Abstract
Brain stimulation has emerged as an effective treatment for a wide range of neurological and psychiatric diseases. Parkinson’s disease, epilepsy, and essential tremor have FDA indications for electrical brain stimulation using intracranially implanted electrodes. Interfacing implantable brain devices with local and cloud computing resources have the potential to improve electrical stimulation efficacy, disease tracking, and management. Epilepsy, in particular, is a neurological disease that might benefit from the integration of brain implants with off-the-body computing for tracking disease and therapy. Recent clinical trials have demonstrated seizure forecasting, seizure detection, and therapeutic electrical stimulation in patients with drug-resistant focal epilepsy. In this paper, we describe a next-generation epilepsy management system that integrates local handheld and cloud-computing resources wirelessly coupled to an implanted device with embedded payloads (sensors, intracranial EEG telemetry, electrical stimulation, classifiers, and control policy implementation). The handheld device and cloud computing resources can provide a seamless interface between patients and physicians, and realtime intracranial EEG can be used to classify brain state (wake/sleep, preseizure, and seizure), implement control policies for electrical stimulation, and track patient health. This system creates a flexible platform in which low demand analytics requiring fast response times are embedded in the implanted device and more complex algorithms are implemented in offthebody local and distributed cloud computing environments. The system enables tracking and management of epileptic neural networks operating over time scales ranging from milliseconds to months.
Keywords: Epilepsy, deep brain stimulation, implantable devices, seizure detection, seizure prediction, distributed computing
Brain Implants integrated with Local and Distributed Computing Devices provide a seamless interface between patients and physicians, and real-time intracranial EEG can be used to classify brain state (wake/sleep, pre-seizure, seizure), implement control policies for electrical stimulation, and track patient health. The system enables tracking and management of epileptic neural networks operating over time scales ranging from milliseconds to months.
I. Introduction
Electrical Brain Stimulation (EBS) via implanted brain electrodes has shown therapeutic benefit in a wide range neurological and psychiatric diseases [1]. Parkinson’s disease and essential tremor have FDA indications for deep brain stimulation (DBS) and epilepsy has FDA indication for responsive neural stimulation (RNS) and DBS of the bilateral electrical stimulation of anterior nucleus of the thalamus for treatment of focal epilepsy.
Preliminary studies also show EBS can improve cognition [2]–[4], depression [5], [6], and post-traumatic disorder [7]. There is now considerable interest in interfacing EBS implants with local and cloud computing resources to link the brain with computational devices [8], [9]. Epilepsy, in particular, is a neurological disease that could benefit from integration of brain implants with off-the-body computing for tracking disease and therapy.
Epilepsy is a common neurological disease affecting approximately 50 million people worldwide. Even in developed countries with access to a wide array of antiseizure medications, approximately 1/3 of people with epilepsy have drug resistant epilepsy (DRE). Epilepsy surgery is an option for some, but many patients are not surgical candidates because their seizures are poorly localized, or originate from multiple foci, or involve eloquent brain regions that cannot be resected. Furthermore, resective surgery is irreversible, and is unsuccessful in achieving seizure freedom in approximately 50% of patients [10], [11]. Advances in neural engineering have led to implantable devices capable of both sensing and electrical stimulation [12], [13]. Duty cycle stimulation of the anterior nucleus of the thalamus (SANTE: [14], [15]) and closedloop, responsive stimulation triggered by detected focal epileptiform activity (RNS: [16]–[18]) reduce seizures, are not destructive, and are reversible if unsuccessful. However, it is rare for patients to achieve long-term seizure freedom [18], [15], and multiple limitations have been recognized using current devices. The current treatment paradigm is limited by the use of stimulation parameters that are not individualized, and adjustments that are driven only by treatment failures. A fundamental limitation of most epilepsy trials is the use of patient diaries for measuring seizure outcome, a serious limitation given the established inaccuracy of patient seizure diaries [19]–[21]. The Medtronic device used in the SANTE trial and the NeuroPace RNS device do not provide electronic seizure diaries. The SANTE trial DBS did not have sensing capability, and thus relied on patient reporting. While the RNS device represents a significant engineering advance over the Medtronic Activa PC device, with continuous sensing, embedded detection algorithms and responsive stimulation, the RNS does not provide accurate seizure diaries. The limited embedded computation power and data storage capacity of the RNS preclude high specificity and sensitivity seizure diaries and seizure analytics. The absence of NeuroPace electronic seizure diaries was the topic of interest and discussed in Epilepsia Gray Matter Letters [22], with follow up response from NeuroPace Inc. clarifying that while the RNS has continuous sensing, the device is not capable of providing high accuracy electronic seizure diaries for the reasons mentioned above [23]. Clinicians, however, can draw inference on the effects of stimulation using RNS counts of detected events, and “long-episodes” of abnormal activity can serve as an imperfect surrogate for possible seizures. But with the limited RNS data storage it is generally not possible to store all the EEG data of the detected events to provide definitive seizure counts. Lastly, while the extended period of time required for treatment optimization is often assumed to be related to brain plasticity and the long-term effects of stimulation, it is possible that since seizures can be relatively infrequent, sporadic, and inaccurately reported this could have significant impact on therapy optimization. Notably, both the SANTE DBS [15] and RNS [18] trials reported multiple years to achieve optimal therapeutic results. We argue that it is time for an accurate seizure diary to guide therapeutic electrical brain stimulation.
