Abstract
The purpose of this article is to review six important inflammatory dermatoses of the vulva and to update readers on the new advancements in treatment of these mucosal conditions. Psoriasis, lichen sclerosis, lichen simplex chronicus and lichen planus are common vulvar conditions that cause pruritis and/or pain. Plasma cell vulvitis and desquamative inflammatory vaginitis are rare and challenging to be recognized, which often remain undiagnosed.
Vulvar Psoriasis: Pathophysiology and Epidemiology
Psoriasis is a chronic, inflammatory skin condition that affects approximately 2% of the general population.1 The pathophysiology is multifactorial, with polygenetic factors playing a role in abnormal T cell activation favoring the TH17 profile. Vulvar psoriasis (VP) often occurs in the setting of generalized plaque psoriasis or inverse psoriasis. Involvement of the genital skin occurs in 29–46% of patients with psoriasis.2 Only 2–5% of psoriatic patients present with involvement limited to the genital area.1 Psoriasis has a bimodal onset with peaks in the third and sixth decades, however, vulvar psoriasis occurs in patients of all ages.
History and Clinical Presentation-VP
Vulvar psoriasis may present with classic plaque type or inverse psoriasis with mild to severe pruritis. Vulvar psoriasis often presents with a nonspecific, poorly demarcated erythema. Scale can be difficult to appreciate in this location. Pain and/or burning may become more severe in the setting of increased inflammation secondary to friction, perspiration and maceration at this site. Vulvar psoriasis may be associated with considerable morbidity, discomfort and embarrassment that may impair psychosexual wellbeing.2
Diagnosis-VP
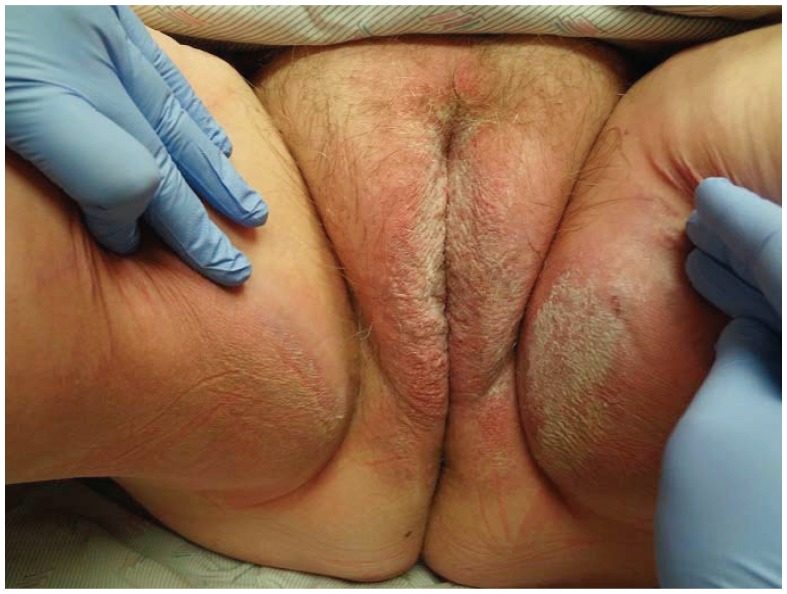
Diagnosis is based on clinical morphology. It often affects hair-bearing cutaneous vulva including the mons pubis and labia majora while sparing the vulvar mucosa, with poorly demarcated erythematous plaques with minimal to no scale. Maceration or fissures may also be appreciated.2 Clinical confirmation is often provided by whole-body cutaneous exam, which reveals features of generalized or inverse psoriasis. Patients with genital psoriasis are more likely to have nail findings compared to those who lack genital involvement.3 Diagnosis may be complicated by superimposed infection, contact dermatitis or changes of lichen simplex chronicus4 (See Figure 1). Differential diagnosis includes vulvovaginal candidiasis, dermatophyte infection, lichen simplex chronicus, extramammary Paget’s disease, and contact dermatitis.4 If clinical diagnosis is unclear or lesions are unresponsive to treatment, skin biopsy may be warranted.
Figure 1.
Vulvar psoriasis complicated with contact dermatitis.
Pathology-VP
Histologic features of vulvar psoriasis are similar to those from non-genital psoriasis, however, typical characteristics may be subtle.1 It features parakeratosis, collections of neutrophils within the stratum corneum (Munro microabscesses), collections of neutrophils within the epidermis (spongiotic pustules of Kujol), regular epidermal acanthosis, thinning of suprapapillary plates, loss of the granular layer and dilatation of vessels in the papillary dermis.
