Abstract
Objective
The prognostic importance of extracapsular extension (ECE) in breast cancer is not yet clear, especially in patients with pathological T1-2 and N1 (pT1-2N1) disease. We aimed to investigate whether the extent of ECE was an independent prognostic factor for survival outcomes in patients with pT1-2N1 breast cancer.
Materials and Methods
A total number of 131 patients with pT1-2N1 breast cancer treated between 2009 and 2015 were retrospectively evaluated. A single pathologist re-analyzed the histologic examples of all cases. The extent of ECE was graded from 0 to 4.
Results
There was a significant correlation between the number of lymph nodes involved and ECE grade (p=0.004). According to the univariate analysis, lymphovascular invasion (LVI) and ECE grade were the significant prognostic factors for overall survival (OS); age, number of metastatic lymph nodes, menopausal status, and ECE grade were the prognostic factors for disease-free survival (DFS). With a median follow-up of 46 months, grade 3–4 ECE seems to be notably associated with a shorter OS and DFS in multivariate analysis. The mean OS was 85 months for the patients with grade 0–2 ECE vs 75 months for the patients with grade 3–4 ECE (p=0.003). The mean DFS was 83 months for the patients with grade 0–3 ECE vs 68 months for the patients with grade 4 ECE (p=<0.0001).
Conclusion
This research has shown that the extent of ECE is an important prognostic factor for survival in pT1-2N1 breast cancer patients and grade 3–4 ECE seems to be notably associated with a shorter OS and DFS.
Keywords: Axillary lymph node metastases, breast cancer, extracapsular extension, radiotherapy, prognosis
Introduction
The most important prognostic factor for early stage breast cancer is the involvement of axillary lymph nodes (1, 2). Extracapsular extension (ECE) frequently identified as invasive cancer appearing at least invasion of the lymph node capsule or passing through the nodal capsule into the perinodal tissue (3). The association between the presence of ECE and poorer outcomes in breast cancer was shown in the 1970s and numerous studies further investigated this issue (3–13). Mambo and colleagues revealed that the presence of ECE had a detrimental impact on prognosis only for patients with three or fewer metastatic axillary lymph nodes (4). Some researchers claimed that the presence of ECE is an indicator of worse locoregional recurrence (7, 10), while others claimed that the existence of ECE is associated with poorer survival outcomes and increased distant metastasis (6, 9, 10) and still others suggested that it has no effect (8).
Otherwise, the absence or presence of ECE has been mostly registered as ‘no’ or ‘yes’, with no specification and without any quantitation in these studies. The extent of ECE was defined as a 5-level scale (on a scale from 0 to 4) by Lewis and colleagues in head and neck cancer and they showed a better correlation with survival outcomes than ECE as a dichotomous (14).
In this study, we aimed to investigate whether the extension of ECE grading is an independent prognostic factor for survival outcomes in patients with pathologically T1-2 and N1 (pT1-2N1) breast cancer and whether this type of scale can be used for breast cancer.
Materials and Methods
Patient population
The data of 169 women with pT1-T2N1 breast cancer who had been treated postoperatively after breast conserving surgery (BCS) or modified radical mastectomy (MRM) and axillary lymph node dissection (ALND) between 2009 and 2015 were retrospectively reviewed. Patients received neoadjuvant chemotherapy, <10 axillary lymph nodes removed (incomplete lymph node dissection), had another concurrent cancer, had initially distant metastases and follow-up period <12 months were excluded. The 7th American Joint Committee on Cancer (AJCC) staging system was used for tumor staging. Finally, 131 patients with pT1-2N1 breast cancer were included in this study. We gathered not only treatment method information such as type of chemotherapy, type of hormonotherapy, type of surgery, and radiotherapy, but also clinicopathologic prognostic factors such as histologic type and grade, tumor stage, number of excised and metastatic axillary lymph nodes, size of metastatic lymph nodes, age, menopausal status, status of estrogen/progesterone receptor (ER/PR), status of human epithelial growth factor receptor family 2 (Her2) and existence of lymphovascular invasion (LVI). The Nottingham combined histologic grade (Elston-Ellis modification of Scarff-Bloom Richardson grading system) were used for grading and the World Health Organization (WHO) classification of breast cancer were used for classification of invasive carcinomas. A single pathologist re-analyzed the histologic examples of all cases (F.S.) without information of the patient survival outcomes and the histologic slides were graded for the highest degree of ECE using the scale published by Lewis and colleagues (14). The grading of ECE was as follows: grade 0= tumour within the side of lymph node or tumour within subcapsular sinus without thickening of the lymph node capsule; grade 1=tumour encompassing subcapsular sinus with thickening of the lymph node capsule; grade 2=tumour spreading ≤1mm beyond the lymph node capsule; grade 3=tumour spreading ≥1 mm beyond the lymph node capsule; grade 4=no residual lymph node habit with soft tissue masses. This research was approved by the institutional review board and conducted according to the ethical principles of the declaration of Helsinki. Because of the retrospective design of study, the informed consent was not received.
