Abstract
This retrospective case series analyzed 45 malpractice claims for delayed detection of esophageal intubation from the Anesthesia Closed Claims Project. Inclusion criteria were cases from 1995–2013, after adoption of identification of CO2 in expired gas to verify correct endotracheal tube position as a monitoring standard by the American Society of Anesthesiologists. Forty-nine percent (95% CI 34%−64%) occurred in the operating room or other anesthesia location where CO2 detection equipment should have been available. The most common factors contributing to delayed detection were not using, ignoring, or misinterpreting CO2 readings. Misdiagnosis, as with bronchospasm, occurred in 33% (95% CI 20%−49%).
Introduction:
Esophageal intubation was a major source of anesthesia patient death and brain damage as well as a major source of liability in the 1980s.1 Esophageal intubation accounted for 6% of all closed anesthesia malpractice claims and 18% of those associated with adverse respiratory events in the earliest closed claims analysis.1 Nearly all (98%) of those claims resulted in death or brain damage and most (82%) resulted in malpractice claim payments, with average payments significantly higher than non-respiratory claims. In 1991, identification of CO2 in the expired gas to verify correct position of the endotracheal tube became an American Society of Anesthesiologists (ASA) standard of basic anesthetic monitoring for general anesthesia.2 This study aims to identify factors associated with delayed detection of esophageal intubation in anesthesia malpractice claims after this change in monitoring standards.
Methods:
After IRB approval, we analyzed trends in delayed detection of esophageal intubation from 1970–2013. We analyzed in detail the 45 claims for delayed detection of esophageal intubation that occurred in 1995 or later in the Anesthesia Closed Claims Project Database of 10,811 claims.3,4 Personnel performing the intubation, location, and factors associated with delayed detection were abstracted from claim narratives. Payment data was adjusted to 2015 dollar values using the Consumer Price Index, with median and interquartile range (IQR) reported. Esophageal intubations between groups were compared by Fisher’s exact test for proportions and Mann Whitney U Test for payment amount, with p<0.05 for statistical significance. Confidence intervals for proportions were calculated according to Fleiss.5
Results:
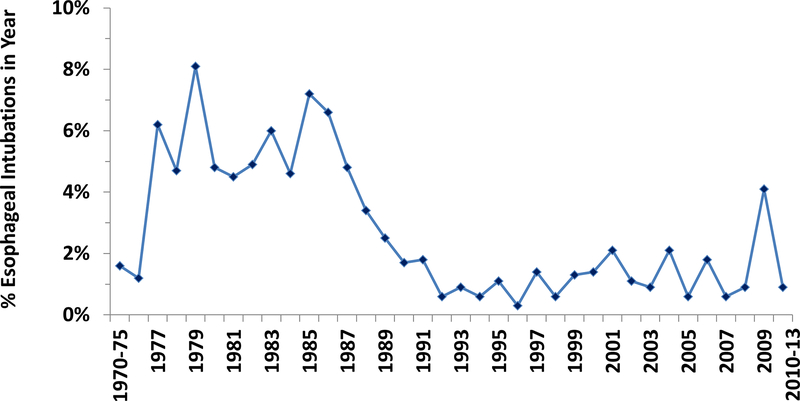
Delayed detection of esophageal intubation declined as a proportion of anesthesia malpractice claims from approximately 3%−8% of claims per year before 1990 to 1–2% per year in 1990 and later (Figure 1). There were a total of 45 cases that occurred in the year 1995 or later, with 31 (69%) occurring in the year 2000 or later.
Trends in esophageal intubation over time. The proportion of claims for delayed detection of esophageal intubation by year of event in the Anesthesia Closed Claims Project Database. The years 1970–75 and 2010–2013 were collapsed due to small total numbers of claims in the database for those years.