Treatment-VP
Guidelines for treatment of intertriginous psoriasis including vulvar psoriasis were recently published.5 Firstline treatment includes topical steroids. Topical vitamin D analogs or calcineurin inhibitors are a good option for long term therapy and do not carry the risks of atrophy, telangiectasia, striae, and ulceration. Topical steroids and steroid-sparing agents are often used in combination to minimize irritation. Any concomitant infection should be treated accordingly. Second-line treatments include emollients and topical tar-based products. Severe vulvar psoriasis may require systemic therapy, such as methotrexate, oral retinoids or biologic agents.5
Lichen Sclerosis: Pathophysiology and Epidemiology
Lichen sclerosis (LS) is a chronic inflammatory condition with an unknown pathophysiology. It appears to be multifactorial involving genetic, autoimmune, and environmental factors.4 Circulating antibodies directed against extracellular matrix protein 1 have been identified in 74% of patients with lichen sclerosus.6 There is a bimodal age peak at premenarche and postmenopausal, however most patients are 50 years and older.4 Malignant transformation to squamous cell carcinoma has been reported in 2%–5% of patients.4, 7
History and Clinical Presentation-LS
Most patients complain of pruritus, burning and/or pain, however some may be asymptomatic. Dyspareunia may occur in the setting of scarring that leads to narrowing of the vaginal introitus. Perianal involvement may lead to pain on defecation with resultant constipation.8 Symptoms may be progressive or relapsing and remitting, and spontaneous remission is rare.6 Extragenital involvement occurs in less than 20% of patients.7 One should consider screening for a history of autoimmune disease, such as thyroid disease, alopecia areata and vitiligo.6,8
Diagnosis-LS
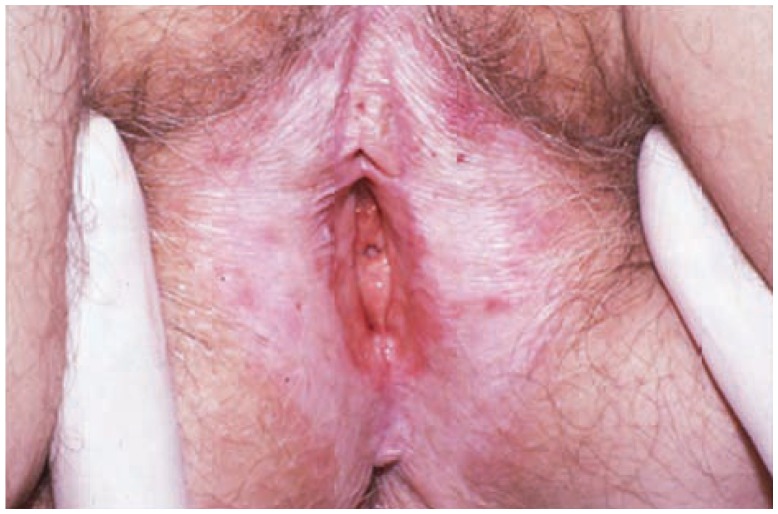
Lichen sclerosus may involve the labia minora, labia majora, clitoris, clitoral hood, perineum and perianus, characteristically forming a figure-of-eight around the vulva and anus6, 7 (See Figure 2). It presents as circumscribed or widespread pallor with a wrinkled texture. Additionally, fissures, erosions, ecchymoses, and pigmentary alteration may be present. Scarring may result in shrinkage or loss of the labia minora, stenosis of the vaginal introitus and scarring of the clitoral hood.7 Of note, the vagina and cervix are typically spared. Differential diagnosis includes lichen planus, psoriasis, lichen simplex chronicus, vitiligo, immunobullous disease and mucous membrane pemphigoid.4, 6 Biopsy can aid in distinguishing lichen sclerosis from lichen planus and in ruling out malignant transformation to squamous cell carcinoma.6, 7
Figure 2.
Lichen sclerosus with shiny glistering cigarette paper-like texture.
