to increase their adherence to physical activity, a key health behavior in this population. Health behavior adherence was measured by the MOS-SAS (range 0–18) items regarding diet, physical activity, and medication adherence (DiMatteo et al, 1992). Participants were eligible if they scored ≤ 15, which suggested suboptimal health behavior adherence. Participants were excluded if they exhibited significant cognitive impairment (as measured by a 6-item questionnaire) (Callahan et al, 2002), had medical illnesses likely lead to death within 6 months, were unable to participate in physical activity, did not have a telephone, or were unable to speak English, read, or write.
Procedures
Patients receiving medical care at an academic medical center with a documented history of HF were identified through systematic searches of the healthcare system’s electronic medical record system using a secure, online query tool. After confirming likely eligibility via medical record review and contact with the patient’s cardiologist (if necessary), patients were sent opt-out letters describing the study’s purpose and procedures and asking them to inform us if they did not wish to be contacted further. Study staff then called patients who did not opt out from the study to inform them about the study, screen interested patients for eligibility, and schedule an initial screening/enrollment visit in-person if the person appeared to be eligible.
At the initial enrollment visit, eligibility was confirmed, and informed consent was obtained. Participants completed baseline measures of psychological health, functional status, health-related quality of life, and health behavior adherence. They also received an accelerometer to wear for a week, as a baseline measure of physical activity. Finally, following the completion of baseline measures, participants were introduced to the PP-based health behavior intervention.
Study Intervention
Intervention Development and Theoretical Model:
The PP-based health behavior intervention paired the use of PP exercises with education and systematic goal-setting related to physical activity, low sodium diet, and medication adherence, three key health behaviors in patients with HF (Belardinelli et al, 1999; O’Connor et al, 2009; Pina et al, 2003; Wright et al, 2003; Wu et al, 2008). The intervention utilized established PP exercises (Miller et al, 2002; Moskowitz et al, 2012; Seligman et al, 2005), which were adapted for a HF population based on our group’s work in other patients with significant medical illness (Dubois et al., 2016; Huffman et al., 2016). Systematic goal-setting was added based on our experience optimizing a similar intervention in patients with acute coronary syndrome (Celano et al., 2018). Finally, qualitative interviews were performed to confirm the main intervention components and to identify important barriers to health behavior completion in HF.
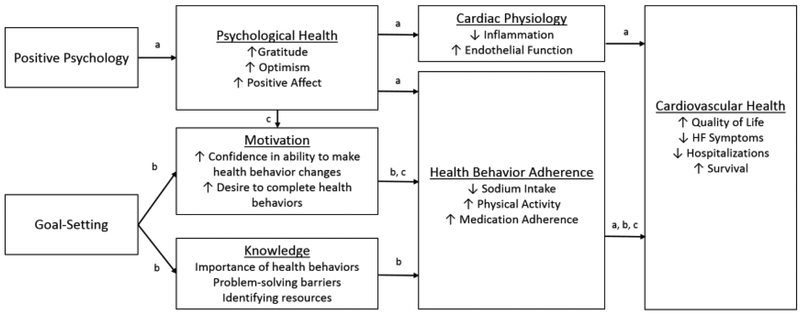
From a theoretical standpoint, PP exercises were combined with systematic goal-setting for several reasons. First, PP itself may impact health behavior adherence and cardiovascular health (see Figure 1, Pathway a). Psychological well-being is prospectively associated with both improved adherence to health behaviors and superior medical outcomes, including mortality (Chida et al, 2008; DuBois et al, 2015; Giltay et al, 2007). Furthermore, PP-like interventions increase physical activity following percutaneous coronary interventions and improve medication adherence in patients with hypertension (Ogedegbe et al, 2012; Peterson et al, 2012). Finally, psychological well-being has been associated with reduced levels of inflammation and improved endothelial function, which may translate to improvements in cardiac health (Brouwers et al, 2013; Celano et al, 2017).
Figure 1: Theoretical Framework for a Combined PP and Goal-setting Intervention.
a=pathway by which PP may impact cardiovascular health
b=pathway by which education and goal-setting may impact cardiovascular health
c=pathway by which the PP and goal-setting portions of the program may work together to impact cardiovascular health HF=heart failure
Second, the use of HF education and systematic goal-setting may improve health behavior adherence (see Figure 1, Pathway b). Patients with HF often exhibit limited knowledge of their HF symptoms and the details of how best to manage those symptoms and adhere to treatment, including medications, physical activity, and dietary changes (Hope et al, 2004; Lainscak et al, 2007). Providing information about physical activity, medication adherence, and a low sodium diet may help patients to better understand the importance of these behaviors and make changes to improve adherence (Corotto et al, 2013). Furthermore, systematic goal-setting, which utilizes several motivational interviewing (MI) tools and techniques, may improve adherence. In patients with HF, MI-based interventions have been associated with improvements in confidence in HF self-care (Paradis et al, 2010), maintenance of self-care (Masterson Creber et al, 2016), and physical activity (Brodie et al, 2005). MI tools and assistance with goal-setting may help participants become more confident in their ability to make changes to their self-care and then maintain these changes over time.