Automated seizure detection, electronic seizure diaries, and seizure forecasting have been demonstrated using a novel system (NeuroVista Inc.) consisting of an implanted device providing continuous intracranial EEG (iEEG) telemetry coupled with off-the-body analytics in humans [20] and canines [22]–[27]. The ability to track behavioral state [28] and the correlation of electrophysiological biomarkers with seizure probability [29], [30] have also been demonstrated in humans using iEEG recordings. These advances suggest a new treatment paradigm where stimulation parameters are dynamically adjusted based on seizure probability in order to prevent seizures. The potential to dynamically adjust therapeutic stimulation in response to biomarkers that serve as surrogates for seizures, e.g. pathological interictal epileptiform discharges (IED), pathological high frequency oscillations [31], and other electrophysiological measures of brain excitability [32]–[34], make rational selection and adjustment of stimulation parameters to prevent seizures feasible.
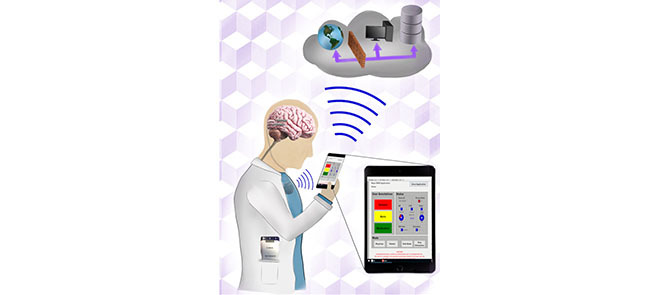
Here we describe a next generation epilepsy therapy system in canines with naturally occurring epilepsy. A handheld epilepsy personal assist device (EPAD) provides bidirectional wireless connectivity with the cloud via cellular and Wi-Fi and with an implantable, investigational neurostimulator (Summit System RC+S: Medtronic Inc.) with embedded scientific payloads (sensors, iEEG telemetry, electrical stimulation, classifiers, and control policy implementation). The epilepsy therapy system (Fig. 1.) provides automated seizure detection [22], [35], seizure forecasting [20], [26] and behavioral state classification [28] running locally on the EPAD or distributed in the cloud. Similarly, electrical stimulation control policy algorithms can run on the implanted device, the technology pioneered in the Neuropace RNS device, the EPAD, and in the cloud. This system provides control policies operating at time scales ranging from milliseconds, again similar to the Neuropace Inc. RNS technology, to months using forecasting algorithms applied to a large database of patient iEEG recordings. A seizure detector embedded on the RC+S device provides rapid (msec) responsive electrical stimulation. Brain state classifiers and control policies running on the EPAD and in the cloud provide seizure forecasting and behavioral state tracking over longer time scales (minutes, hours, days) and could prove useful for intelligent stimulation to prevent seizures and disrupt consolidation of seizure engrams [36], [37]. The multiple computing environments (embedded on device, EPAD, and Cloud) create a distributed computing and data platform for analytics that can track disease and inform therapy [8].
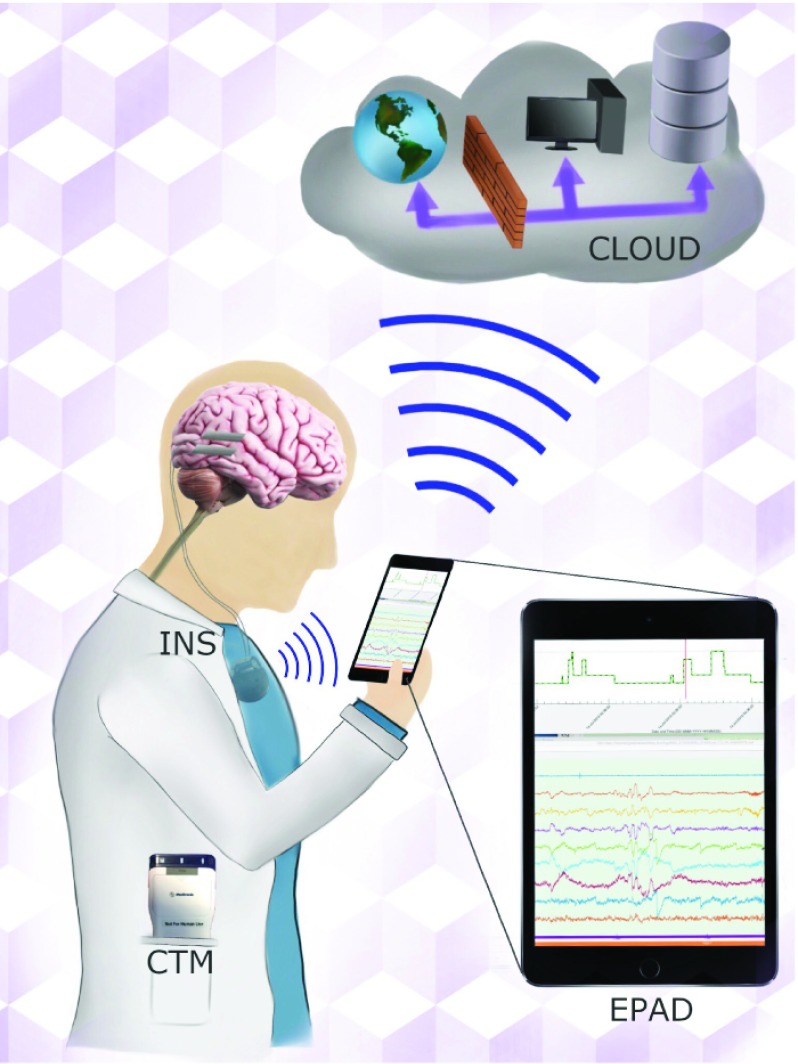
FIGURE 1.
Schematic of next generation epilepsy management system using Medtronic investigational Summit RC+S. The implanted neural stimulator (INS) combines flexible stimulation paradigms, continuous telemetry of intracranial EEG, bi-directional communication between the INS and the Mayo Epilepsy Patient Assistant Device (EPAD) and provided by the Clinician Telemetry Module (CTM) and Summit RDK. The EPAD seamlessly interfaces with the INS and cloud to create a flexible platform with local and distributed computing, analytics, and data storage.