Photo courtesy of Libby Edwards, MD
Pathology-LS
Histologic features can vary depending upon the chronicity of the lesion, however, a lichenoid inflammatory infiltrate is characteristic. Early lesions often reveal interface or lichenoid dermatitis. In contrast, well-developed lesions typically have a thin atrophic epidermis, superficial dermal edema with a wide band of hyalinization just below the dermoepidermal junction.7 Dilatation of blood vessels and red blood cell extravasation are also features. Hyperkeratosis may also be appreciated in some specimens. Superimposed changes of lichen simplex chronicus can also be seen.4
Treatment-LS
First line treatment includes potent and ultrapotent topical corticosteroids. Established guidelines recommend daily application of ultrapotent topical corticosteroid for one month, followed by every other day application for one month and then twice weekly application for an additional month.6, 7 Maintenance therapy with less frequent application is recommended. Second-line therapies include topical calcineurin inhibitors 6, 7 For severe disease, systemic retinoids and other immunosuppressant medications may be considered.6
Lichen Simplex Chronicus: Pathophysiology and Epidemiology
Lichen simplex chronicus (LSC) is one of the most common causes of vulvar pruritus and is seen in patients of all ages.7 The cause of LSC is unknown. While many patients have a personal or family history of atopy, the mechanism by which an atopic diathesis results in the development of LSC is not clear. In most circumstances, pruritus occurs secondary to irritation. Common causes of irritation include warmth, moisture, excessive cleaning, and friction from tight clothing. Rubbing or scratching provides immediate yet temporary relief, however ultimately results in skin thickening, which in turn leads to continued rubbing and scratching. This pattern has been coined “the itch-scratch cycle,” which defines LSC. LSC can occur primarily or secondary to underlying dermatoses affecting the vulva.
History and Clinical Presentation-LSC
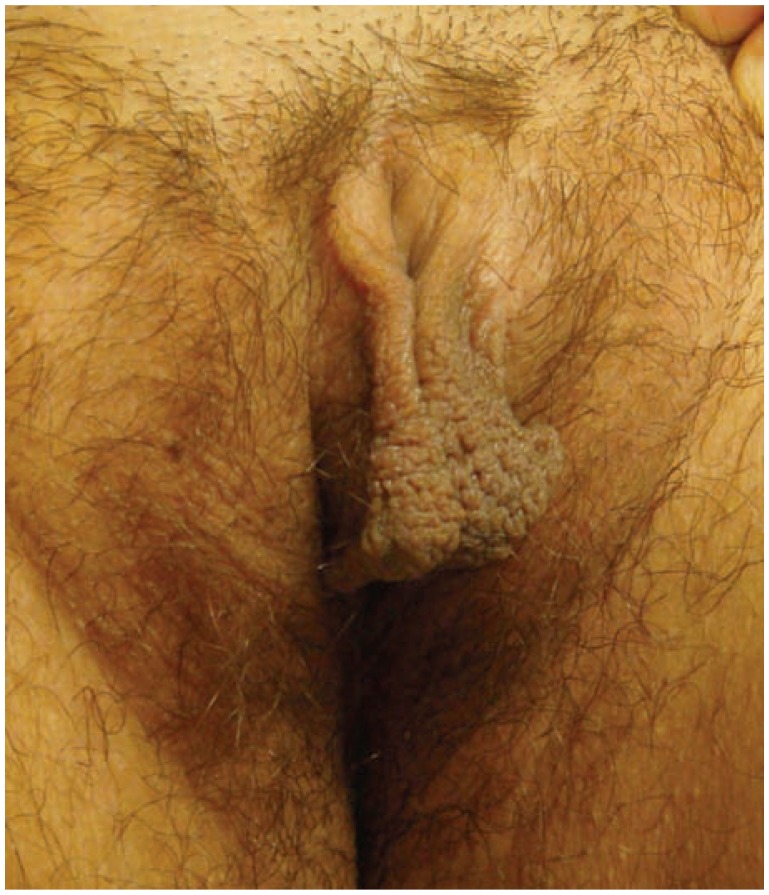
Patients complain of chronic or intermittent pruritus of the vulva that has been present for weeks to years. Many patients identify an inciting event that coincided with onset of pruritus, commonly a yeast infection or other stress, however, note that pruritus persisted after resolution of the triggering event.9 Most patients are conscious of the itch-scratch cycle and are able to minimize rubbing and scratching throughout the day.9 Pruritus is often worse in the evening or during the night, when scratching provides pleasure and relief of symptoms. Frequent nightly rubbing and scratching leads to erosions that cause burning and pain. Patients may try various topical medications or use more aggressive hygiene routines in effort to relieve symptoms; however, these practices often exacerbate irritation and pruritus. (See Figure 3.)
Figure 3.
Lichen simplex chronicus with hyperpigmented lichenified plaques secondary to rubbing.