Finally, PP, education, and goal-setting may interact to improve health behavior adherence and subsequent cardiovascular health (see Figure 1, Pathway c). PP interventions enhance optimism, hope, and other positive emotions that may help individuals become more willing and confident to make health behavior changes (Bolier et al, 2013; Sin et al, 2009). These positive emotions may make individuals more motivated to set goals to improve their health behaviors and more likely to attain them through both conscious and unconscious means (Cameron et al, 2015; Cameron et al, 2018; Slovinec D’Angelo et al, 2014; Van Cappellen et al, 2018). Furthermore, experiencing positive emotions may strengthen internal resources (e.g., coping strategies to overcome barriers, a greater awareness of options to complete health behaviors) that increase an individual’s likelihood to engage in health behaviors and reach their health behavior goals despite adversity (Fredrickson, 2001; Van Cappellen et al, 2018). Ultimately, improvements in health behavior adherence may translate into substantive cardiovascular benefits, including reduced rates of hospitalization and mortality (Arcand et al, 2011; Belardinelli et al, 1999; O’Connor et al, 2009; Pina et al, 2003; Wright et al, 2003; Wu et al, 2008).
Intervention Delivery:
The PP-based intervention was introduced to participants at the in-person study visit, following the completion of baseline measures. In this session, a study trainer (CC.) described the links between well-being, health behavior adherence, and cardiovascular health and outlined both the PP and goal-setting portions of the program. Participants were encouraged to view the purpose of both the PP and goal-setting portions of the program as building skills to increase their ability to improve their mental and physical health. Participants received a treatment manual, a copy of the Learning to Live with Heart Failure guide (2016), and an Omron pedometer (to keep track of physical activity) and were oriented to their use. Finally, participants were introduced to the first PP exercise and goal-setting topic and instructed to complete the appropriate sections in the manual over the next week.
All subsequent sessions were completed via telephone, with the first part of the call focusing on PP and the latter part on goal-setting. During the PP portion of the session, the study trainer reviewed the prior week’s PP exercise with the participant, reinforced positive thoughts and feelings that arose while performing and reviewing the exercise, and identified ways to integrate that week’s PP activity/skill into daily life. Next, the study trainer and participant discussed the rationale for the next week’s exercise, reviewed the appropriate section of the treatment manual, and made a plan for completing the exercise over the next week. In the goal-setting portion of the call, the study trainer similarly reviewed the participant’s progress towards the health behavior goal set in the previous session, discussed new techniques for improving health behavior adherence, and set a new health behavior goal for the upcoming week. While participants were not actively encouraged to use PP skills to aid in setting goals (e.g., using the personal strength of perseverance to meet their physical activity goal), they were free to do so if they chose. In both the PP and goal-setting portions of the call, the study trainer recorded relevant information related to the PP exercises (e.g., how participants completed them) and goals (e.g., number of steps taken per day, progress towards a dietary goal). This information was discussed during the integration sessions to encourage continued performance of PP activities and revise overall health behavior goals.
Intervention Content:
The PP portion (see Table 1) of the intervention was adapted from prior PP intervention studies (DuBois et al, 2016; Huffman et al, 2016; Moskowitz et al, 2012; Seligman et al, 2005) in both healthy and medically ill populations. The PP exercises were clustered based on their general theme into three groups: gratitude-, strength-, and meaning-based activities. In this way, similar PP exercises were performed sequentially, which allowed each subsequent session to build upon the previous ones. At the end of each group of activities, an “integration” activity was performed, which encouraged participants to find ways to use PP skills in daily life. Finally, during each phone session, participants were encouraged to be more aware of positive feelings as they occur, expand their vocabulary for naming positive emotions, and develop daily habits that would allow for the continued use of PP skills after the intervention was completed.
Table 1:
Description of positive psychology exercises*
| Targeted Construct | PP exercise | Description | |
|---|---|---|---|
| Session 1 | Gratitude | Gratitude for positive events | Participants recall and record three positive events that occurred within the last week. |
| Session 2 | Expressing gratitude | Participants write a letter of gratitude to a person who has done something kind for them. | |
| Session 3 | Integrating gratitude into daily life | Participants practice using a skill related to gratitude in order to integrate it into daily life. | |
| Session 4 | Strengths | Recalling a past success | Participants recall a time when they were successful and write about their role in the success and the positive emotions associated with the success. |
| Session 5 | Using personal strengths | Participants identify a personal strength and then use it in a new way over the next week. | |
| Session 6 | Integrating strengths into daily life | Participants practice using strength-based skills in order to integrate them into daily life. | |
| Session 7 | Meaning | Enjoyable and meaningful activities | Participants complete three activities: an enjoyable activity alone, an enjoyable activity with another person, and a meaningful activity. |
| Session 8 | Performing acts of kindness | Participants complete three kinds acts, which go beyond what they might typically do for others. | |
| Session 9 | Integrating meaning into daily life | Participants practice skills related to meaning in order to integrate them into daily life. | |
| Session 10 | Planning for the future | Participants review their favorite activities and make a plan to perform them in daily life. |
Sessions were performed via weekly phone calls; therefore, sessions also represent the number of weeks the participant has been engaged in the intervention.