II. Methods
A. Experimental Setup
1). Medtronic’s Investigational Summit System
The Medtronic Inc. Summit RC+S [13] is an investigational device built upon FDA approved technology from the Activa PC family of neurostimulators used for treating Parkinson’s disease, essential tremor, and epilepsy, of which over 140,000 devices have been implanted worldwide. The Activa PC is an implantable neurostimulator without sensing capability, and is approved for use in focal epilepsy throughout much of the world based on the clinical trial investigating stimulation of the anterior nucleus of the thalamus for epilepsy [14]. Electrical stimulation of thalamus was recently approved for treatment of epilepsy in the USA. The device is not currently approved for the treatment of epilepsy in the USA. The Summit RC+S is a rechargeable, implantable 16-channel neurostimulation device providing bidirectional communication with arms-length telemetry, electrophysiological and motion sensing, and adjustable, constant-current stimulation. The Summit RC+S is capable of continuous sensing both inertial signals from an embedded 3axis accelerometer and iEEG from 4 channels selectable from 16 implanted electrode contacts. The Summit RC+S has relatively limited data storage on the device (~3 minute buffer), but provides continuous iEEG (up 1000 Hz sampling rate) telemetry off the device. Basic spectral analysis algorithms (Fast Fourier Transforms and powerband summation) are embedded in device and can trigger realtime, closed-loop, and responsive electrical stimulation.
The implantable RC+S is a component of the investigational Summit System, shown in Figure 2, which provides instruments to wirelessly recharge the INS (PTM – patient telemetry module with RTM – radio telemetry module and antenna) and communicates with the implantable neural stimulator (INS). The Summit system provides a bi-directional interface between the INS and EPAD using the clinician telemetry module (CTM) for wireless communication and data streaming. The INS and CTM communicate via U-Band and the CTM and EPAD communicate via secure Bluetooth. The communication channel between the PC and the RC+S through the CTM are encrypted and secured using out-of-band key exchanges in order to reduce the possibility of interference with the Summit System components. The PC and CTM need to be connected over USB before Bluetooth can be used, and the CTM must be placed directly on top of the RC+S to inductively exchange keys before transitioning to using distance telemetry. By requiring a physical (or inductive) connection between each device before a session can begin, the chance of interference from an unknown source is greatly diminished. To ensure patient safety, the stimulation electrode contacts and parameters can only be programmed by a clinician using the Research Lab Programmer (RLP). The RLP communicates with the INS using CTM and can also be used to define safe parameter ranges that limit how embedded algorithms or connected devices can adjust stimulation. In practice, the safe stimulation parameter range is established in a facetoface encounter between the patient and a clinician who will directly observe clinical symptoms, signs or electrographic abnormalities associated with stimulation before the parameters are programmed into the RC+S. The Summit System also provides an application programming interface (API) that allows researchers to develop their own offthebody device applications to control the Summit RC+S sensing parameters and adjust stimulation within the safe parameter space.
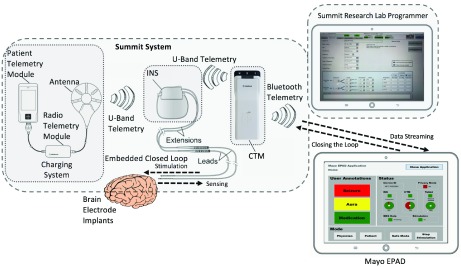
FIGURE 2.
The Medtronic Investigational Summit RC+S system includes: implantable neural stimulator (INS), patient telemetry unit (PTM) and radio telemetry module (RTM) for wireless charging, clinician telemetry module (CTM) for wireless interface between INS and the epilepsy patient assistant device (EPAD) and research lab programmer (RLP). The RLP is used to program INS settings, stimulation and safety parameters. The CTM provides the interface with the EPAD and enables streaming of data from the INS to EPAD and to control the INS (closing the loop). The integrated system provides a flexible platform for device embedded, EPAD, cloud-based analytics and closed loop responsive stimulation.
2). Mayo Epilepsy Personal Assistant Device (EPAD)
The Mayo EPAD is a custom software application running on a tablet device (HP Tablet; Windows 10) with Bluetooth communication with the Medtronic Summit RC+S through a research development kit API. The Mayo EPAD bidirectional communication capability with the implanted RC+S provides an interface for controlling iEEG data acquisition and storage, real-time analysis, and closed loop responsive stimulation. The embedded and external algorithms and control policies are limited to the defined, safe stimulation parameter space. The EPAD also serves as a more powerful local computational node with larger data storage that provides capabilities not possible with the limited computational capabilities of the RC+S device. The EPAD communicates with the cloud infrastructure for large volume data storage, remote data viewing, and large-scale computing for advanced analytics. The EPAD is designed as a communication tool for the patient to enter notes, medications, auras, symptoms, and seizures. The EPAD also provides statistics on connection, data transmission and communication with the INS and resolves connectivity problems if the connection is unstable. The EPAD provides patient alerts to check system components, battery levels, and wireless network connectivity in case issues can’t be solved automatically (e.g. charge a system component). This integrated system provides epilepsy management capabilities needed to optimize therapy and track outcomes (Fig. 1 and 2).
3). Canine Epilepsy
The preclinical testing was performed in normal and epileptic canines. There are many animal epilepsy models induced by chemical or physical insults, but these models may differ significantly from human epilepsy [38]. In contrast, epilepsy occurs naturally in dogs with prevalence, age of onset, and clinical presentation similar to human epilepsy [39]. Canine epilepsy has a prevalence of 0.5 to 5.7% [40], with 65% of seizures characterized as focal onset without secondary generalization [39]. The EEG in canine and human epilepsy is similar [22], [41]–[44]. In summary, the clinical [39], [45], electroencephalographic [22], [46], [47], and pharmacological [41]–[44] features of naturally occurring canine epilepsy are similar to human epilepsy. Naturally occurring canine epilepsy is an excellent vehicle for preclinical translation since the dogs are large enough to accommodate devices designed for humans.