Diagnosis-LSC
Diagnosis of LSC is based on history and clinical findings. LSC typically affects the hair-bearing portion of the labia majora.7 Examination shows epidermal thickening or lichenified papules and plaques with accentuation of skin markings. Ill-defined erythema, linear excoriations, erosions, ulcers, fissures and crusting may also be appreciated. Scale may or may not be present in the warm, moist environment of the vulva. Pubic hair in the affected areas may be broken or decreased in density as a result of chronic friction. Depending upon the patient’s natural skin color and the severity and chronicity of the symptoms, pigment alteration including post-inflammatory hyper- and/or hypopigmentation may also be appreciated.7
Differential diagnosis of LSC includes psoriasis, extramammary Paget’s disease, candidiasis, dermatophytosis and Streptococcus A infection. A thorough whole-body mucocutaneous exam can aid in the diagnosis if the patient has lesions characteristic of other dermatoses elsewhere. Biopsy can also provide valuable information if the clinical diagnosis is unclear or if the patient is not responding to treatment as expected.
Pathology-LSC
Histology reveals hyperkeratosis, parakeratosis, acanthosis, a prominent granular layer, lengthened rete ridges and a variable chronic inflammatory infiltrate. In addition, lamellar thickening of the papillary dermis and perineural fibrosis may be seen.7
Treatment-LSC
There is no evidence-based data concerning the treatment of LSC.7 However, most experts agree that treatment of LSC requires a multi-faceted approach. First and foremost, identifying and eliminating suspected irritant and allergic contact exposures should be discussed with the patient. A bland skin care regimen is highly recommended. Secondly, the itch-scratch cycle that perpetuates symptoms must be interrupted. This is often accomplished by application of potent or ultrapotent topical corticosteroid. Topical corticosteroids applied once daily for three to four weeks often results in significant improvement in pruritus. While some experts use a tapering schedule over three to six months or transition to a lower potency steroid to help prevent relapse, others advise use of topical corticosteroids as needed. Sedating antihistamines help prevent scratching during sleep and topical anesthetics are alternative options if topical corticosteroids do not provide significant symptomatic relief. Topical calcineurin inhibitors are second-line treatment for patients who are unable to tolerate or are resistant to topical corticosteroids.7 Lastly, it is important to identify underlying conditions or infections that may result in secondary LSC and treat appropriately.8 Patients should be followed closely with monthly exams initially while using daily high potency topical corticosteroids, with less frequent follow-up during maintenance treatment.
Lichen Planus: Pathophysiology and Epidemiology
