Abstract
Nicotine and soluble semi-stable aldehydes and ketones in cigarette smoke are key mediators of the elevated risks for vascular disease, cancer, and chronic obstructive pulmonary disease observed in smokers. Nicotine, via sympathetic stimulation, increases risk for both vascular disease and cancer. Comprehensive suppression of sympathetic activity with the well-tolerated drug carvedilol, which inhibits beta1, beta2 and alpha1 adrenergic receptors, may be protective to smokers and other nicotine addicts. The soluble aldehydes and ketones in tobacco smoke appear to exert their adverse effects through activation of NADPH oxidase complexes in vascular tissues and in the lungs. The phytochemical phycocyanobilin (PhyCB), richly supplied by the edible cyanobacterium spirulina, in studies on rodents and in human cell cultures has shown the ability to safely mimic intracellular bilirubin’s physiological role as an inhibitor of NADPH oxidase activity. It therefore may have potential for mitigating the pro-oxidative effects of tobacco smoke aldehydes and ketones. Joint administration of carvedilol and spirulina merits exploration as a strategy for moderating the pathogenic impact of smoking in chronic tobacco users who either fail to quit or refuse to try cessation of tobacco. Carvedilol may be appropriate for those who manage a nicotine addiction in other ways (smokeless tobacco, e-cigarettes, nicotine gum). Further clinical studies to evaluate the impact of carvedilol on cardiovascular risk factors in nicotine addicts, and rodent studies to assess markers of lung inflammation in smoke-exposed rodents fed PhyCB, are recommended.
Adverse Impact of Semi-Stable Organic Oxidants in Tobacco Smoke
The adverse effects of smoking tobacco on vascular health appear to be mediated primarily by nicotine and a range of soluble and relatively stable organic compounds in the smoke capable of undergoing spontaneous addition reactions with thiols and other nucleophiles within vascular cells.1 Notably, these compounds include alpha, beta-unsaturated aldehydes or ketones such as acrolein and crotonaldehyde, and are the major constituents of cigarette smoke. The insoluble tars in tobacco smoke that contribute importantly to mutagenesis in the lung, upper respiratory tract and oral cavity have limited access to the systemic vasculature.
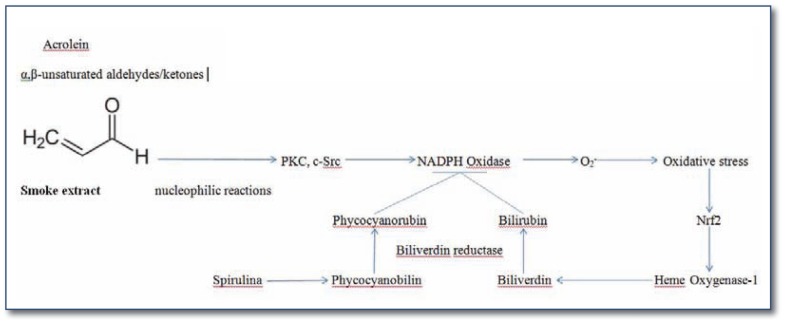
A number of studies have observed that exposure of endothelial or vascular smooth muscle cells, in vitro or in vivo, to cigarette smoke extract (CSE) rich in such soluble compounds leads to induction of oxidative stress generated primarily by NADPH oxidase complexes.1–7 Concurrent inhibition of these complexes largely alleviates the adverse effects of CSE exposure on endothelial or smooth muscle function. CSE exposure has likewise been shown to boost NADPH oxidase activity in airway epithelia, tracheal smooth muscle, keratinocytes, and glioma cells.8–16 Activation of various PKC isoforms, and/or c-Src, by the reactive compounds in CSE, appears to mediate NADPH oxidase activation; activation of protein kinase C (PKC) does not appear to be secondary to activation of phospholipase C and diacylglycerol generation, and so might reflect direct interaction with PKC protein.15 These compounds are also likely to be key mediators of the inflammatory lung damage that markedly increases chronic obstructive pulmonary disease (COPD) risk in smokers, and may contribute to lung carcinogenesis. Highly active oxidants in cigarette smoke, too unstable to exert systemic effects directly, also contribute to this lung inflammation. (Summarized in Figure 1.)
Figure 1.
Semi-stable compounds in cigarette smoke induce oxidative stress throughout the body via NADPH oxidase activation. The phycocyanobilin in spirulina can mimic bilirubin’s physiological role as a feedback inhibitor of NADPH oxidase.