4). Electrode Implantation in Dogs
The study was approved by institutional IACUC committees of Mayo Clinic, University of Minnesota, and University of California Davis where canines were implanted and housed. Electrodes were implanted intracranially in canines under anesthesia using framed stereotaxis with a custom head frame. Subjects underwent a 3.0T MRI (GE Medical Systems) using a stereotactic T1-weighted MPRAGE sequence and a diagnostic coronal T2 FLAIR sequence to screen for abnormalities. Targets and trajectories were planned using stereotactic software (Compass  Stereotactic Systems) adapted for our animal head frame. Burr holes were drilled into the skull for each of the four depth electrodes (Medtronic models 3391 and 3387), which were inserted to depth and secured with clinical wire anchors and bone screws. The electrode tails were tunneled to the RC+S device in a pocket behind the right scapula. The canine underwent a post-op x-ray CT scan (Somatom Flash, Siemens Inc.), which was then co-registered to the stereotactic MRI (Analyze 12.0, BIR, Mayo Foundation) in order to verify targeting accuracy.
Stereotactic Systems) adapted for our animal head frame. Burr holes were drilled into the skull for each of the four depth electrodes (Medtronic models 3391 and 3387), which were inserted to depth and secured with clinical wire anchors and bone screws. The electrode tails were tunneled to the RC+S device in a pocket behind the right scapula. The canine underwent a post-op x-ray CT scan (Somatom Flash, Siemens Inc.), which was then co-registered to the stereotactic MRI (Analyze 12.0, BIR, Mayo Foundation) in order to verify targeting accuracy.
B. Device Performance Metrics
1). Battery Life – Smart Sensing Paradigms
The rechargeable Summit RC+S has battery capacity that provides 24 hours of continuous data streaming and therapeutic stimulation. However, recharging is a patient burden that must be minimized. The sensing & telemetry paradigms on the EPAD include 4-channel continuous sensing with selectable sampling rates (250 1000 Hz) and a smart sensing paradigm designed to optimize battery longevity. The smart sensing paradigm makes use of continuous loop recording (3-minute buffer) of the iEEG on the device and adjustable periodic iEEG telemetry off the device combined with triggered telemetry using an iEEG power threshold to capture putative seizures. The iEEG telemetry performance was evaluated and various sensing and stimulation paradigms designed to extend battery life were explored and evaluated for real-time seizure detection, forecasting, and brain state classification. The performance of seizure detection and forecasting algorithms were compared prospectively using both continuous iEEG telemetry and smart sampling paradigms.
2). Embedded and Hybrid Detection Strategy
Seizure detection was evaluated using an RC+S device embedded linear discriminant analysis (LDA) algorithm alone and a hybrid 2stage algorithm that combined a hypersensitive detector embedded on the RC+S coupled with a more complex classification algorithm running on the EPAD system or in the cloud [35]. To evaluate the detector’s performance, we performed a statistical measure of the binary classification using testing data that was not used for training on hourly basis as follows: True Negative is an hour with no seizure and no detections; False Negative is a verified seizure when there is no device detection; False Positive is detection on the device that was not associated with a seizure; True Positiveis detection on device and an associated seizure. Results are reported as sensitivity (SS) and specificity (SP), and false positive detections rate per day (FPR). The device embedded detector was tuned for hypersensitive detection to capture electrographic seizures. A previously validated seizure detection system requiring a significant computational resource [35] was used on the offthebody EPAD system and on the cloud. The patient specific classifier was trained using data from the initial seizures and interictal data from randomly chosen segments, all collected within the first months of recording.
3). Closed Loop Responsive Stimulation Delays
The bidirectional coupling between the RC+S device and Mayo EPAD provides closedloop stimulation using algorithms embedded directly on the implanted device, or algorithms running on the EPAD, or within a cloud environment. We tested delays in closed loop on the RC+S device and Mayo EPAD. The suitability of these two approaches is driven by the minimum time delay needed to implement an effective therapy and the computational complexity of the algorithm and independence of data connectivity to cloud. The simple detection algorithms embedded on the device achieve fast responsiveness but poorer accuracy, whereas more complex algorithms running on the tablet will show greater latency. We tested timing delays for various closed loop stimulation approaches.
C. Machine Learning and Performance Evaluation
1). Seizure Forecasting With Offthebody Analytics
The computation required for seizure forecasting is considerable, and not possible to run in an embedded fashion on the RC+S device itself. An offthebody forecasting system was implemented using the best performing algorithm in a recent seizure forecasting competition [27]. Classifiers to identify interictal and preictal brain states were trained off-line using one week of interictal data and two to three onehour preictal data segments directly preceding visually verified seizures for each epileptic canine. The algorithm selects the best performing classifier (SVM, random forest, or a linear or logistic regression model) using training data crossvalidation, and then the classifier coefficients are uploaded to the EPAD. Results are reported as sensitivity (SS), mean false positive rate per day (FPR), and time in warning (TIW) [48].
2). A Cloud Platforms for Analyzing Streaming Data
Implantable neurodevices face challenges for data management, including storage, retrieval, and review of recorded signals. Continuous iEEG generates large amounts of data which may need to be accessed across institutions and analyzed both manually and algorithmically. For this project Medtronic ORCA Digital Heath system, Mayo Clinic Electrophysiology Laboratory (MSEL) cloud system, and Blackfynn Inc. (https://www.blackfynn.com) were used for visualizing iEEG data, diaries, annotating seizures and behavioral states, and running algorithms. Seizure markings were validated by board certified epileptologists. For the hybrid detection, during smart sensing, iEEG clips were streamed from MSEL cloud to the Blackfynn cloud platform. Once uploaded to the Blackfynn platform, we retrieved data via a custom python client and ran higher performing, computationally intensive seizure detection algorithms [35].