Half (49%) of these 45 cases occurred during anesthesia care in surgical locations in an operating room (OR, 40%) or a non-operating room anesthesia (NORA) location (9%). The other most common locations were the intensive care unit (ICU, 20%), resuscitation in the cardiac catheterization lab (cath lab) (11%), and reintubation or resuscitation in the post-anesthesia care unit (PACU, 9%). The remainder occurred in emergency room (ER) and ward (4% each) or outside of the hospital (2%). Purely elective cases accounted for 29% and resuscitation for 38%.
Of the 45 claims for delayed detection of esophageal intubation, 29 (64%) of the intubations were performed by an anesthesiologist and 13 by other healthcare providers (three were unspecified, but most likely not by an anesthesiologist). Among the 29 esophageal intubations by anesthesiologists, 13 were detected by an anesthesiologist, five by another (non-anesthesiologist) physician, and four were not diagnosed until autopsy. Among the 16 claims where the esophagus was intubated by a non-anesthesiologist, nine were detected by an anesthesiologist, two by another type of physician, and two not diagnosed until autopsy. In total, 76% of esophageal intubations were recognized during resuscitation and 13% were not detected until autopsy.
Among the 28 cases in which there was information on the method used to erroneously confirm proper endotracheal tube placement, 15 used one technique, while 11 used two techniques and two used three techniques. The most common technique used to confirm endotracheal tube placement was auscultation of breath sounds (21 cases, 46% of 45 total cases). Other common techniques were colorimetric CO2 detection devices (n=8, 18%), capnography (n=6, 13%), visualization of the larynx (n=5, 11%), and condensation in the endotracheal tube (n=3, 7%).
CO2 monitoring issues were factors in delayed detection in three-quarters of claims (73%). The most common CO2 monitoring issues were not using or ignoring end-tidal CO2 (ETCO2, Table). In 10 of the 12 cases in which a CO2 monitoring device was not used, it was not clear that a CO2 monitoring device was available. Among the nine cases where ETCO2 readings were ignored, overconfidence in endotracheal tube placement was sometimes observed (Table). In seven cases CO2 readings were misinterpreted and in six there was equivocal color change of the colorimetric CO2 device. Confirmation bias was a factor in some of these cases. For example in one case a positive CO2 waveform was seen for a short period of time, leading the team to think the endotracheal tube was correctly placed, but the trace then disappeared. In another case the CO2 device was in the process of calibrating, so the team observed an erroneous positive reading. In one case of equivocal color change the endotracheal tube was removed and replaced, with multiple opinions sought to confirm placement.
Table:
Factors Associated with Delay in Detection of Esophageal Intubation
| Factor | n (% of 45) |
Examples1 |
|---|---|---|
| CO2 Detection Issues | 33 (73%) | |
| Did not use CO2 device | 12 (27%) | CO2 device available in two cases: induction in OR and extubation in PACU; CO2 device not available in three cases: ER, cath lab, NORA intraoperative conversion from MAC to GA; Missing information on CO2 device availability in seven cases. |
| Ignored ETCO2 reading | 9 (20%) | 1: A 50+ yo male post cystoscopy developed respiratory insufficiency during transport to the PACU and was apneic and pulseless upon arrival. The anesthesiologist reintubated the patient, observing the tube pass through the cords. The PACU nurse noted bilateral coarse distant breath sounds on chest auscultation. An ER physician noted absence of breath sounds, the presence of breath sounds in the abdomen, and lack of CO2, but was unable to convince the anesthesiologist to replace the tube until the chest x-ray was obtained that showed esophageal intubation. When the tube was replaced, breath sounds were heard, CO2 was detected, and the patient improved, was transferred to the ICU where he died. |
| Saw positive CO2/misinterpreted CO2 detection | 7 (16%) | 2: A 45+ yo female was returned to the OR for evacuation of a neck hematoma. Laryngoscopy was difficult. After placing the endotracheal tube, the anesthesiologist saw CO2 on the monitor. Within one minute, the CO2 trace disappeared. The patient then arrested. The endotracheal tube was replaced during resuscitation, with a good ETCO2 waveform, and surgery performed. The patient sustained brain damage |