Treatment
After surgery, 4 cycles of cyclophosphamide and doxorubicin followed by 4 cycles of docetaxel were suggested to all patients with pN1, except for patients who were aged ≥70 years with positive hormone receptor or who had poor performance status. Adjuvant hormonotherapy was applied to all patients with positive ER/PR.
A standard two parallel opposing tangential fields and a single ipsilateral anterior supraclavicular fossa (SCF) field were delivered using three-dimensional conformal radiotherapy technique (3D-CRT) for a total dose of 50 Gy to the breast/chest wall and SCF. In BCS patients, 10–16 Gy boost dose was applied to the tumor bed additionally.
The end points
The primary endpoint of this research was to investigate whether the extent of ECE grading was an independent prognostic factor for survival outcomes in pT1-2N1 breast cancer patients. The time from the date of finishing of RT to the date of the recurrence or distant metastases was defined as the disease-free survival (DFS) and the time from the date of finishing of RT to the date of last follow-up or death was defined as overall survival (OS). Patients were routinely evaluated for tumor control in 3-month intervals in the first two years and in 6-month interval for the next three years, and annually thereafter.
Statistical Analysis
SPSS Statistic program version 13 was used for statistical analysis. The median value, the mean value, the proportion value and the standard deviation values were performed for descriptive statistics. Pearson’ s Chi-square test was carried out to compare the categorical variables and independent sample t-test and ANOVA test were carried out to compare the continuous variables. Kaplan-Meier survival analysis was performed to estimate survival analysis. In univariate analysis, survival curves of subgroups were compared with the two-sided long rank test. Cox proportional regression analysis was performed for prediction of 95% confidence intervals (CIs) and hazard ratios. All the variables with statistical significance (p≤0.05) in univariate analysis were added as covariates in multivariate analysis.
Results
Pathologic assessment
The median number of removed axillary lymph nodes was 18 (range, 10–47). 68 patients (52%) had one, 40 patients (31%) had two and 23 patients (17%) had three involved lymph nodes. The median pathological lymph node size was 1.3 cm (range, 0.3–5). Pathologic assessment of the axillary lymph node dissection samples showed that there were 10 ECE grade 0, 47 ECE grade 1, 19 ECE grade 2, 38 ECE grade 3, and 17 ECE grade 4 cases. There was no correlation between pathological lymph node size and grade of ECE but there was a correlation between involved axillary lymph node number and grade of ECE (p=0.004) (Table 1). The median tumor size was 2.5 (range; 0.9–5) cm. There was no correlation between tumor size and grade of ECE. Tumor characteristics are summarized in Table 2.
Table 1.
Correlation of ECE grade with the number of lymph nodes involved
| ECE grade | Number of lymph nodes involved* | Total (%) | ||
|---|---|---|---|---|
| 1 (%) | 2 (%) | 3(%) | ||
| Grade 0 | 8 (80) | 2 (20) | 0 (0) | 10 (8) |
| Grade 1 | 31(66) | 13 (28) | 3 (6) | 47 (36) |
| Grade 2 | 12 (63) | 5 (26) | 2 (10) | 19 (15) |
| Grade 3 | 12 (32) | 14 (37) | 12 (31) | 38 (29) |
| Grade 4 | 5 (29) | 6 (35) | 6 (35) | 17 (13) |
| Total (%) | 68 (52) | 40 (30) | 23 (18) | 131 (100) |
ECE: extracapsular extension.