3). Active Probing of Neural Networks
We used the Medtronic Summit RC+S device for active probing of brain neuronal networks with subthreshold evoked potentials. Four canines (two epilepsy & two controls) were selected for testing. Stimulation parameters such as electrode contacts for stimulation and sensing, stimulation frequency, pulse width, and amplitude were tested and adjusted manually during visual contact with the dogs to ensure there were no clinical signs, seizures, or IED during stimulation. Each canine was stimulated for a period of 48 hours using 30 seconds of stimulation followed by 30 seconds with stimulation off.
4). Automated Behavioral State Classification
To test the feasibility of automated behavioral state classification running on the EPAD with and without background electrical stimulation, we compared awake/deep sleep classification results over 48 hours with a video recording. Data acquired from one dog over a 48hour video iEEG recording (four electrodes sampled at 250 Hz) was analyzed, and a linear discriminant classifier using power in band features [28] was applied and compared in clear wake and deep sleep periods using 48 hours of video iEEG data. We have also evaluated the feasibility of using an unsupervised clustering method and power in band features to separate awake and deep sleep 30-second epochs during electrical stimulation of the brain (frequency of stimulation 2Hz, stimulation amplitude 3mA, pulse width 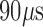 ). These were manually selected twelve 30-seconds epochs within a period of clear wakefulness (captured by the video monitoring) and another 12 segments of 30second epochs were selected from within periods of putative sleep (confirmed by video and iEEG). We extracted power in band (PIB) features during stimulation and used multifactorial analysis and unsupervised K-means clustering to classify awake and deep sleep.
). These were manually selected twelve 30-seconds epochs within a period of clear wakefulness (captured by the video monitoring) and another 12 segments of 30second epochs were selected from within periods of putative sleep (confirmed by video and iEEG). We extracted power in band (PIB) features during stimulation and used multifactorial analysis and unsupervised K-means clustering to classify awake and deep sleep.
III. Results
A. Implantation and Monitoring
Seven dogs (4 epileptic & 3 controls) were implanted with the Medtronic RC+S INS device and 4 intracranial electrodes with 4 contacts each (for a total of 16 recording/stimulation contacts) targeting the neocortex, hippocampus, and anterior nucleus of thalamus (Table 1). There are currently 2 epileptic dogs and 2 control dogs living implanted with the RC+S system. The average post-implant survival duration for all dogs was 376 days. Three dogs died during the study. Two dogs were euthanized due to a progressing infection that did not respond to antibiotics. One dog was found deceased in the kennel after a weekend, following 644 days of monitoring with no indication of illness or distress. Review of the continuous video and iEEG demonstrated that during a 12-hour period before the death the dog had multiple unwitnessed seizures, and a prolonged seizure preceded death.
TABLE 1. Summary of Implanted Subjects. Electrode Configuration, Survival, Days of Recording, and Telemetry Performance.
| Dog # | Epilepsy | Electrode Placement | Survival [days] | Recording [days] | Data [%] |
|---|---|---|---|---|---|
| 1 | Yes | L&R hippocampus, L&R Anterior Nucleus | 644 | 304 | 97.3 |
| 2 | Yes | L&R hippocampus, L&R Anterior Nucleus | 498 | 463 | 95.8 |
| 3 | Yes | L&R NeoCortex | 283 | 240 | 99.1 |
| 4 | Yes | L&R NeoCortex | 303 | 237 | 98.7 |
| 5 | No | L&R NeoCortex | 289 | 252 | 99.4 |
| 6 | No | L&R hippocampus, L&R Neocortex | 224 | 162 | 95.7 |
| 7 | No | L&R NeoCortex | 393 | 392 | 95.24 |
To further evaluate the Epilepsy Management System, we recently implanted two pet dogs with epilepsy that went home with their owners after device implantation. The pet dogs are the first animals to our knowledge to be living outside of the hospital with their owners with data streaming from the EPAD to a cloud environment. The electrode configuration for these two implants was bi-lateral anterior nucleus of thalamus and bilateral hippocampus. We are now managing epilepsy in a real-world use case with dog owners charging the devices (RC+S, CTM & EPAD) regularly, annotating medications and witnessed seizures to create patient diaries. The iEEG data and annotations are transmitted to the cloud and remotely reviewed by physicians to create gold standards annotations of electrographic events and seizures. We are currently gathering seizure data for training of seizure detection and forecasting algorithms.
B. Device Performance Metrics
1). Battery Life - Smart Sensing Paradigms
The iEEG data (sampling rate 250 Hz) was acquired from a fourelectrode configuration for multiple months, with electrode placement unique to each subject. Data were collected based on the smart sensing paradigms described above. The iEEG telemetry performance during continuous and smart sensing paradigm was quantified for each dog by calculating the amount of analyzable iEEG collected (see Table 1). We tested the battery depletion rate for continuous sensing, smart sensing without detection (periodic loop recording), and smart sensing (Fig. 3). The battery depletion rate for continuous telemetry (4 channels at 250 Hz sampling rate) was 4.33 ± 1.66% per hour compared to periodic telemetry with continuous sensing embedded detector battery depletion of 2.72 ± 1.00% per hour (30 seconds on and 90 seconds off), 2.80 ± 1.78% per hour (60 second on and 60 seconds off) and 1.61 ± 0.62% per hour (30 seconds on and 150 second off). The battery depletion rate with these sensing and telemetry paradigms was stable over long term monitoring (Fig. 3A). Figure 3B shows a data plot of the battery level over a period of one month showing a similar battery discharge rate when the same sensing paradigm is maintained. The results suggest that a device using a smart sensing mode - collecting one minute of data separated by one-minute breaks - must be charged every other day, while a device using continuous streaming needs charging every day. The charging latency can be prolonged by decreasing the duration of and extending the interval between data telemetry sessions depending on the application.