Lichen planus (LP) is a mucocutaneous inflammatory disease of unknown etiology, which may affect the skin, hair, nails, and mucosal surfaces including the oral cavity and genitalia. It is estimated that approximately 50% of women affected by oral LP have vulvar involvement.10,11 The pathogenesis of LP is poorly understood, although increasing evidence suggests it is autoimmune in nature, governed by CD8+ cytotoxic T lymphocytes.12,13 Antigenic triggers remain unknown.
History and Clinical Presentation-LP
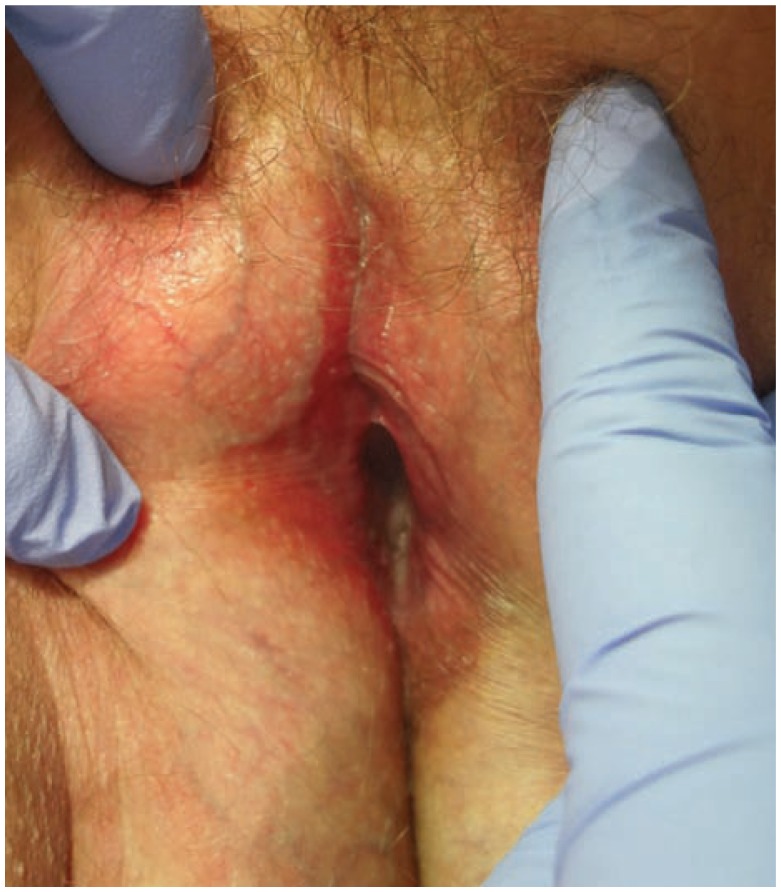
Vulvar LP presents as one or more of three clinical patterns: classic papulosquamous, hypertrophic and erosive.14 Classic lesions usually occur on the labia majora in a similar presentation to other sites of cutaneous LP with pruritic, polygonal, violaceous flat-topped papules with lacelike reticulated white patches called Wickham’s striae.15 The hypertrophic form shows lesions of classic LP with overlying features of lichen simplex chronicus.14 The erosive type is the most common genital form of LP in women and typically affects the labia minora and vaginal introitus.14 (See Figure 4) Its clinical appearance demonstrates bright erythema and erosions and may be surrounded by a white reticular rim. Chronic erosions may lead to scarring in up to 95% of affected patients and subsequent vaginal stenosis, strictures and agglutination.11,15 Erosive LP is frequently characterized by vulvar pain, pruritus, burning and associated dysuria and dyspareunia.16 Rare cases of malignant SCC transformation have been reported, however the link between LP and vulvar neoplasia is not well established.17,18
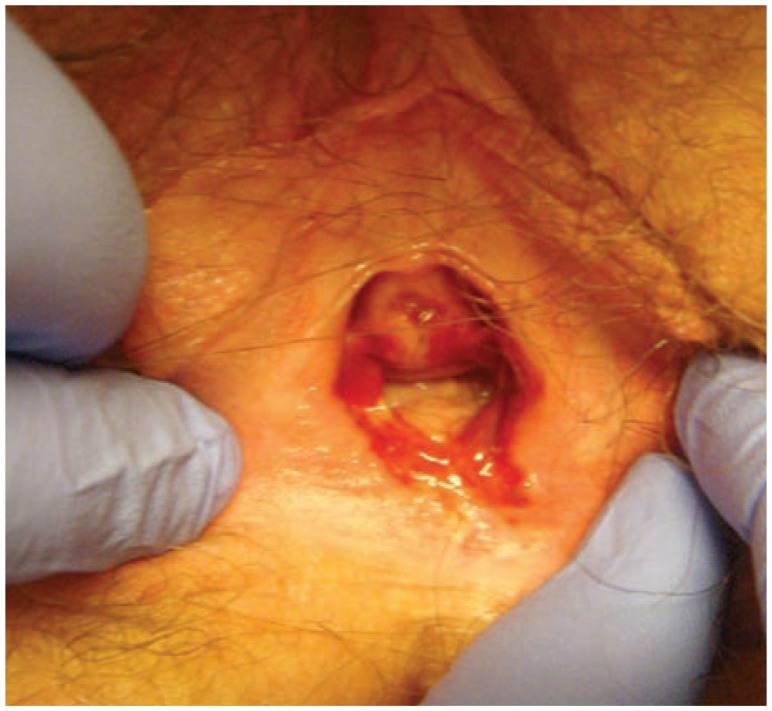
Figure 4.
Erosive Lichen planus with significant scarring and agglutination.
Diagnosis-LP
Clinical diagnosis of vulvar LP is complex, and should always be suspected given bright red and painful vulvar erosions. According to a recent consensus, diagnosis of erosive LP requires at least three of the following: 1) scarring, 2) hyperkeratotic border or Wickham’s striae, 3) other mucosal involvement, 4) well-demarcated erosions at the vaginal introitus, 5) pain/burning symptoms, 6) vaginal inflammation, 7) band-like infiltrate at the dermal-epidermal junction, 8) lymphocyte predominance, and 9) evidence of basal degeneration.19
Pathology-LP
Classic LP lesions show compact orthokeratosis, wedge-shaped hypergranulosis, acanthosis of the epidermis, necrotic keratinocytes within the epidermis (colloid bodies/Civatte bodies), basilar vacuolization, rete ridges with a “saw-tooth” appearance, and a dense band-like lymphocytic infiltrate in the papillary dermis obscuring the dermal-epidermal junction. When located in mucosal sites, LP may feature parakeratosis and a thinned epidermis, while the “saw tooth” rete ridge appearance and “wedge-shaped” hypergranulosis may be attenuated.14 Hypertrophic LP displays similar features to those mentioned above, in addition to pronounced irregular epidermal acanthosis. Erosive LP shows epidermal erosion or ulceration with prominent underlying inflammation; a clue to diagnosis includes typical LP changes at the edge of the ulcer.15 For this reason, a biopsy obtained to help aid in the diagnosis of erosive LP should be taken from the edge of an eroded area.