| Equivocal color change | 6 (13%) | 3: A 50+ yo male for urgent embolization of splenic artery in IR under GA was intubated by the anesthesiologist, who thought the tube passed through the vocal cords. The CO2 indicator changed color slowly, then bradycardia occurred. The tube was removed, mask ventilation applied, and atropine administered. The tube was replaced and the anesthesiologist thought it was visualized through the cords. Multiple physicians and nurses auscultated the lungs and the CO2 detection device changed color slowly. Bradycardia occurred again, but was attributed to splenic bleeding. The patient was taken to the OR. When the abdomen was opened, there was no bleeding, but the stomach was ruptured with air coming out from the ventilator. The patient died. |
| Thought device broken | 3 (7%) | 4: A 60+ yo female was scheduled for thyroidectomy. After intubation, breath sounds were assessed as equal bilaterally, gastric sounds negative, and ETCO2 thought to be malfunctioning. Over 10 min, SpO2 decreased to 90% and HR decreased to 60/min. The patient then had bradycardia at 40 to 25 BPM, followed by asystole, treated with CPR. AIMS data showed no ETCO2 and falling FiO2 over 20 min. The patient was extubated and mask ventilated; SpO2 went to 95–98%, but ETCO2 remained <5 (but not zero). After reintubation (20 min after intubation) and continued CPR, ETCO2 went to 38–40 about 13 min after reintubation. The patient sustained hypoxic brain damage and did not regain consciousness. |
| Differential Diagnosis | 15 (33%) | |
| Bronchospasm | 11 (24%) | 5: A 40+ yo male was readmitted for cauterization of nasal bleed after sinus surgery. After intubation, wheezing was noted on the right greater than left, with progressively falling SaO2 and ETCO2. The patient received diphenhydramine and hydrocortisone for poor lung compliance, with fixation on a diagnosis of bronchospasm. About 10–15 min after initial intubation, the patient was reintubated. The patient developed bradycardia. A code was called with resumption of vital signs after 10 min. The emergency surgery proceeded, but the patient did not wake up and died two months later. |
| Other | 4 (9%) | Pneumothorax, hypovolemia, severe bilateral pneumonia, and restrictive pericarditis with pleural effusion (n=1 each) |
| Cardiac arrest (presumably with lack of cardiac output) | 6 (13%) | 6: A 75+ yo male underwent cardiac catheterization with sedation by cath lab nurses. He remained responsive until he became hypotensive, bradycardic, and apneic 4 min after coronary angioplasty. He was treated with bag-mask ventilation, CPR, and pacing wire. Anesthesiologist #1 arrived and intubated; breath sounds were confirmed by a cardiology fellow, with condensation in the ETT and low ETCO2 on the detector. The patient was cyanotic and required CPR. Anesthesiologist #1 left before ABG results were obtained (pH =7.09, PO2=6, PCO2=100), and interpretation was low cardiac output. Repeat ABGs 17 min after arrest were pH=7.02, PO2=8, PCO2=103. The patient was reintubated by anesthesiologist #2. Color immediately improved. Repeat ABGs 6 min later were pH=7.28, PO2=92, PCO2=93. The patient died. |
| Communication Problems | 12 (27%) | 7: An anesthesiologist responded to a code in the ICU after intubation of a 20+ yo patient by an ER physician. The anesthesiologist found clear signs of esophageal intubation, however the ER physician refused to allow anesthesiologist to check via laryngoscopy, insisting on repeating laryngoscopy himself. After >10 min of heated discussion between anesthesiologist and ER physician, the anesthesiologist was finally allowed to check the tube position and detected it in the esophagus. He easily reintubated the patient, but she sustained severe brain damage and died. |
Note: Totals sum to >100% and >45 due to multiple factors in many claims. Ages are rounded to lowest age within 5 yr increments to maintain confidentiality.
Numbered examples are individual cases selected to illustrate the factor. Examples that are not numbered represent a summary of the cases with that factor.