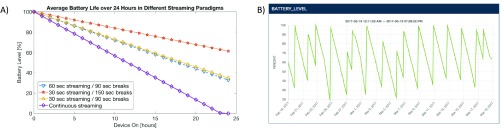
FIGURE 3.
A) Graphical representation of different sensing paradigms and battery depletion rates showing that smart sensing (red, yellow, and blue lines) can run approximately 2.5time longer than continuous recording (purple) while still capturing 100% of the seizures recorded with the embedded detector on. B) Battery level and battery consumption and charging trend shown in a Medtronic Digital Health Dashboard web application over approximately month of recording on one of the study subjects. It displays the approximately linear and regular discharging of the INS battery when maintaining the smart sensing paradigm.
2). Seizure Detection Using Embedded and Hybrid Detection Strategy
a: Embedded Device Detector
The Summit RC+S embedded LDA classifier was used to differentiate iEEG anomalies as interictal or seizure events. Seizure detector performance using a single optimized iEEG channel, power in band (central frequency and range), and canine specific classifier threshold was tested using previously recorded interictal and seizure data. Optimization of detectors for each epileptic dog consumed a substantial amount of recording time and required several seizures (on average 21±26 seizures) to setup suitable contacts, determine optimal frequency band for power calculation, and to find the optimal hypersensitive threshold with 100% sensitivity and maximal possible specificity. Results for the hypersensitive detection on the device for all epileptic dogs in the cohort are shown in Table 2. An embedded detection example is shown in canine #1 (Fig. 4). A graphical display of the number and temporal distribution of seizures of embedded detector events in a long-term recording is displayed using a heat map figure on a Medtronic Digital Health Dashboard web application to see the temporal distribution of seizures’ frequency (Fig. 4).
TABLE 2. Comparison of Embedded and EPAD/Cloud Detectors - Dogs With Naturally Occurring Epilepsy.
| Dog # | Num. of seizures detected on device | Embedded Accuracy [%] | Embedded Sensitivity [%] | Embedded Specificity [%] | Embedded FPR per day | EPAD Accuracy [%] | EPAD Sensitivity [%] | EPAD Specificity [%] | EPAD FPR per day |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 99.45 | 100 | 99.45 | 7.9 | 100 | 100 | 100 | 0 |
| 2 | 10 | 93.56 | 100 | 90.56 | 99.2 | 97.5 | 100 | 95.5 | 0.67 |
| 3 | 12 | 99.9 | 100 | 99.9 | 0.07 | Not Set | |||
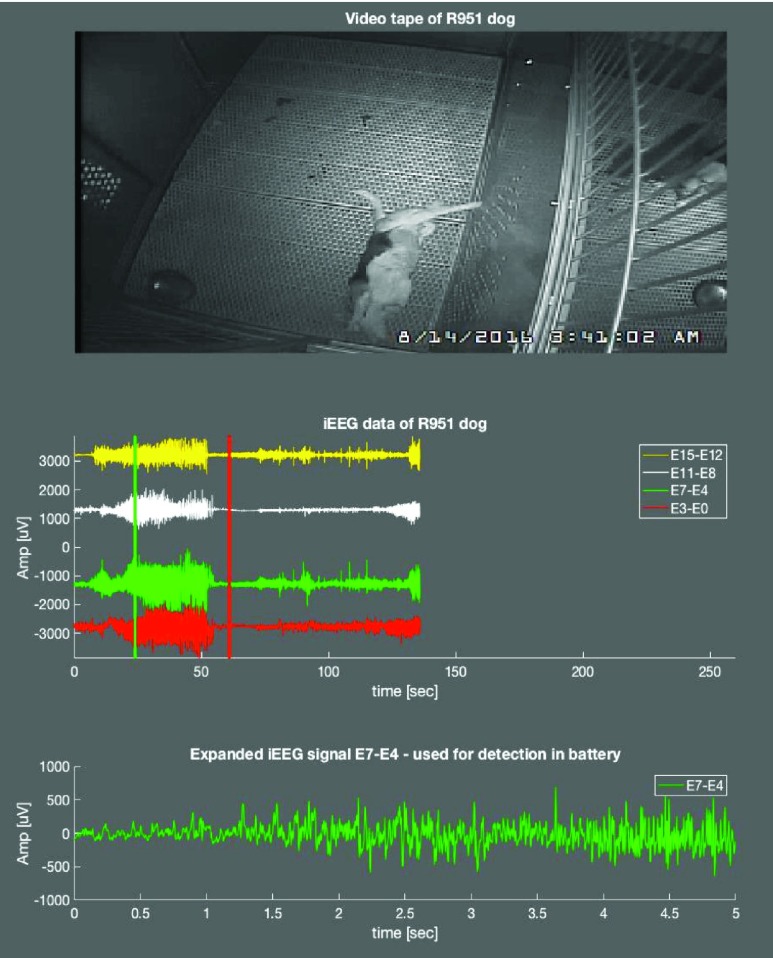
FIGURE 4.