Treatment-LP
High-potency corticosteroids are first-line treatment; however, monotherapy is rarely sufficient.20 Topical calcineurin inhibitors have also shown good response in select case series.17,21 Alternative therapies to consider in treatment-resistant disease include systemic corticosteroids, immunosuppressants, retinoids and in rare cases, biological agents. Vaginal dilators may be employed to treat introital narrowing.
Plasma Cell Vulvitis: Pathophysiology and Epidemiology
Plasma cell vulvitis (PCV), also known as Zoon’s vulvitis and vulvitis circumscripta plasmacellularis, was first described in 1957 by Garnier and is known as the female counterpart to Zoon’s balanitis.22 It is a rare, benign, chronic inflammatory condition, which is aptly named for its plasma cell rich infiltrate. It tends to affect middle age and elderly postmenopausal women. The origin is unclear, though several hypotheses have been put forth suggesting a triggering factor such as trauma, virus, hormones, and autoimmunity. Its pathogenesis predominantly relies on distinct histopathologic features.
History and Clinical presentation-PCV
Lesions of PCV present as red or orange-brown, glistening, sharply demarcated patches or plaques, and may mimic punctate purpur (See Figure 5). Distribution is most often bilateral and symmetrical, most commonly found on the vestibule and labia minora. Erosions and tissue fragility/friability has been seen.23 It is typically associated with vulvar pruritus, pain, burning, bleeding and discharge. The natural course of disease is characterized by recurrent periods of relapse and remission.
Figure 5.
Rusty red, glistering well demarcated plaques of plasma cell vulvitis.
Diagnosis-PCV
Diagnosis is suggested by clinical examination and confirmed by biopsy. Definitive diagnosis of PCV is based on distinct histologic findings.
Pathology-PCV
The characteristic histopathological features of PCV include superficial erosions with sparse neutrophils scattered in the upper epidermis, moderate spongiosis, effacement of rete ridges, red blood cell extravasation and a mixed lichenoid infiltrate in the papillary dermis rich in plasma cells. This high density of plasma cells is a key feature; they must comprise over 50% of the inflammatory infiltrate, or be accompanied by epidermal atrophy and hemosiderin deposition when comprising only 25–50% of the infiltrate.24 Over time, epidermal atrophy may ensue and subepidermal clefts along with dermal fibrosis may develop. Suprabasilar keratinocytes are notably “diamond-” or “lozenge-shaped.” 25 Vertically oriented dermal blood vessels may be another diagnostic clue.
Treatment-PCV
Asymptomatic patients do not require treatment. When symptoms ensue, potent topical corticosteroids are the first-line treatment.26 Alternative treatments consist of intralesional corticosteroids, imiquimod and topical cyclosporine.27 Topical tacrolimus has not been shown to be effective.28
Desquamative Inflammatory Vaginitis: Pathophysiology and Epidemiology
Desquamative inflammatory vaginitis (DIV) is a relatively common sterile inflammatory vaginitis causing vulvovaginal irritation with accompanying purulent discharge. It was first described by Scheffey, et al. in 1956.29 DIV was initially believed to be a presentation caused by any noninfectious erosive mucosal skin disease, particularly lichen planus, manifesting in the vagina. Current opinion, however, favors that DIV is a distinct clinical entity in itself, characterized by vaginal wall erythema and copious discharge in the absence of infection, erosions or other mucosal involvement.30 The cause of DIV is unknown, though autoimmune etiology has been purported. DIV occurs in both pre- and postmenopausal women.31
History and Clinical Presentation-DIV
Patients with DIV often complain of vulvar burning, irritation and dyspareunia. A yellow or green vaginal discharge is common. Unlike lichen planus, the oral mucosa is spared. Physical examination demonstrates variable erythema of the vestibule, caused by irritation from purulent secretions. Erosions and scarring are absent. The vagina may display red macules and micropapules, mimicking the “strawberry cervix” of trichomonas.30 Vestibular edema may be present in severe cases.