Correlation of extracapsular extension grade with the number of lymph nodes involved, p=0.004
Table 2.
Tumor characteristics
| Variables | No. of patients (total:131) | % |
|---|---|---|
| Histopathology | ||
| Invasive ductal carcinoma | 117 | 90 |
| The others | 14 | 10 |
| Tumor grade | ||
| Grade 1 | 6 | 5 |
| Grade 2 | 77 | 59 |
| Grade 3 | 48 | 36 |
| Hormonal status | ||
| ER (+) PR (+) c-erbB2 (−) | 75 | 57 |
| ER (+) PR (+) c-erbB2 (+) | 26 | 20 |
| ER (−) PR (−) c-erbB2 (+) | 18 | 14 |
| Triple (−) | 12 | 9 |
| Tumor size | ||
| Median (cm) | 2.5 (0.9–5) | |
| Dissected lymph node number | ||
| Median | 18 (10–47) | |
| The number of lymph nodes involved | ||
| 1 | 68 | 52 |
| 2 | 40 | 31 |
| 3 | 23 | 17 |
| Involved lymph node size | ||
| Median (cm) | 1.3 (0.3–5) | |
| LVI | ||
| Yes | 59 | 45 |
| No | 52 | 40 |
| Unknown | 20 | 15 |
| ECE grade | ||
| Grade 0–2 | 76 | 58 |
| Grade 3–4 | 55 | 42 |
ECE: extracapsular extension; ER: estrogen receptor; LVI: lymphovascular invasion; PR: progesterone receptor
Patients characteristics
The median patient age was 50 (27–82) years. Mastectomy+ ALND was applied 71% of the patients. 43% of the patients were in a premenopausal status. 9% of the patients had triple (−) hormonal status. 96% of the patients used chemotherapy, 78% of the patients used hormonotherapy and 30% of the patients used trastuzumab. All the patients received adjuvant RT to the chest wall/breast and ipsilateral SCF. Treatment and patient characteristics are summarized in Table 3.
Table 3.
Patients and treatment characteristics
| Variables | No. of patients (total:131) | % |
|---|---|---|
| Age (years) | ||
| Median | 50 (27–82) | |
| <45 | 32 | 24 |
| ≥45 | 99 | 76 |
| Menopausal status | ||
| Premenopausal | 63 | 48 |
| Peri/postmenopausal | 68 | 52 |
| Surgery type | ||
| Mastectomy+ALND | 93 | 71 |
| BCS+ALND | 38 | 29 |
| Chemotherapy | ||
| Yes | 126 | 96 |
| No | 5 | 4 |
| Hormonotherapy | ||
| Yes | 102 | 78 |
| No | 29 | 22 |
| Trastuzumab | ||
| Yes | 39 | 30 |
| No | 92 | 70 |
MRM: modified radical mastectomy; ALND: axillary lymph node dissection; BCS: breast conserving surgery
Survival Analysis
The median follow-up time was 46 (range; 14–86) months. Eight of the 131 patients (6%) died and sixteen of the 131 patients (12%) had distant metastases during the follow-up period. None of the patients had loco-regional recurrence. Distribution of distant metastasis and number of died patients according to ECE grade is shown in Table 4.
Table 4.