The goal-setting portion (see Table 2) of the intervention was designed to educate participants about the importance of health behavior adherence and systematically set goals related to these health behaviors utilizing MI techniques (Miller et al, 2002). It was divided into three health behavior-specific modules: physical activity, low-sodium diet, and medication adherence. For each health behavior, participants were encouraged to track the behavior using tracking sheets included in the manual, set SMART (Specific, Measurable, Attainable, Relevant, and Time-based) goals, identify barriers to health behavior completion and problem-solve around them, and identify resources to attain their health behavior goals. Educational information was drawn from the Learning to Live with Heart Failure guide (2016), which was provided to all participants. Participants were able to choose the order in which they completed the goal-setting modules. Once they completed a module, participants continued to set goals for the health behavior for the remainder of the study. This allowed participants to work on the health behavior they identified as being most important for a full 9 weeks.
Table 2:
Description of goal-setting sessions*
| Health Behavior** | Goal-setting topic | Description | |
|---|---|---|---|
| Session 1 | Physical Activity | Introduction to increasing physical activity | Participants learn about the benefits of being physically active and begin to track activity using a pedometer. |
| Session 2 | Setting a SMART physical activity goal | Participants learn to set SMART (Specific, Measurable, Attainable, Relevant, and Time-based) physical activity goals. | |
| Session 3 | Physical activity resources | Participants learn how to identify and overcome barriers and find resources to stay active. | |
| Session 4 | Heart-Healthy Diet | Introduction to a heart-healthy diet | Participants learn how to reduce dietary sodium. |
| Session 5 | Setting a SMART heart-healthy diet goal | Participants learn how to set SMART goals related to diet. | |
| Session 6 | Heart-healthy diet resources | Participants learn how to identify and overcome barriers and find resources to adhere to a heart-healthy diet. | |
| Session 7 | Medication Adherence | Introduction to medication adherence | Participants learn about the importance of medication adherence for heart health. |
| Session 8 | Setting a SMART medication goal | Participants learn how to set a specific medication-related SMART goal. | |
| Session 9 | Medication resources | Participants learn how to identify and overcome barriers and find resources to manage medication adherence. | |
| Session 10 | Planning for the future | Participants review their progress and make a plan for continuing these healthy behaviors in the future. |
Sessions were performed via weekly phone calls; therefore, sessions also represent the number of weeks the participant has been engaged in the intervention.
The order in which health behaviors were presented could be altered based on participant preference.
Data collection and outcomes
Participant baseline sociodemographic information, current medications, medical history, and current medical status were obtained from participant report and medical records.
Feasibility (primary study outcome).
Intervention feasibility was measured by the number of intervention sessions completed by participants. Phone sessions were considered to be complete if they included a review of the last week’s exercise, assignment of a new PP exercise, and setting of a health behavior goal. To examine the feasibility of study outcomes, rates of questionnaire completion and adequate accelerometer use were measured.
Acceptability and immediate impact.
To examine acceptability of the PP sessions, participants were asked to rate the ease and utility of the PP session on a scale of 0 (very difficult/not helpful) to 10 (very easy/very helpful) immediately after completing the exercise (and prior to the weekly phone session). The immediate impact of each PP exercise on optimism and happiness also were measured by pre-and post-exercise participant ratings of happiness and optimism on 10-point Likert scales (0 = not happy/optimistic, 10 = very happy/optimistic), which similarly were recorded by participants in the manual before and after completing the exercise.
Exploratory analyses: psychological and health behavior outcomes.
To examine the impact of the PP sessions on psychological health, optimism, positive affect, depression, and anxiety were measured. Dispositional optimism was measured using the Life Orientation Test – Revised (LOT-R), a well-validated 6-item instrument (Scheier et al, 1994). Positive affect was assessed using the positive affect items of the Positive and Negative Affect Schedule (PANAS) (Watson et al, 1988), a well-validated scale previously used in patients with HF (Brouwers et al, 2013). Anxiety and depression were measured using the Hospital Anxiety and Depression Scale (HADS) (Bjelland et al, 2002). This scale examines cognitive symptoms of depression and anxiety, which reduces the likelihood of confounding by somatic HF symptoms.
Physical activity was assessed using Actigraph GT3X+ accelerometers (Actigraph LLC, Pensacola, FL), which have been used in multiple studies of physical activity in patients with medical illness (Kelly et al, 2013; Webel et al, 2017). Participants wore the accelerometer for one week at the start of the intervention (baseline) and again following completion of the intervention (10 weeks). We required at least 5 valid days (defined as at least 480 minutes of wear time) for the accelerometer data to be included in analyses, based on established recommendations and prior studies (Cain et al, 2011; Huffman et al, 2017). We measured both steps and average daily minutes of moderate to vigorous physical activity (MVPA), defined as ≥ 1952 counts/minute (Cain et al, 2011).