Protective Potential of Spirulina
The intracellular antioxidant activity of bilirubin, generated by activation of the inducible heme oxygenase-1 (HO-1) in response to oxidative stress, has been traced to the capacity of low nanomolar concentrations of bilirubin to inhibit certain NADPH oxidase complexes.17–20 The many prospective epidemiological studies correlating elevated serum bilirubin with favorable health outcomes may reflect either a direct impact of circulating bilirubin on NADPH oxidase activity in cells (possibly important when bilirubin levels are very high, as in Gilbert syndrome), or the ability of serum bilirubin to serve as a marker for more efficient activation of HO-1 in oxidant-stressed cells.21–23
In a twenty year follow-up of the health status of over 500,000 adults monitored by primary care physicians in the British Health Service, Horsfall and colleagues reported that incidence rate of lung cancer per 10,000 person years in men in the top decile of serum bilirubin at baseline was 1.5, as contrasted with 5.0 in those in the bottom decline.24 The inverse association of bilirubin with lung cancer risk held up after adjustment for multiple relevant covariates, including smoking status. The findings in women were quite similar (0.8 vs. 3.0). With respect to risk for COPD in men, the incidence in the top decile of baseline bilirubin was 7.5, as compared to 19.5 in the bottom decile. These findings likewise were validated after appropriate statistical adjustments for covariates, and the findings in women were similar (7.5 vs. 14.8). The findings on lung cancer are confirmative of a previous Belgian prospective study, in which lung cancer mortality in men was 60% lower in the upper vs. lower category of baseline bilirubin, after multivariate adjustment.25 Similarly, in a Taiwanese prospective cohort of over 400,000 individuals, incidence rate per 10,000 was 3.73 and 7.02 in the upper and lower bilirubin quartiles, respectively (P=0.003); mortality rates in those quartiles were 2.46 and 4.84, respectively (P<0.001).26 These findings were significant for those that had ever-smoked, but not non-smokers. With respect to risk for cardiovascular disease, this also tends to correlate inversely with baseline bilirubin levels in a number of prospective studies.27–29
In aggregate, these considerations suggest that the antioxidant effects of bilirubin have considerable potential to alleviate the increased risk for lung cancer, COPD, and vascular disorders associated with smoking. Although there are no rich natural sources of bilirubin or its more soluble precursor biliverdin that could be employed for nutraceutical use (and these complex molecules are expensive to synthesize), cyanobacteria such as spirulina can contain about six parts per thousand dry weight of a biliverdin derivative, phycocyanobilin (PhyCB). This can be converted within cells to a bilirubin homolog, phycocyanorubin, which appears to share bilirubin’s capacity to inhibit NADPH oxidase complexes.30–32 This likely explains why, in a large number of rodent studies, oral administration of spirulina, or of phycocyanin, the spirulina protein to which PhyCB is covalently attached, has exerted anti-inflammatory and cytoprotective effects, including marked inhibition of early atherogenesis in hamsters.30, 33–35 Administration of free PhyCB has recently been shown to inhibit nephropathy in diabetic mice, and to exert marked anti-inflammatory and antioxidant effects on the brain after simulated stroke in rats.32, 36 Hence, we propose that regular oral administration of adequate amounts of spirulina, phycocyanin, or of concentrated PhyCB may have potential for mitigating the adverse effects of the semi-stable soluble compounds in cigarette smoke on vascular health, lung integrity, and lung cancer induction. Surprisingly, there appears to be only one published study to have evaluated the impact of spirulina/phycocycanin on lung health in rodents; this found that oral phycocyanin suppressed induction of paraquat-induced alveolitis and fibrosis in rats.37
Nicotine Contributes to Vascular and Cancer Risk in Smokers