Next Generation Epilepsy Management System: Canine with epilepsy undergoing continuous video-intracranial EEG monitoring and electrical brain stimulation. Top: Video of seizure onset captured on device. Middle: Automated seizure classification algorithm embedded on Summit RC+S captured seizure (green/red vertical line). Four channels of iEEG showing electrographic seizure onset. Bottom: Blow-up of channel showing seizure evolution.
b: Hybrid Detection System
Classifiers were deployed prospectively on all future outofsample data. The seizure detection algorithm was trained on 6 seizures from canine #1 comprising a total of 193 seconds of seizure data, and 1390 seconds of interictal data (51 randomly selected segments). Seizure clips were detected by the algorithm with probability scores >90%. The trained detector was then run in a prospective manner consisting all data (180 days). The detector had accurate detections of 4 unique seizures. There were no false positives (specificity 100%). The same seizure detection algorithm [35] was trained on 6 seizures from canine #2 comprising 176 seconds of seizure data and 1216 seconds of interictal data (21 randomly selected segments). The detector was evaluated in a prospective manner on all future data collected (60 days) using the smart sampling scenario with engaged embedded detector and periodic 60-second data streaming with 60-second breaks. The performance of the seizure detector on outofsample prospective data was sensitivity 100% (10/10 seizures detected), specificity 95.5%, and a false positive rate of 0.67/day (Table 2). The results show that seizure detection from an embedded classifier can be improved with offthebody analytics either on local or distributed computing. Currently the detector trained for canine #2 is running online. During smart sensing and hybrid detection, iEEG clips are streamed from the MSEL cloud to the Blackfynn cloud platform. Once uploaded to the Blackfynn platform, data are retrieved via a custom python client and analyzed with a higher performing, computationally intensive seizure detection algorithm. The results of the seizure detection algorithms are sent back to the Blackfynn platform as automated time-synced annotations, allowing any user to review automatically detected seizures shortly after the data was collected. Automated seizure detections can also be used to generate seizure diaries on the cloud analytics system.
3). Closed Loop Responsive Stimulation Delay
a: Detector Embedded on the RC+S Device
In the embedded closed-loop scenario, the RC+S device samples LFPs from the implanted electrodes and performs a fast-Fourier transform (FFT) to convert the time domain data into the frequency domain. Key recording factors affecting delay time include the iEEG sampling rate and the FFT configuration size, windowing, and update. The RC+S then can be configured to sum the FFT output frequency bins into band-power estimates, which are used as input into a programmable linear-discriminate (LD) classifier. The RC+S device will then change the output stimulation parameters based on changes in the signal determined by the LD classifier. Given the large number of parameters within the signal processing components, the delays in the embedded scenario are largely driven by researcher-configured parameters. To measure the system delay of the embedded closed-loop functionality, we configured a benchtop RC+S device to turn stimulation on in response to a frequency burst created by a signal generator while using different time-domain sampling rates and FFT sizes. The signal generator was configured to provide 15 pulses of 27Hz input into the RC+S. The test was performed in each test case 60 times and the final delays ranged 123.2 ± 14.0 msec (64-point FFT, 1kHz sampling rate) to 1432 ± 15.msec (1024-point FFT, 250Hz).
b: Detector Running on the EPAD
For the distributed case, which the Mayo EPAD will use for off-the-body analysis, we tested the system delay by again making use of a benchtop RC+S device, signal generator, and oscilloscope. The Summit API was used to create a test application that used a simple edge detector on the streamed time-domain data to trigger various stimulation commands on the RC+S device. We measured the time between the signal generator’s edge being input into the RC+S and the stimulation adjustment on the lead using an oscilloscope to gain a measure of the full system latency. Each test was done 10 times. Compared to the embedded case, it’s important to note that there are several other important system parameters that influence these delay measurements. First the stream frame interval, which is how often the RC+S sends a packet of data back to the user. Increasing the streaming interval will increase the delay as packets are sent less frequent, but shortening it too far increases bandwidth usage due to additional packet header overhead. Second, stimulation updates like increments and decrements take effect on the 2nd pulse after the command is sent, so a low stimulation frequency will result in longer delays. For this work we used ratio 5:1, 125Hz stimulation, and 50 msec frame size. Delay was measured for therapy on/off commands (310±21 msec), stimulation amplitude increments (163.7 ± 22.3 msec), and a go-to command (169.6±20.3 msec), which updates amplitude and frequency on the INS concurrently.
C. Machine Learning and Performance Evaluation
1). Seizure Forecasting Using EPAD
The seizure forecasting system was initially tested for canine #3 with a linear regression classifier using all available features. Training using only two lead seizures (lead seizures defined as separated from other seizures by at least four hours) and a prediction horizon of four hours, we obtained the following results: SS = 100%, FPR = 0.31 and TIW = 10%. In the final experiment, we increased the number of lead seizures in the training to three with the following performance SS = 100%, FPR = 0.21, and TIW = 7%. Additionally, we performed seizure forecasting in a pseudo-prospective manner for dog # 1 (SS = 92%, FPR = 0.62, TIW = 0.09%) and dog #2 (SS = 81%, FPR = 0.8, TIW = 0.3%). These results suggest that a robust, reliable, subject specific, seizure forecasting algorithm with high SS, low FPR, and low TIW can be achieved (Brinkmann 2016). The algorithm’s performance is influenced by the basic experimental parameters (definition of lead seizure and prediction horizon) [20], [26], and [48]. The high sensitivity and relatively low TIW demonstrates the potential clinical viability of seizure forecasting.
2). Active Probing of Neural Networks
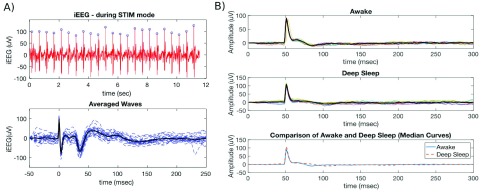
Low frequency electrical stimulation over 48 hours on each dog was used to test active probing. There was no significant decline in telemetry quality during the experiment, with more than 97% of data analyzable. Figure 5A shows an example of stimulation and evoked related potentials. We also analyzed ERP data in two distinct behavioral states (awake and deep sleep) and objectively compared results between those two states in canine #1 (Fig. 5B). Based on the pattern of iEEG data and concurrent video, we analyzed 12 segments (30 seconds long) in each state. We recorded iEEG data from a hippocampal electrode (1kHz sampling rate) while stimulating in the parahippocampus (stimulation frequency 2Hz, amplitude 3.0mV, pulse width 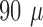 sec) and calculated ERP response in awake versus sleep. The peak of the ERP during awake was earlier in wake (36 msec) than in sleep (72 msec), but the amplitude of the response was similar.
sec) and calculated ERP response in awake versus sleep. The peak of the ERP during awake was earlier in wake (36 msec) than in sleep (72 msec), but the amplitude of the response was similar.