Medication adherence was measured using a Self-Reported Medication Adherence scale (SRMA) adapted from the literature (Lu et al, 2008). Participants were asked to state what percentage of the time (in 10% increments) they took their medications as prescribed in the past two weeks. Sodium intake was assessed using the Scored Sodium Questionnaire (SSQ), a validated scale which assesses sodium intake over the past month (Mason et al, 2014). Finally, self-reported adherence to physical activity, medications, and diet were assessed using the MOS-SAS items for these health behaviors (DiMatteo et al, 1992). The MOS-SAS has been used in multiple studies assessing adherence in cardiac patients (Huffman et al, 2011b; Ziegelstein et al, 2000).
Data Analysis
For the assessment of feasibility, descriptive statistics were used to report the proportion of exercises completed. A priori, we determined that the intervention would be considered feasible if at least 5 of 9 PP sessions were completed by a majority of participants. For acceptability (ease and utility) of each PP exercise, we performed mixed effects regression models, which allowed us to control for intra-individual variability, given that participants could complete up to 9 PP sessions. Based on our prior work (Huffman et al, 2016), PP sessions were considered to be acceptable if mean ease and utility scores were at least 6/10. The immediate impact of exercises was calculated by comparing pre-and post-exercise ratings of optimism and happiness using paired t tests.
In addition to examining the acceptability of the PP exercises as a group, we also attempted to determine whether any specific PP exercises were rated by participants as easier to complete or more helpful, or were associated with greater immediate improvements in optimism and happiness. To accomplish this, differences in the ease, utility, and immediate impact on optimism and happiness between specific PP exercises were examined using analyses of variance.
To examine the impact of the intervention on psychological outcomes and health behavior adherence (including physical activity), we used paired t tests to explore mean differences between baseline and 10-week values. Effect sizes (Cohen’s d) were calculated by dividing the mean change in outcome by the standard deviation of the change in outcome. All statistical tests were two-tailed, and p<.05 was considered to be significant. We did not control for multiple comparisons given the exploratory nature of the analyses. All statistical tests were performed using Stata 15.0 statistical software (College Station, TX).
Results
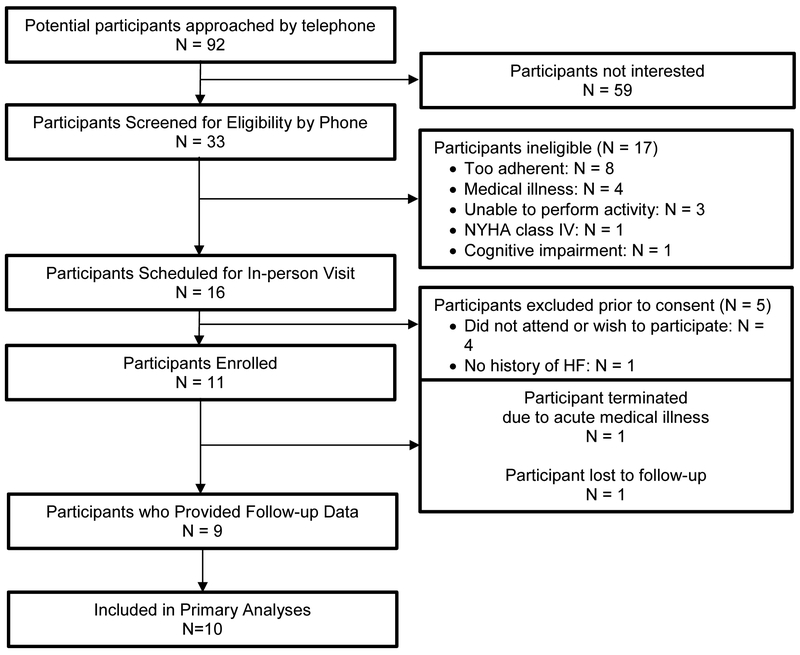
Ninety-two potential participants were contacted via telephone to describe the study and assess eligibility (see Figure 2 for study flow). Of those, 33 were screened for eligibility, and 11 ultimately enrolled. The most common reasons for exclusion were being too adherent to health behaviors and having significant medical illness that limited physical activity. One participant developed an acute medical illness (unrelated to the study) shortly after enrollment and was unable to continue participation in the study or provide follow-up data. The remaining ten participants were included in our primary analyses.
Figure 2: Recruitment and study flow.
Baseline data.
Baseline demographic information, medical variables, and outcome measures are listed in Table 3. Participants were 67.1 (SD 10.8) years of age on average, and 60% were men. All participants had either NYHA class I or II HF symptoms. Participants had relatively high levels of optimism (mean = 20.9 [SD 2.4]) and positive affect (38.7 [SD 3.5]), with low levels of depression (1.4 [SD 1.3]) and anxiety (4.2 [SD 2.2]). HF symptom and quality of life scores on the KCCQ suggested mild symptoms of HF (84.1 [SD 15.4]) and relatively high quality of life (85.8 [SD 11.8]).