ABG = arterial blood gas; AIMS = anesthesia information management system; BP = blood pressure; bpm = beats per minute; cath lab = cardiac catheterization lab; CPR = cardio-pulmonary resuscitation; ER=emergency room; ETT = endotracheal tube; GA=general anesthesia; HR = heart rate; ICU = intensive care unit; IR = interventional radiology suite; MAC= monitored anesthesia care; min=minutes; NORA =non-operating room location; OR= operating room; PACU =post anesthesia care unit; yo = year old
In one third of cases (33%), late detection was associated with confusion over differential diagnosis, most often bronchospasm (Table). These cases often exemplified fixation errors, with focus on treating a comorbidity rather than questioning endotracheal tube placement.
Cardiac arrest (presumably with lack of cardiac output) contributed to delayed detection in 13% of esophageal intubation claims (Table). Communication problems occurred in 27% of esophageal intubations and were more common when the anesthesiologist was called to help in a non-anesthesia location (43%) than during anesthesia care in the OR/NORA (9%, p=0.017).
Nearly all esophageal intubations with delayed detection resulted in patient death or severe brain damage (96%). Most (67%) resulted in payment made on behalf of the anesthesiologist, with median payment of $665,000 (IQR $236,000 to $1,213,500). Payment on behalf of the anesthesiologist was more common when the anesthesiologist had performed the intubation (86% paid) than when performed by others (50% paid, p=0.041), but the payment amount did not differ between groups.
Discussion:
Although advanced anesthetic monitoring has been a standard of care for confirmation of endotracheal tube placement during general anesthesia since 1991, CO2 detection issues contributed to persistence of delayed detection of esophageal intubation in these malpractice claims for events that occurred well after adoption of these standards (1995 or later, with most occurring in the 2000s). Half of these cases (49%, 95% CI 34%−64%) arose in the operating room or another procedural (NORA) location, where CO2 detection equipment should have been readily available. Although malpractice claims data is limited by retrospective non-random sampling, bias toward severe outcomes, and lack of a denominator for risk estimation, valuable information on relatively rare adverse events can be gained.4 This study found that errors in differential diagnosis and communication problems also contributed to delayed detection (Table). Three cognitive factors contributing to delayed detection of esophageal intubation were observed in this case series: fixation error, confirmation bias, and overconfidence.6 Fixation error is a focus on a single issue at the expense of all others, such as seen in case example five where the team focused on bronchospasm and failed to consider esophageal intubation (Table). Confirmation bias involves focus on information that supports a working diagnosis while ignoring conflicting information. Diagnostic methods such as auscultation of breath sounds used in conjunction with CO2 detection may have contributed to confirmation bias. Air flowing through a tube in the esophagus, especially with the faster gas flows and higher tidal volumes during mechanical ventilation, may be misinterpreted as breath sounds in the lungs on auscultation.7 Vesicular breath sounds may be transmitted to the epigastric area in patients with thin or small body habitus, rendering epigastric auscultation error unreliable for detection of esophageal intubation.7 Confirmation bias was also observed when colorimetric CO2 detection devices showed equivocal color change, such as illustrated by case three (Table), with the team preferring to accept a working diagnosis of correct endotracheal tube placement in the face of ambiguous or potentially conflicting information. Overconfidence with inaccurate self-assessment results in misplaced certainty about diagnoses. In cases one and seven overconfidence was exhibited when the intubating physician refused to allow another physician to check endotracheal tube placement, even in the absence of CO2. Training and education, workplace strategies, and forcing functions to prevent confirmation bias and fixation errors, remedy overconfidence,8 and improve anesthesiologists’ non-technical skills9 have potential to reduce catastrophic patient injury from esophageal intubation. Training can prevent future errors, while workplace strategies and forcing functions can catch errors in present time.8 Education about cognitive errors, including “consider the opposite” procedures, can reduce confirmation bias and fixation errors.8 Workplace strategies to address overconfidence include group decision strategies as a norm in complex situations.8 Forcing functions to catch errors include standing rules that require ruling out “must-not-miss” diagnoses, ROWS (Rule Out Worst-Case Scenario) and “consider the opposite.”8 However, prevention of error through such strategies is entirely theoretical as they have not been tested in the complex, high stakes, real-world environment. Application of these strategies during critical events under conditions of time pressure and uncertainty is fraught with difficulty. Hence, the magnitude of potential improvement in detection of esophageal intubation is impossible to predict. The occurrence of delayed detection related to lack of ETCO2 monitoring devices, especially outside of the operating room, suggests that availability of ETCO2 monitoring devices in all locations where intubation might occur has additional potential to address the continued occurrence of catastrophic injury resulting from delayed detection of esophageal intubation.