Distribution of distant metastasis and number of died patients according to ECE grade
| ECE grade | n (%) | Number of distant metastasis* | Number of died patientso |
|---|---|---|---|
| Grade 0 | 10 (8) | 1 | 0 |
| Grade 1 | 47 (36) | 1 | 1 |
| Grade 2 | 19 (15) | 1 | 0 |
| Grade 3 | 38 (29) | 6 | 2 |
| Grade 4 | 17 (13) | 7 | 5 |
| Total (%) | 131 (100) | 16 | 8 |
ECE: extracapsular extension
Correlation of ECE grade with number of distant metastasis, p=0.001
Correlation of ECE grade with number of died patients, p=0.001
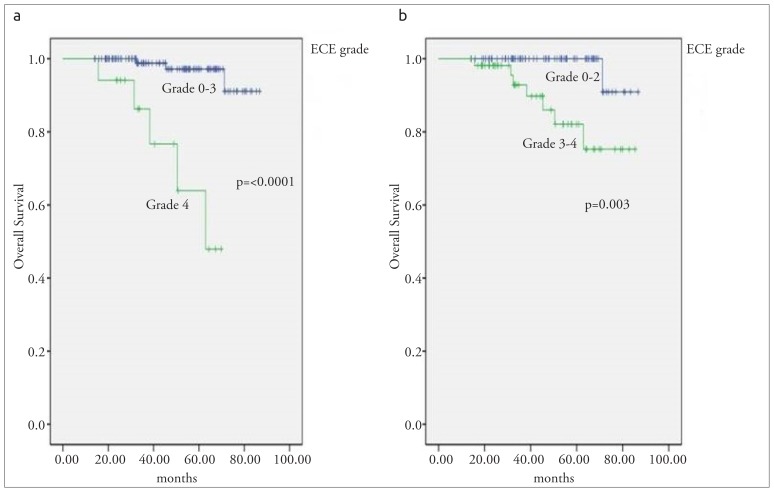
The mean OS was 81 (range; 78–85) months for all the patients. 2- and 5-year OS rates were 99% and 93%, respectively. According to the Kaplan Meier analysis, LVI and ECE grade were the significant prognostic factors for pT1-2 N1 breast cancer. Patients with LVI had a poorer OS than patients with no-LVI (p=0.019). ECE grade was the other prognostic factor for OS (p=<0.0001). The mean OS was 84 months for the patients with grade 0–3 ECE vs 57 months for the patients with grade 4 ECE (p=<0.0001; Figure 1a) and the mean OS was 85 months for the patients with grade 0–2 ECE vs 75 months for the patients with grade 3–4 ECE (p=0.003; Figure 1b) Cox proportional hazard regression analysis was also assessed. According to multivariate analysis, where age, menopausal status, hormonal status, number of metastatic axillary lymph nodes, tumor size, and ECE grade were entered as predictive variables, the ECE grade (HR=12.4, 95 CI, 1.1–133.7, p=0.03) was the only prognostic factor for OS. The statistical analysis results are shown in Table 5.
Figure 1. a, b.
Overall survival curve according to ECE grade (a) Grade 0–3 vs Grade 4 (b) Grade 0–2 vs Grade 3–4
Table 5.
Univariate and multivariate proportional hazard regression analysis related with OS
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | ||||||
| ≥45 years | 1 | 0.4–8.21 | 1 | 0.1–9.5 | 0.7 | |
| <45 years | 1.96 | 0.3 | 1.3 | |||
| Menopausal status | ||||||
| Postmenopausal/perimenopausal | 1 | 0.86–21.57 | 0.07 | 1 | 0.6–34 | 0.1 |
| Perimenopausal | 4.32 | 4.5 | ||||
| Triple negative | ||||||
| No | 1 | 0.59–14.85 | 0.1 | 1 | 0.1–6.1 | 0.9 |
| Yes | 2.96 | 1.1 | ||||
| Tumor size | 1.54 | 0.77–3.07 | 0.2 | 1.3 | 0.5–3.1 | 0.5 |
| The number of lymph nodes involved | ||||||
| 1 | 1 | 0.3 | 1 | 0.6 | ||
| 2 | 1.49 | 0.24–8.94 | 0.6 | 0.8 | 0.1–6.1 | 0.3 |
| 3 | 3.54 | 0.67–18.71 | 0.1 | 1.3 | 0.1–10.5 | 0.7 |
| ECE grade | ||||||
| ECE grade 0–2 | 1 | 1 | ||||
| ECE grade 3–4 | 12.3 | 2.5–100 | 0.01* | 12.4 | 1.1–133.7 | 0.03* |
| ECE grade 0–3 | 1 | |||||
| ECE grade 4 | 19.5 | 3.8–101 | <0.0001* | |||
ECE: extracapsular extension
Statistically significant
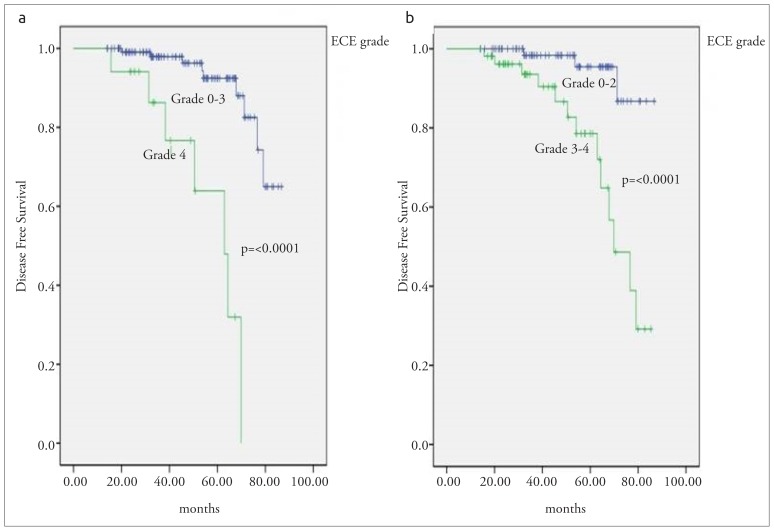