Table 3:
Baseline characteristics of participants (N=10)
| Characteristic | Value |
|---|---|
| Sociodemographic characteristics; N (%), unless otherwise noted | |
| Age; mean (SD) | 67.1 (10.8) |
| Female gender | 4 (40) |
| Caucasian race | 9 (90) |
| Lives alone | 1 (10) |
| Married | 7 (70) |
| Highest education High school graduate College graduate Advanced degree |
3 (30) 5 (50) 2 (20) |
| Medical characteristics; N (%), unless otherwise noted | |
| New York Heart Association (NYHA) class NYHA class 1 NYHA class 2 |
4 (40) 6 (60) |
| Coronary artery disease | 6 (60) |
| Type 2 diabetes | 3 (30) |
| Hyperlipidemia | 6 (60) |
| Hypertension | 9 (90) |
| Current smoking | 1 (10) |
| Age-adjusted Charlson Comorbidity Index; mean (SD) | 5.4 (2.6) |
| Body mass index; mean (SD) | 30.1 (9.9) |
| NT-proBNP; mean (SD) | 616 (1170) |
| Left ventricular ejection fraction; mean (SD) | 55 (10.0) |
| Medications (N [%]) | |
| Aspirin | 5 (50) |
| Beta blocker | 7 (70) |
| ACE inhibitor / ARB | 9 (90) |
| Diuretic | 7 (70) |
| Statin | 6 (60) |
| Antiplatelet agent | 5 (50) |
| Antidepressant | 1 (10) |
| Anxiolytic | 0 (0) |
| Health outcome measures; all mean (SD) | |
| Positive affect (PANAS; range 10-50) | 38.7 (3.5) |
| Dispositional optimism (LOT-R; range 0-24) | 20.9 (2.4) |
| Depression (HADS-D; range 0-21) | 1.4 (1.3) |
| Anxiety (HADS-A; range 0-21) | 4.2 (2.2) |
| Health behavior outcomes; all mean (SD) | |
| Self-reported adherence (MOS-SAS; range 3-18) | 12.7 (1.3) |
| Self-reported medication adherence | 9.6 (0.5) |
| Sodium intake (SSQ) | 56.7 (22.4) |
| Physical activity/day (minutes)* | 243.4 (156.8) |
| Moderate to vigorous physical activity/day (minutes)* | 28.9 (45.3) |
| Steps/day* | 6,435 (6649) |
N=9
BMI=body mass index; CAD=coronary artery disease; HADS-A=Hospital Anxiety and Depression Scale, Anxiety subscale; HADS-D=Hospital Anxiety and Depression Scale, Depression subscale; HF=heart failure; LOT-R=Life Orientation Test-Revised; LVEF=left ventricular ejection fraction; MI=motivational interviewing; MOS-SAS=Medical Outcomes Study Specific Adherence Scale; PANAS=Positive And Negative Affect Schedule; PP=positive psychology; QoL=quality of life; SD=standard deviation.
Feasibility, acceptability, and immediate impact.
On average, participants completed 7.8 out of 9 (87%) possible PP exercises, and 9 of 10 participants (90%) completed a majority of PP exercises. These results were driven primarily by one participant who withdrew from the study after completing only one phone session and exercise. Nine participants (90%) provided self-report outcome data, and eight (80%) provided accelerometer data. Participants found the PP exercises to be relatively easy to complete (ease score = 7.5 [SD 2.1]) and helpful (utility score = 7.8 [SD 1.8]). There were no significant differences in ease or utility among different PP exercises (ease: F=0.82, p=.58; utility: F=0.80, p=.61).
PP exercises led to a significant increase in happiness (7.8 [SD 1.5; pre-exercise] vs. 8.6 [SD 1.1; post], t=5.4, p<.001) and a marginally significant increase in optimism (8.2 [SD 1.5; pre-exercise] vs. 8.4 [SD 1.3; post], t=1.7, p=.09). When comparing the immediate impact of individual PP exercises on happiness, analyses of variance revealed a marginally significant difference (F=1.98, p=.06), with the “Expressing Gratitude” exercise leading to the greatest improvement (1.7 [SD 1.7] points increase) and the “Integrating Meaning into Daily Life” exercise leading to no change in happiness (0.0 [SD 0.8] points). Individual exercises did not differentially impact optimism (F=1.31, p=.26).
Exploratory outcomes: overall impact on psychological and health behavior outcomes.
The PP intervention led to significant, large pre-post improvements in dispositional optimism (d=0.80, t=2.40, p=.04) and anxiety (d=−0.92, t=−2.75, p=.02), a marginally significant improvement in positive affect (d=0.69, t=2.07, p=.07), and no change in depressive symptoms (d=0.10, t=0.29, p=.78; see Supplementary Table 2). Similarly, post-intervention, participants reported large improvements in adherence to health behaviors (d=1.19, t=3.57, p=.007), medication adherence (d=0.84, t=2.53, p=.04), and sodium intake (d=−0.86, t=−2.44, p=.04). The intervention led to a small- to medium-sized increase in activity (~700 steps per day), though this was not significant (d=0.44, t=1.24, p=.26), and virtually no change in MVPA (d=0.02, t=.04, p=.97).