Acknowledgments:
The authors acknowledge the closed claims reviewers from the ASA and participation of the following liability insurance companies who have given permission to be acknowledged: Anesthesia Service Medical Group, Inc., San Diego, CA; COPIC Insurance Company, Denver, CO; ISMIE Mutual Insurance Company, Chicago, IL; MAG Mutual Insurance Company, Atlanta, GA; Medical Liability Mutual Insurance Company, New York, NY; Midwest Medical Insurance Company, Minneapolis, MN; NORCAL Mutual Insurance Company, San Francisco, CA; Physicians Insurance A Mutual Company, Seattle, WA; Preferred Physicians Medical Risk Retention Group, Overland Park, KS; Risk Management Foundation, Cambridge, MA; State Volunteer Mutual Insurance Company, Brentwood, TN; The Doctors’ Company, Napa, CA; The University of Texas System, Austin, TX.
Funding:
Supported in part by the American Society of Anesthesiologists (ASA) and the Anesthesia Quality Institute (AQI), Schaumburg, IL. All opinions expressed are those of the authors and do not reflect the policy of the ASA or AQI. REDCap (Research Electronic Data Capture) electronic data capture tools hosted at University of Washington was provided by the Institute of Translational Health Science (ITHS) through UL1 RR025014 from NCRR/NIH.
Preliminary findings were accepted for presentation at the American Society of Anesthesiologists annual meeting in Chicago IL, October 22, 2016. (Honardar MR, et al: Delayed Detection of Esophageal Intubation in Anesthesia Malpractice Claims. Abstract BOC03.)
Footnotes
Conflicts of Interest:
None
Contributor Information
Marzieh R. Honardar, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA.
Karen L. Posner, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA.
Karen B. Domino, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA.
References:
- 1.Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: a closed claims analysis. Anesthesiology 1990;72:828–33 [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists. Standards for Basic Intra-Operative Monitoring. Amended 10/23/1990, effective 1/1/1991.
- 3.Cheney FW, Posner K, Caplan RA, Ward RJ. Standard of care and anesthesia liability. JAMA 1989;261:1599–603. [PubMed] [Google Scholar]
- 4.Cheney FA. The American Society of Anesthesiologists Closed Claims Project: what have we learned, how has it affected practice, and how will it affect practice in the future? Anesthesiology 1999;91:552–6. [DOI] [PubMed] [Google Scholar]
- 5.Fleiss JL. Statistical Methods for Rates and Proportions, 2nd edition. New York, John Wiley & Sons, 1981, pp 14–15. [Google Scholar]
- 6.Stiegler MP, Tung A. Cognitive processes in anesthesiology decision making. Anesthesiology 2014;120:204–17. [DOI] [PubMed] [Google Scholar]
- 7.Birmingham PK, Cheney FW, Ward RJ. Esophageal intubation: a review of detection techniques. Anesth Analg 1986;65:886–91. [PubMed] [Google Scholar]
- 8.Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf 2013;22:ii65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flin R, Maran N. Basic concepts for crew resource management and non-technical skills. Best Pract Res Clin Anaesthesiol 2015;29:27–39. [DOI] [PubMed] [Google Scholar]