Nicotine, via its stimulatory effects on sympathetic activity, also seems likely to play a mediating role in the vascular risks, and perhaps even cancer risks, associated with smoking. Elevated resting heart rate, a rough correlate of systemic sympathetic activity, correlates with increased risk for cardiovascular mortality and cancer mortality in prospective epidemiology; these correlations persist after adjustment for established risk factors that can modulate heart rate, such as BMI, smoking, and exercise training.38–44 While the impact of sympathetic activity and nicotine on cardiovascular risk evokes little surprise – the use of smokeless tobacco has been linked to increased risk for myocardial infarction and stroke, albeit less dramatic than the increase associated with smoking45, 46 - the impact on cancer risk has been less understood. Recent studies establish that a high proportion of cancers express beta2-adrenergic receptors whose activation makes these cancers more aggressive and harder to kill.47–49 This may explain why both psychological stress and cigarette smoking have been linked to poorer cancer prognosis in a number of studies, and why concurrent use of non-specific beta-blocker drugs has been associated with improved prognosis in some cancers, including lung cancer.50–65 Hence, the propensity of nicotine to activate beta2 receptors in cancer-prone tissues may help to explain why smoking increases cancer risk in many organs not directly exposed to cigarette smoke. With respect to lung cancer, the American Cancer Society Prevention Study II found that, when comparing smokers who switched to chewing tobacco with those who quit nicotine altogether, the former were at higher risk not only for mortality from coronary disease and stroke, but also lung cancer (HR=1.46, 95% CI 1.24–1.73).66
Protective Potential of Carvedilol
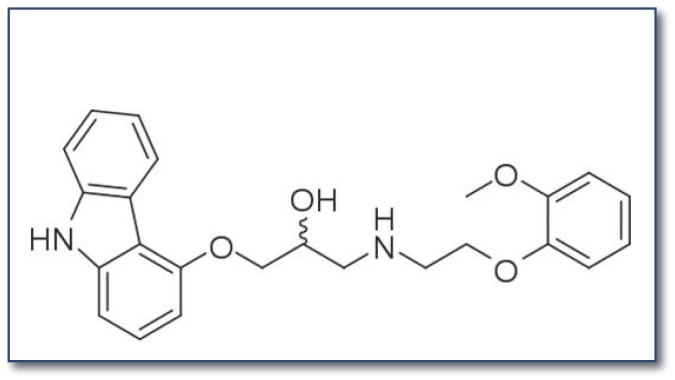
If a nicotine-mediated increase in sympathetic activity is responsible for much of the vascular risk and even some of the cancer risk associated with smoking, then comprehensive suppression of sympathetic activity in smokers may be protective. In particular, the drug carvedilol (see Figure 2), which inhibits beta1, beta2, and alpha1 receptors, and is generally well tolerated in recommended dose ranges, may have potential in this regard. Likely because of its ability to concurrently inhibit alpha1 receptors, thereby inducing arteriolar vasodilation, carvedilol does not appear to have the adverse impact on cardiac output, insulin sensitivity or diabetes risk seen with many other beta-blockers.67, 68 To date, only one published study has examined the effects of carvedilol in abstinent smokers receiving a bolus dose (4 mg) of nicotine.69 The subjects, who had not smoked since the previous evening, received placebo or one of two doses of carvedilol at 8:00 a.m. followed by a light breakfast. Two-and-a-half hours later, a 4 mg nicotine lozenge was administered. Both doses of carvedilol blunted the stimulatory impact of nicotine on heart rate and blood pressure. Importantly, carvedilol did not suppress the pleasurable sensations which the subjects reported after nicotine administration, whereas it did inhibit some of the perceived “bad effects” of the potent nicotine dose. Carvedilol did not influence ratings of tobacco withdrawal symptom severity. These findings suggest that carvedilol may have potential for alleviating much of the health risk associated with smoking or other types of nicotine usage (smokeless tobacco, e-cigarettes, nicotine gum), while not impeding nicotine’s ability to provide positive psychic reward and prevent withdrawal symptoms. As these authors state their findings “may have treatment implications given the cardiovascular risks associated with smoking, some of which are thought to be mediated by the noradrenergic system.” 69 Unfortunately, this insightful report appears to have received little attention and, to date, no follow up.
Figure 2.
Carvedilol provides comprehensive inhibition of nicotine-triggered sympathetic activity by blocking beta1, beta2, and alpha1 receptors. (Failure to inhibit alpha2 receptors is not a drawback, as these act presynaptically to provide feedback suppression of sympathetic activity.)