Discussion
Our findings suggest that a telephone-delivered, PP-based health behavior intervention is feasible, well-accepted, and associated with improvements in proximal psychological outcomes in patients with HF and suboptimal health behavior adherence. These findings are consistent with the literature supporting the feasibility and efficacy of PP interventions at improving psychological health. In patients with and without significant medical illness, PP interventions have been associated with significant improvements in well-being (Bolier et al, 2013; Sin et al, 2009). Furthermore, in patients who have experienced an acute coronary syndrome, a similar, telephone-based PP intervention was feasible, acceptable, and associated with moderate improvements in positive affect, depression, and anxiety (Huffman et al., 2016). The current study suggests that the effects of PP-based treatments on psychological health may extend to HF.
This study also lays the foundation for future research to examine the effects of PP-based interventions on psychological and physical health and engagement in health behaviors in patients with HF. Though our exploratory analyses should be interpreted with caution given the small sample size, lack of blinding, and absence of a control condition, they suggest that a PP-based intervention may improve psychological well-being and health behavior adherence in patients with HF. Future studies should explore the impact of the intervention on these physical, psychological, and health behavior outcomes utilizing an attentional control group that could mitigate any placebo effects that may have been present in this single-arm trial.
Furthermore, future study into the identification of which components of the intervention are most important to improving health is critical. Based on our theoretical model, and consistent with other theories of health behavior change (e.g., the upward spiral theory of lifestyle change (Van Cappellen et al, 2018), the broaden and build theory (Fredrickson, 2001) we hypothesize that the PP and goal-setting portions of the program may work synergistically to improve health behavior adherence by helping participants to feel more positive when performing health behaviors, be more aware of the benefits of those changes, and be more optimistic that their efforts will lead to improvements in health. To test if this is the case, future studies might compare the combined PP and goal-setting intervention to an attentional control condition, as well as to conditions involving PP or goal-setting alone.
Finally, future research should be performed to determine optimal methods of delivery (individual vs. group, in-person vs. telephone vs. online), examine the degree to which PP and goal-setting exercises are integrated, quantify the duration of the effects of the intervention and identify ways to prolong its effects, and identify those health behaviors that are most changeable in response to this type of intervention. These areas of inquiry may help to optimize this or other behavioral interventions to have the greatest positive impact on patients while remaining as efficient and cost-effective as possible.
Our study has several notable limitations. First, it was performed at a single academic medical center and had a largely White population. Second, its small sample size limited our ability to see statistically significant improvements on psychological and health behavior outcomes. Third, at baseline participants were generally very positive, which raises the question of how such an intervention might be received by individuals who are less positive at baseline. Finally, there was no control condition in this initial trial, and participants were not blinded to the main purpose of the trial.
In conclusion, this single-arm, proof-of-concept trial suggests that a telephone-delivered, PP-based intervention was feasible, acceptable, and associated with improvements in both psychological and health behavior outcomes in patients with mild to moderate HF. Larger, controlled trials are needed to determine the efficacy of the intervention on psychological and cardiovascular health. Ultimately, if effective, such an intervention has large public health significance, as it has the potential to improve both health behavior adherence and cardiovascular outcomes in a population at high risk for hospitalization and mortality.
Supplementary Material
Source of Funding
Funding: This research project was supported by the National Heart, Lung, and Blood Institute through grant K23HL123607 (to Dr. Celano). Time for analysis and article preparation was also funded by the National Heart, Lung, and Blood Institute through grant R01HL113272 (to Dr. Huffman). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. The sponsor had no role in the design, analysis, interpretation, or publication of the study. Dr. Celano has received honoraria for talks to Sunovion Pharmaceuticals on topics unrelated to this research.
Footnotes
Trial Registration: ClinicalTrials.gov identifier: NCT02938052
Conflicts of Interest
The authors report no other conflicts of interest.