The mean DFS was 77 (range; 73–81) months for all the patients. 2- and 5-year DFS rates were 98% and 88%, respectively. According to the Kaplan Meier analysis, age, number of metastatic axillary lymph node, menopausal status, and ECE grade were the significant prognostic factors for DFS in patients with pathologic T1-2 and N1 breast cancer. Patients with <45 years old had a poorer DFS than ≥45 years old (p=0.004). Premenopausal patients had a poorer outcome than postmenopausal and perimenopausal patients (p=<0.0001). Patients with three involved lymph nodes had poorer outcomes than patients with lesser metastatic lymph node (p=0.03). Patients with grade 4 ECE had the worst survival outcomes than the patients with the other ECE grades. The mean DFS was 80 months for the patients with grade 0–3 ECE vs 56 months for the patients with grade 4 ECE (p=<0.0001; Figure 2a) and the mean DFS was 83 months for the patients with grade 0–2 ECE vs 68 months for the patients with grade 3–4 ECE (p=<0.0001, Figure 2b). According to multivariate Cox regression analysis, to have premenopausal hormone status (HR=6.1, 95 CI, 1.1–35.1, p=0.04) and to have grade 3–4 ECE were the independent prognostic factors for DFS (HR=7.8, 95 CI, 1.1–54.7, p=0.03).
Figure 2. a, b.
Disease-free survival according to ECE grade (a) Grade 0–3 vs Grade 4 (b) Grade 0–2 vs Grade 3–4
Discussion and Conclusion
Stage T1-2 and N1 breast cancer is a unique group that involves a heterogeneous group of tumors characterized by some prognostic clinical, pathological, and molecular factors. Data about the prognostic importance of ECE in breast cancer are conflicting (3–13). Only a few studies have investigated the role of presence of ECE in patients with one to three involved axillary lymph nodes (4–6, 9). On the other hand, absence or presence of ECE has been frequently registered as ‘ no ‘ or ‘ yes ‘ in these studies. This is the first study which evaluates the ECE grade grouping by a scale and using this type of classification seems to be an independent prognostic factor for overall survival in pT1-2N1 breast cancer patients. The results of this research show that the extent of ECE grade is an independent prognostic factor for both DFS and OS in pT1-2N1 breast cancer patients. As referred in the introduction, a correlation between the existence of ECE and survival outcomes was first demonstrated by Fisher and colleagues who reported a significant correlation with an increased risk of failure (3). Mambo and colleagues demonstrated that the presence of ECE has a negative impact on survival outcomes in patients with three or fewer metastatic axillary lymph nodes and no impact on survival outcomes in patients with four or more metastatic axillary lymph nodes (4). Clayton and Hopkins revealed that the presence of ECE had a significant effect on long-term survival in patients with 6 or fewer involved axillary lymph nodes (15). Similarly, Donegan and colleagues reported that the presence of ECE had an influence only in patients with one to three axillary lymph nodes (5). Leonard and colleagues showed that the existence of ECE was associated with a poorer OS (5-year OS rates of 35%, 59%, and 79%, for extensive ECE, focal ECE, and no ECE, respectively) (16). In the present study, 5-year OS rates of 100%, 90%, 100%, 90% and 63%, for Grade 0, Grade 1, Grade 2, Grade 3 and Grade 4 ECE, respectively. In the randomized British Columbia trial, extensive ECE was found to significantly predict for decreased OS and DFS in stage II breast cancer patients (17).