Smokers are of course at increased risk for COPD, and many practitioners are reluctant to use beta-blockers – especially non-selective beta-blockers such as carvedilol – in the context of COPD, owing to concerns regarding triggering of bronchoconstriction or blunting of response to beta2 agonist bronchodilators. This reluctance does not appear to be warranted based on a survey of current clinical evidence. While it is true that forced expiratory volume in 1 second (FEV1) tends to be slightly lower when patients with COPD are treated with carvedilol as opposed to bisoprolol,70 this small difference appears to have little impact on hard endpoints. As Minor and colleagues summarize in a recent review, “In retrospective and observational analyses, both cardioselective and noncardioselective beta-blockers appear to decrease mortality in COPD patients with and without overt cardiovascular disease, including those with hypertension, heart failure, and atherosclerosis, as well as those undergoing major vascular surgery….Beta blocker use did not decrease the effectiveness of beta2 agonists….In general, these findings do not support current recommendations for the preferential use of cardioselective beta-blockers.”71 Hence, concerns regarding COPD should not discourage use of carvedilol in smokers, with or without COPD.
Suggestions for Clinical Research
It is therefore proposed that carvedilol treatment may be a rational strategy for addicted smokers who fail to quit or refuse to try smoking cessation, or for those who use smokeless tobacco, e-cigarettes, or nicotine gum to manage their nicotine addiction. Longer-term controlled studies evaluating carvedilol administration in nicotine users (smokers or non-smokers), focusing on endothelial function and a wide range of other cardiovascular risk factors, assessing risk for hypotensive complications in normotensive subjects, and determining whether carvedilol influences positively or negatively the subjective satisfaction provided by nicotine, appear worthwhile.
Administration of spirulina – or of phycocyanin or other concentrated sources of PhyCB – may have potential for mitigating the pro-oxidant effects of cigarette smoking that contribute to smoking’s adverse impact on vascular health, lung integrity, and cancer risk. Evaluating this latter strategy in rodent models of smoke exposure would be an appropriate first step in assessing spirulina’s potential for protecting smokers. Inasmuch as high intakes of spirulina might be required to achieve an optimal antioxidant effect in humans – an extrapolation from rodent studies suggests that 15–30 g daily may be needed in this regard30 – and most people find the flavor and odor of spirulina disagreeable, development of spirulina extracts highly enriched in PhyCB may be required to enable the full protective potential of spirulina to be tested. Such extracts could also make it feasible to conduct the prospective double-blind studies required to most convincingly assess PhyCB’s antioxidant/anti-inflammatory activity in humans. Alternatively, development of functional foods in which the flavor of spirulina is adequately masked can be contemplated. Ultimately, clinical trials could be designed to examine PhyCB’s effects on lung markers of inflammation and on vascular risk factors in smokers.
Physicians should construe smoking as a medical condition – a nicotine addiction. The first priority should be to persuade people to discontinue smoking or nicotine use, as this will be more protective than any compensatory measures. But if such efforts fail, rational strategies for minimizing the associated risk should be employed.
Other nutraceutical/pharmaceutical strategies may be of benefit to smokers. There is reason to suspect that ample intakes of long-chain omega-3 fatty acids – most notably docosahexaenoic acid (DHA) – may provide important protection to the lungs and vascular system of smokers.72–80 This will be the topic of a separate report. Tobacco smoke increases the body burden of cadmium, which may contribute significantly to the pathogenic impact of smoking;81, 82 high-dose zinc supplementation may have potential for alleviating cadmium toxicity.83
Metabolic syndrome, like nicotine use, is associated with an up-regulation of sympathetic activity and heart rate, stemming from the impact of hyperinsulinemia on brain regulatory centers, that likely contributes to the increased vascular and cancer risks associated with this syndrome.84 Hence, carvedilol therapy may likewise have important protective potential for patients with metabolic syndrome. Analogously, NADPH oxidase activation is a key reason for the oxidative stress associated with this syndrome, pointing to a potential for spirulina to provide protection in this regard.85–91 Curiously, nicotine tends to promote metabolic syndrome and hyperinsulinemia, both by acute effects on insulin sensitivity mediated by increased lipolysis and a direct cholinergic action on muscle fibers, and in the longer term by a tendency to accumulate fat selectively in visceral stores.92–94 Hence, both the peripheral effects of nicotine, and central effects mediated by hyperinsulinemia, contribute to sympathetic over-activity in smokers.
Biography
Mark F. McCarty, BA, is with Catalytic Longevity, Carlsbad, Ca. James H. O’Keefe, MD, MSMA member since 2003, and James J. DiNicolantonio, PharmD, (above) are with Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
Contact: jjdinicol@gmail.com

References
References exceeded space. Email lfleenor@msma.org for the listing.