References
- Arcand J, Ivanov J, Sasson A, Floras V, Al-Hesayen A, Azevedo ER, Mak S, Allard JP & Newton GE (2011) A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. Am J Clin Nutr 93:332–7. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G & Purcaro A (1999) Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99:1173–82. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT & Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52:69–77. [DOI] [PubMed] [Google Scholar]
- Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F & Bohlmeijer E (2013) Positive psychology interventions: a meta-analysis of randomized controlled studies. BioMed Central Public Health 13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie DA & Inoue A (2005) Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs 50:518–27. [DOI] [PubMed] [Google Scholar]
- Brouwers C, Mommersteeg PM, Nyklicek I, Pelle AJ, Westerhuis BL, Szabo BM & Denollet J (2013) Positive affect dimensions and their association with inflammatory biomarkers in patients with chronic heart failure. Biol Psychol 92:220–6. [DOI] [PubMed] [Google Scholar]
- Cain KL, Kelli L & Geremia CM (2011) Accelerometer data collection and scoring manual for adult and senior studies.). San Diego, CA: San Diego State University. [Google Scholar]
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ & Hendrie HC (2002) Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care 40:771–81. [DOI] [PubMed] [Google Scholar]
- Cameron DS, Bertenshaw EJ & Sheeran P (2015) The impact of positive affect on health cognitions and behaviours: a meta-analysis of the experimental evidence. Health Psychol Rev 9:345–65. [DOI] [PubMed] [Google Scholar]
- Cameron DS, Bertenshaw EJ & Sheeran P (2018) Positive affect and physical activity: Testing effects on goal setting, activation, prioritisation, and attainment. Psychol Health 33:258–274. [DOI] [PubMed] [Google Scholar]
- Celano CM, Albanese AM, Millstein RA, Mastromauro CA, Chung WJ, Campbell KA, Legler SR, Park ER, Healy BC, Collins LM, Januzzi JL & Huffman JC (2018) Optimizing a positive psychology intervention to promote health behaviors following an acute coronary syndrome: The Positive Emotions after Acute Coronary Events-III (PEACE-III) randomized factorial trial. Psychosomatic medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano CM, Beale EE, Beach SR, Belcher AM, Suarez L, Motiwala SR, Gandhi PU, Gaggin H, Januzzi JL Jr., Healy BC & Huffman JC (2017) Associations Between Psychological Constructs and Cardiac Biomarkers After Acute Coronary Syndrome. Psychosomatic medicine 79:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano CM & Huffman JC (2011) Depression and cardiac disease: a review. Cardiol Rev 19:130–42. [DOI] [PubMed] [Google Scholar]
- Chida Y & Steptoe A (2008) Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosomatic medicine 70:741–56. [DOI] [PubMed] [Google Scholar]
- Corotto PS, McCarey MM, Adams S, Khazanie P & Whellan DJ (2013) Heart failure patient adherence: epidemiology, cause, and treatment. Heart Fail Clin 9:49–58. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Hays RD & Sherbourne CD (1992) Adherence to cancer regimens: implications for treating the older patient. Oncology 6:50–7. [PubMed] [Google Scholar]
- DuBois CM, Lopez OV, Beale EE, Healy BC, Boehm JK & Huffman JC (2015) Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: A systematic review. Int J Cardiol 195:265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois CM, Millstein RA, Celano CM, Wexler DJ & Huffman JC (2016) Feasibility and Acceptability of a Positive Psychological Intervention for Patients With Type 2 Diabetes. Prim Care Companion CNS Disord 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2001) The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 56:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Zitman FG, Buijsse B & Kromhout D (2007) Lifestyle and dietary correlates of dispositional optimism in men: The Zutphen Elderly Study. J Psychosom Res 63:483–90. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Zitman FG, Hoekstra T & Schouten EG (2004) Dispositional optimism and all-cause and cardiovascular mortality in a prospective cohort of elderly Dutch men and women. Arch Gen Psychiatry 61:1126–35. [DOI] [PubMed] [Google Scholar]
- Hope CJ, Wu J, Tu W, Young J & Murray MD (2004) Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm 61:2043–9. [DOI] [PubMed] [Google Scholar]
- Huffman JC, Albanese AM, Campbell KA, Celano CM, Millstein RA, Mastromauro CA, Healy BC, Chung WJ, Januzzi JL, Collins LM & Park ER (2017) The Positive Emotions after Acute Coronary Events behavioral health intervention: Design, rationale, and preliminary feasibility of a factorial design study. Clin Trials 14:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Mastromauro CA, Boehm JK, Seabrook R, Fricchione GL, Denninger JW & Lyubomirsky S (2011a) Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart International 6:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Mastromauro CA, Sowden G, Fricchione GL, Healy BC & Januzzi JL (2011b) Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes 4:198–205. [DOI] [PubMed] [Google Scholar]
- Huffman JC, Millstein RA, Mastromauro CA, Moore SV, Celano CM, Bedoya CA, Suarez L, Boehm JK & Januzzi JL (2016) A Positive Psychology Intervention for Patients with an Acute Coronary Syndrome: Treatment Development and Proof-of-Concept Trial. Journal of happiness studies 17:1985–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LA, McMillan DG, Anderson A, Fippinger M, Fillerup G & Rider J (2013) Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes CL, Shmotkin D & Ryff CD (2002) Optimizing well-being: the empirical encounter of two traditions. Journal of personality and social psychology 82:1007–22. [PubMed] [Google Scholar]