We found that grade 3 and grade 4 ECE significantly correlates with distant metastasis and poorer survival outcomes. 13 of the 16 patients (81%) with distant metastasis were in grade 3–4 ECE group. Seven of the 17 patients (41%) with Grade 4 ECE had distant metastasis, whereas only nine of the remaining 114 patients (7%) with Grade 0–3 ECE had distant recurrence. Additionally, seven of 8 patients (87%) who died were in grade 3–4 ECE group. Five of 17 patients (29%) with grade 4 ECE died, whereas three of 114 patients with grade 0–3 ECE (2%) died. Additionally, ECE grade strongly associated with the number of involved axillary lymph node that known as a significant prognostic factor for early stage breast cancers. Similarly, Hetelekidis and colleagues showed that the presence of ECE correlated with the number of involved axillary lymph node in early stage breast cancer, but the authors did not find any correlation between the existence of ECE and survival outcomes (8). In this study, they may not have found any association because of the binary registration of ECE status as ‘ no ‘ or ‘ yes ‘. However, Leonard and colleagues showed that the presence of ECE associated with the number of involved axillary lymph nodes, and correlated with a poorer OS (16).
There are some important inferences that can be obtained from the present investigation. First, there is not any standard definition of ECE in the literature. Most researchers have used microscopy/histology, but most simply in a binary registration as ‘no’ or ‘yes’. Some authors have used ‘focal’ or ‘extended’ definition with no description. Standard definition of ECE must be identified, because of the ECE status can be considered by future staging systems. In colorectal cancers, the presence of ECE has been involved in the TNM staging system and it was named a specific subcategory of N group, as N1c (17). The presence of ECE was also recognised for breast cancer in the 5th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual and it was named with a specific subcategory, as pN1biii. But, it was removed from the 6th edition of the AJCC because of the usefulness of this nodal staging (18, 19). It is possible that some proofs against its benefit in the staging system and some difficulties during the standard definition of ECE influenced the consideration of its prognostic effect. However, based on the current study results, we recommend that ECE grade according to the scale published by Lewis and colleagues can be used for patients with T1-2 and N1 breast cancer and we also suggest that ECE status can be reconsidered for breast cancer as a prognostic factor. The prognostic importance of ECE grade must be confirmed by future clinical trials and must be investigated for the other stages of breast cancer.
In conclusion, the present research showed that the extent of ECE is an independent prognostic factor for survival outcomes in pT1-2N1 breast cancer patients and grade 3–4 ECE seems to be notably associated with a shorter OS and DFS.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Necmettin Erbakan University Meram Medicine School.
Informed Consent: Informed consent was not received due to the retrospective nature of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - K.G.,; Design - K.G., F.S.; Supervision - K.G., F.S.; Resources - K.G., F.S., Y.B.B., A.M.; Materials - K.G., F.S., Y.B.B.,A.M.; Data Collection and/or Processing - K.G., F.S., Y.B.B., A.M.; Analysis and/or Interpretation - K.G., F.S.; Literature Search - K.G.; Writing Manuscript - K.G.; Critical Review - K.G., F.S., Y.B.B., A.M.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R, Poisson R, Shibata H, Volk H. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Berg JW, Robbins GF. Factors influencing the short and long-term survival of breast cancer patients. Surg Gynecol Obstet. 1966;122:1311–1316. [PubMed] [Google Scholar]
- 3.Fisher ER, Gregorio RM, Redmond C, Kim WS, Fisher B. Pathologic findings from the national surgical adjuvant breast project. (Protocol no. 4). III. The significance of extranodal extension of axillary metastases. Am J Clin Pathol. 1976;65:439–444. doi: 10.1093/ajcp/65.4.439. [DOI] [PubMed] [Google Scholar]