Diagnosis-DIV
Diagnosis of DIV is based on clinical findings of vaginal erythema and purulent vaginal secretions in a female with no other mucous membrane involvement, normal estrogen levels and cultures negative for bacteria, viruses and yeast. In addition, it is necessary to rule out trichomoniasis. Wet mount shows parabasal cells, increased polymorphonuclear leukocytes, and decreased lactobacilli, with an elevated vaginal pH greater than 5.30 Vaginal cultures may show group B streptococcus, however their pathogenic role is unclear. The diagnosis may be missed if a speculum exam to visualize the vaginal vestibule is not performed, and it is often misdiagnosed as dysesthetic vulvodynia.
In all cases of suspected DIV, an investigation for infection and estrogen deficiency should be sought and corrected if identified.
Pathology-DIV
Biopsy of the vaginal wall mucosa may show one of two histologic patterns. In one study, the majority displayed a lichenoid infiltrate without basilar cell damage, while others showed a nonspecific mixed inflammatory infiltrate with lymphocytes, eosinophils and plasma cells.31
Treatment-DIV
First-line therapy for DIV includes topical clindamycin and/or hydrocortisone acetate 25mg rectal suppositories per vagina.31 Sobel reported improvement of symptoms in over 95% of subjects with clindamycin 2% suppositories, however relapse rate was 30%.32 For recalcitrant disease, potent topical corticosteroids may be used. Postmenopausal patients with DIV may require estrogen therapy to achieve remission. 32 It is advised to consider weekly fluconazole to prophylactically prevent secondary candidiasis.30
Acknowledgment
Dr. Simonetta and Dr. Burns have equal contributions to this paper.
Biography
Residents Cassandra Simonetta, MD, Erin K. Burns, MD, and Assistant Professor Mary A. Guo, MD, (above),are in the Department of Dermatology at the Saint Louis School of Medicine.
Contact: aguo@slu.edu

Footnotes
Disclosures
None reported.
References
- 1.Meeuwis KA, de Hullu JA, Massuger LF, van de Kerkhof PC, van Rossum MM. Genital psoriasis: A systematic literature review on this hidden skin disease. Acta Derm Venereol. 2011;91:5–11. doi: 10.2340/00015555-0988. [DOI] [PubMed] [Google Scholar]
- 2.Meeuwis KA, de Hullu JA, Inthout J, et al. Genital Psoriasis Awareness Program: Physical and Psychological Care for Patients with Genital Psoriasis. Acta Derm Venereol. 2015;95:211–216. doi: 10.2340/00015555-1885. [DOI] [PubMed] [Google Scholar]
- 3.Meeuwis KA, van de Kerkhof PC, Massuger LF, de Hullu JA, van Rossum MM. Patients’ experience of psoriasis in the genital area. Dermatology. 2012;224:271–276. doi: 10.1159/000338858. [DOI] [PubMed] [Google Scholar]
- 4.Hoang MP, Reuter J, Papalas JA, et al. Vulvar inflammatory dermatoses: an update and review. Am J Dermatopathol. 2014;36:689–704. doi: 10.1097/DAD.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 5.Kalb RE, Bagel J, Korman NJ, et al. Treatment of intertriginous psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:120–4. doi: 10.1016/j.jaad.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14(1):27–47. doi: 10.1007/s40257-012-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyal-Barracco M, Wendling J. Vulvar dermatosis. Best Pract Res Clin Obstet Gynaecol. 2014 Oct;28(7):946–58. doi: 10.1016/j.bpobgyn.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Doyen J, Demoulin S, Delbecque K, et al. Vulvar skin disorders throughout lifetime: about some representative dermatoses. Biomed Res Int. 2014 doi: 10.1155/2014/595286. 595286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart KM. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28(4):669–80. doi: 10.1016/j.det.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Le Cleach L, Chosidow O. Lichen Planus. New Engl J Med. 2012;366:723–32. doi: 10.1056/NEJMcp1103641. [DOI] [PubMed] [Google Scholar]
- 11.Cooper SM, Wojnarowska F. Influence of treatment of erosive lichen planus of the vulva on its prognosis. Arch Dermatol. 2006;142:289–94. doi: 10.1001/archderm.142.3.289. [DOI] [PubMed] [Google Scholar]