- Kim ES, Smith J & Kubzansky LD (2014) Prospective study of the association between dispositional optimism and incident heart failure. Circulation. Heart failure 7:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainscak M, Cleland JG, Lenzen MJ, Keber I, Goode K, Follath F, Komajda M & Swedberg K (2007) Nonpharmacologic measures and drug compliance in patients with heart failure: data from the EuroHeart Failure Survey. Am J Cardiol 99:31D–37D. [DOI] [PubMed] [Google Scholar]
- (2016). In Sueta CA & Rodgers JE (Eds)). Chapel Hill, NC: University of North Carolina. [Google Scholar]
- Lemon SC, Olendzki B, Magner R, Li W, Culver AL, Ockene I & Goldberg RJ (2010) The dietary quality of persons with heart failure in NHANES 1999–2006. J Gen Intern Med 25:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H & Wilson IB (2008) Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav 12:86–94. [DOI] [PubMed] [Google Scholar]
- Mason B, Ross L, Gill E, Healy H, Juffs P & Kark A (2014) Development and validation of a dietary screening tool for high sodium consumption in Australian renal patients. J Ren Nutr 24:123–34 e1–3. [DOI] [PubMed] [Google Scholar]
- Masterson Creber R, Patey M, Lee CS, Kuan A, Jurgens C & Riegel B (2016) Motivational interviewing to improve self-care for patients with chronic heart failure: MITI-HF randomized controlled trial. Patient Educ Couns 99:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR & Rollnick S (2002) Motivational interviewing : preparing people for change. (2nd ed, pp xx, 428 p.). New York: Guilford Press. [Google Scholar]
- Moskowitz JT, Carrico AW, Duncan LG, Cohn MA, Cheung EO, Batchelder A, Martinez L, Segawa E, Acree M & Folkman S (2017) Randomized controlled trial of a positive affect intervention for people newly diagnosed with HIV. J Consult Clin Psychol 85:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz JT, Hult JR, Duncan LG, Cohn MA, Maurer S, Bussolari C & Acree M (2012) A positive affect intervention for people experiencing health-related stress: development and non-randomized pilot test. J Health Psychol 17:676–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikrahan GR, Suarez L, Asgari K, Beach SR, Celano CM, Kalantari M, Abedi MR, Etesampour A, Abbas R & Huffman JC (2016) Positive Psychology Interventions for Patients With Heart Disease: A Preliminary Randomized Trial. Psychosomatics 57:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL & Investigators H-A (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogedegbe GO, Boutin-Foster C, Wells MT, Allegrante JP, Isen AM, Jobe JB & Charlson ME (2012) A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Arch Intern Med 172:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis V, Cossette S, Frasure-Smith N, Heppell S & Guertin MC (2010) The efficacy of a motivational nursing intervention based on the stages of change on self-care in heart failure patients. J Cardiovasc Nurs 25:130–41. [DOI] [PubMed] [Google Scholar]
- Peterson JC, Charlson ME, Hoffman Z, Wells MT, Wong SC, Hollenberg JP, Jobe JB, Boschert KA, Isen AM & Allegrante JP (2012) A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Arch Intern Med 172:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN & Sullivan MJ (2003) Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107:1210–25. [DOI] [PubMed] [Google Scholar]
- Rasmussen HN, Scheier MF & Greenhouse JB (2009) Optimism and physical health: a meta-analytic review. Ann Behav Med 37:239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND & Wylie-Rosett J (2011) Heart disease and stroke statistics−−2011 update: a report from the American Heart Association. Circulation 123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge T, Reis VA, Linke SE, Greenberg BH & Mills PJ (2006) Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 48:1527–37. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS & Bridges MW (1994) Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 67:1063–78. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Rashid T & Parks AC (2006) Positive psychotherapy. American Psychologist 61:774–88. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Steen TA, Park N & Peterson C (2005) Positive psychology progress: empirical validation of interventions. American Psychologist 60:410–421. [DOI] [PubMed] [Google Scholar]
- Sin NL & Lyubomirsky S (2009) Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol 65:467–487. [DOI] [PubMed] [Google Scholar]
- Slovinec D’Angelo ME, Pelletier LG, Reid RD & Huta V (2014) The roles of self-efficacy and motivation in the prediction of short- and long-term adherence to exercise among patients with coronary heart disease. Health Psychol 33:1344–53. [DOI] [PubMed] [Google Scholar]
- The Criteria Committee of the New York Heart Association (1994) Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels.). Boston: Little, Brown & Co. [Google Scholar]
- Tindle HA, Chang YF, Kuller LH, Manson JE, Robinson JG, Rosal MC, Siegle GJ & Matthews KA (2009) Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation 120:656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cappellen P, Rice EL, Catalino LI & Fredrickson BL (2018) Positive affective processes underlie positive health behaviour change. Psychol Health 33:77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA & Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- Webel AR, Perazzo J, Longenecker CT, Jenkins T, Sattar A, Rodriguez M, Schreiner N & Josephson RA (2017) The Influence of Exercise on Cardiovascular Health in Sedentary Adults With Human Immunodeficiency Virus. J Cardiovasc Nurs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SP, Walsh H, Ingley KM, Muncaster SA, Gamble GD, Pearl A, Whalley GA, Sharpe N & Doughty RN (2003) Uptake of self-management strategies in a heart failure management programme. Eur J Heart Fail 5:371–80. [DOI] [PubMed] [Google Scholar]
- Wu JR, Moser DK, Lennie TA & Burkhart PV (2008) Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am 43:133–53; vii-viii. [DOI] [PubMed] [Google Scholar]
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP & Bush DE (2000) Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 160:1818–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.