- 4.Mambo NC, Gallagher HS. Carcinoma of the breast: the prognostic significance of extranodal extension of axillary disease. Cancer. 1977;39:2280–2285. doi: 10.1002/1097-0142(197705)39:5<2280::aid-cncr2820390548>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Donegan WL, Stine SB, Samter TG. Implications of extracapsular nodal metastases for treatment and prognosis of breast cancer. Cancer. 1993;72:778–782. doi: 10.1002/1097-0142(19930801)72:3<778::aid-cncr2820720324>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Pierce LJ, Oberman HA, Strawderman MH, Lichter AS. Microscopic extracapsular extension in the axilla: is this an indication for axillary radiotherapy? Int J Radiat Oncol Biol Phys. 1995;33:253–259. doi: 10.1016/0360-3016(95)00081-9. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BJ, Perera FE, Cooke AL, Opeitum A, Dar AR, Venkatesan VM, Stitt L, Radwan JS. Extracapsular axillary node extension in patients receiving adjuvant systemic therapy: an indication for radiotherapy? Int J RadiatOncol Biol Phys. 1997;38:551–559. doi: 10.1016/S0360-3016(97)89483-7. [DOI] [PubMed] [Google Scholar]
- 8.Hetelekidis S, Schnitt SJ, Silver B, Manola J, Bornstein BA, Nixon AJ, Recht A, Gelman R, Harris JR, Connolly JL. The significance of extracapsular extension of axillary lymph node metastases in early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2000;46:31–34. doi: 10.1016/S0360-3016(99)00424-1. [DOI] [PubMed] [Google Scholar]
- 9.Stranzl H, Mayer R, Ofner P, Peintinger F, Prettenhofer U, Hackl A. Extracapsular extension in positive axillary lymph nodes in female breast cancer patients. Patterns of failure and indications for postoperative locoregional irradiation. Strahlenther Onkol. 2004;180:31–37. doi: 10.1007/s00066-004-1170-0. [DOI] [PubMed] [Google Scholar]
- 10.Neri A, Marrelli D, Roviello F, De Stefano A, Guarnieri A, Pallucca E, Pinto E. Prognostic value of extracapsular extension of axillary lymph node metastases in T1 to T3 breast cancer. Ann Surg Oncol. 2005;12:246–253. doi: 10.1245/ASO.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Geng W, Zhang B, Li D, Liang X, Cao X. The effects of ECE on the benefits of PMRT for breast cancer patients with positive axillary nodes. J Radiat Res. 2013;54:712–718. doi: 10.1093/jrr/rrt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooch J, King TA, Eaton A, Dengel L, Stempel M, Corben AD, Morrow M. The extent of extracapsular extension may influence the need for axillary lymph node dissection in patients with T1–T2 breast cancer. Ann Surg Oncol. 2014;21:2897–2903. doi: 10.1245/s10434-014-3752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nottegar A, Veronese N, Senthil M, Roumen RM, Stubbs B, Choi AH, Verheuvel NC, Solmi M, Pea A, Capelli P, Fassan M, Sergi G, Manzato E, Maruzzo M, Bagante F, Koc M, Eryilmaz MA, Bria E, Carbognin L, Bonetti F, Barbareschi M, Luchini C. Extra-nodal extension of lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur J Surg Oncol. 2016;42:919–925. doi: 10.1016/j.ejso.2016.02.259. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Ekstracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carsinoma. Mod Pathol. 2011;24:1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayton F, Hopkins CL. Pathologic correlates of prognosis in lymph node-positive breast carcinomas. Cancer. 1993;71:1780–1790. doi: 10.1002/1097-0142(19930301)71:5<1780::aid-cncr2820710512>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Leonard C, Corkill M, Tompkin J, Zhen B, Waitz D, Norton L, Kinzie J. Are axillary recurrence and overall survival affected by axillary extranodal tumor extension in breast cancer? Implications for radiation therapy. J Clin Oncol. 1995;13:47–53. doi: 10.1200/JCO.1995.13.1.47. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A, editors. AJCC Cancer staging manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 18.Flaming ID, Cooper JS, Henson DE, Hutter VPR, Kennedy BJ, Murphy GP, Osullivan B, Sobin LH, Yarbro JW, editors. AJCC Cancer staging manual. 5th ed. Philadelphia: Lippincott Raven Publishers; 1997. [Google Scholar]
- 19.Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC cancer staging manual. CA Cancer J Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. [DOI] [PubMed] [Google Scholar]