- 12.Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case-control study. Arch Dermatol. 2008;144:1432–5. doi: 10.1001/archderm.144.11.1432. [DOI] [PubMed] [Google Scholar]
- 13.Thornhill MH. Immune mechanisms in oral lichen planus. Acta Odontol Scand. 2001;59:174–177. doi: 10.1080/000163501750266774. [DOI] [PubMed] [Google Scholar]
- 14.Lewis FM. Vulval lichen planus. Br J Dermatol. 1998;138:569–75. doi: 10.1046/j.1365-2133.1998.02164.x. [DOI] [PubMed] [Google Scholar]
- 15.Kirtschig G, Wakelin SH, Wojnarowska F. Mucosal vulval lichen planus: outcome, clinical and laboratory features. JEAVD. 2005;19:301e7. doi: 10.1111/j.1468-3083.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy CM, Galask RP. Erosive vulvar lichen planus: retrospective review of characteristics and outcomes in 113 patients seen in a vulvar specialty clinic. J Reprod Med. 2007;52:43–47. [PubMed] [Google Scholar]
- 17.Kirtschig G, Van Der Meulen AJ, Ion Lipan JW, Stoof TJ. Successful treatment of erosive vulvovaginal lichen planus with topical tacrolimus. Br J Dermatol. 2002;147:625–6. doi: 10.1046/j.1365-2133.2002.488713.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis FM, Harrington CI. Squamous cell carcinoma arising in vulva1 lichen planus. Br JDermatol. 1994;131:703–5. doi: 10.1111/j.1365-2133.1994.tb04987.x. [DOI] [PubMed] [Google Scholar]
- 19.Simpson RC, Thomas KS, Leighton P, et al. Diagnostic criteria for erosive lichen planus affecting the vulva: an international electronic-Delphi consensus exercise. Br J Dermatol. 2013;169:337–343. doi: 10.1111/bjd.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper SM, Haefner HK, Abrahams-Gessel S, Margesson LJ. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520–1. doi: 10.1001/archderm.144.11.1520. [DOI] [PubMed] [Google Scholar]
- 21.Byrd JA, Davis MD, Rogers RS., 3rd Recalcitrant symptomatic vulvar lichen planus: response to topical tacrolimus. Arch Dermatol. 2004;140:715–20. doi: 10.1001/archderm.140.6.715. [DOI] [PubMed] [Google Scholar]
- 22.Garnier G. Benign plasma cell erythroplasia. Br J Dermatol. 1957;69:77–81. doi: 10.1111/j.1365-2133.1957.tb13232.x. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Leopold K, Carlson JA. Chronic vulvar purpura: persistent pig-mented purpuric dermatitis (lichen aureus) of the vulva or plasma cell (Zoon’s) vulvitis? J Cutan Pathol. 2003;30:572–6. doi: 10.1034/j.1600-0560.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 24.Virgili A, Levratti A, Marzola A, Corazza M. Retrospective histopathologic reevaluation of 18 cases of plasma cell vulvitis. J Reprod Med. 2005;50:3–7. [PubMed] [Google Scholar]
- 25.Selim MA, Hoang MP. A Histologic review of vulva inflammatory dermatoses and intraepithelial neoplasm. Dermatol Clin. 2010;28:649–67. doi: 10.1016/j.det.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Botros SM, Dieterich M, Sand PK, Goldberg RP. Successful treatment of Zoon’s vulvitis with high potency topical steroid. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:178–9. doi: 10.1007/s00192-005-1289-2. [DOI] [PubMed] [Google Scholar]
- 27.Hautmann G, Geti V, Difonzo EM. Vulvitis circumscripta plasmacellularis. Int J Dermatol. 1994;33:496–7. doi: 10.1111/j.1365-4362.1994.tb02863.x. [DOI] [PubMed] [Google Scholar]
- 28.Virgili A, Mantovani L, Lauriola MM, Marzola A, Corazza M. Tacrolimus 0.1% ointment: is it really effective in plasma cell vulvitis? Report of four cases. Dermatology. 2008;216:243–6. doi: 10.1159/000112935. [DOI] [PubMed] [Google Scholar]
- 29.Scheffey L, Rakoff A, Lang W. An unusual case of exudative vaginitis (hydrorrhe vagainalis) treated with local hydrocortisone. Am J Obstet Gynecol. 1956;72:210–1. doi: 10.1016/s0002-9378(16)37537-8. [DOI] [PubMed] [Google Scholar]
- 30.Edwards L, Lynch P. Genital Dermatology Atlas. 2nd ed. Philadelphia: Lippincott Williams Wilkens; 2011. Print. [Google Scholar]
- 31.Murphy R, Edwards L. Desquamative inflammatory vaginitis: what is it? J Reprod Med. 2008;88:331–6. [PubMed] [Google Scholar]
- 32.Sobel J. Desquamative inflammatory vaginitis: a new subgroup of prurulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol. 1994;171:1215–20. doi: 10.1016/0002-9378(94)90135-x. [DOI] [PubMed] [Google Scholar]