FIGURE 5.
A) Evoked related potential for subject 1. Top) Raw iEEG data captured on sensing electrode (1 kHz sampling rate) using stimulation parameters: frequency 2 Hz, pulse width 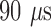 , amplitude 3.0 mV (blue dots show automated detections of stimulation peaks). Bottom) Detected stimulation waveforms aligned by peak in stimulation artifact. Bold black line shows averaged waveform. B) Evoked related potentials during wake (Top) and deep sleep (middle) using continuous parahippocampus electrical stimulation and hippocampus sensing in canine #2. Bottom graph shows comparison of median curves calculated in awake versus in deep sleep. Note a difference in ERP delay in awake versus deep sleep.
, amplitude 3.0 mV (blue dots show automated detections of stimulation peaks). Bottom) Detected stimulation waveforms aligned by peak in stimulation artifact. Bold black line shows averaged waveform. B) Evoked related potentials during wake (Top) and deep sleep (middle) using continuous parahippocampus electrical stimulation and hippocampus sensing in canine #2. Bottom graph shows comparison of median curves calculated in awake versus in deep sleep. Note a difference in ERP delay in awake versus deep sleep.
3). Automated Behavioral State Classification
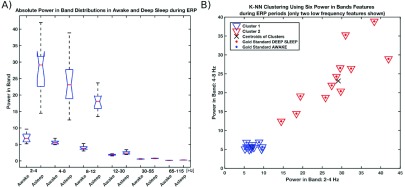
All the phases of deep sleep classified by the classifier were visibly matched with behavioral signs of the dog (data from early AM hours (1AM–3AM), including kennel lights off, prolonged period of immobility, slow and deep respirations, and eyes closed). Awake states corresponded to signs of obvious wakefulness (eyes opened, moving around, jumping, etc.). Transitional phases of sleep (drowsy, N2, REM) could not be evaluated using this methodology due to lack of scalp and associated sleep (e.g. eye, chin) electrodes. For the ERP study, the results of automated behavioral state classification suggest that distributions in spectral power are not overlapping for several bands in awake and deep sleep (Fig. 6A), thus ERP might be useful for automated separation of deep sleep and awake. We were able to automatically separate data into two clusters using a simple rule (higher delta power in deep sleep) to classify awake or deep sleep states. The results show 100% correct classification for these two distinct behavioral classes in the 24 epochs analyzed (Fig. 6B).
FIGURE 6.
A) Distribution of absolute power in six iEEG bands (2–4 Hz, 4–6 Hz, 8–12 Hz; 12 – 30 Hz, 30 – 55Hz, 65 – 115 Hz) for 12 30-second segments during stimulation for awake versus deep sleep (selected manually). Note several bands with no overlap in power. B) K-NN clustering to two clusters using six absolute power in band features (note only two features are displayed in 2-D graph). Clear separation between two classes (wake & deep sleep) was found using all six features. The class with higher delta power is assigned to deep sleep.
IV. Discussion and Conclusion
This manuscript describes an Epilepsy Management System designed around Medtronic’s investigational Summit RC+S System and a hand-held EPAD. The EPAD serves as a local computing device with bi-directional connectivity to the internet and an implanted Medtronic RC+S device. The system demonstrates multiple advances, and includes automated seizure detection, electronic seizure diary features, active electrical probing of neural networks, behavioral state classification, seizure forecasting and implementation of control policies driven by embedded and off-the-body analytics that can be used to inform therapeutic electrical stimulation. The primary causes of data drops we encountered are losing Bluetooth connectivity, e.g the dog wandering beyond the range of EPAD antenna, or by EPAD to CTM connection interruptions. These data drops are sporadic and short and didn’t affect algorithm performance. Further, a smart sensing paradigm was demonstrated where iEEG telemetry is intermittently activated based on detected iEEG anomalies and user-defined background periodic data sampling to significantly prolong RC+S device battery life while maintaining seizure detection and forecasting performance. Seizure detection and closed-loop stimulation was accomplished using an embedded detector. The detector was tuned to be hypersensitive (sensitivity 100%) so that all seizures were captured. This generated a high number of false positive detections, but triggered reliable data recording in all cases when seizures occurred during the periodic breaks in data streaming (for battery saving). High accuracy classifications between false positives and true seizures are subsequently made by a seizure detector using advanced signal processing and machine learning methods running on the EPAD or cloud. In the EPAD system we implemented the best seizure detection algorithm from Baldassano et al. [35]. We show that the integration of the detection algorithm on the EPAD achieves 100% sensitivity with low false positive rates (Table 2). We demonstrated the feasibility of active probing and brain state tracking and showed that closed loop stimulation based on a device embedded iEEG algorithms perform at millisecond timescales and that an off-the-body EPAD and cloud based algorithm can close the loop with delays on the order of a second. Our testing evaluated the time lag for automated seizure detection and expert visual review for the implanted device embedded detector and the EPAD, a remaining challenge will be responsive stimulation implementation and redetection after stimulus delivery (e.g. as described for RNS system in [49]). These tests are underway in freely behaving canines where subject specific stimulation protocols are likely to be useful.
The bi-directional connectivity of the EPAD with internet services allow for the creation of a cloud based centralized data and analytics platform. In this study, the cloud services Medtronic ORCA Digital Heath system, Mayo Clinic Electrophysiology Laboratory cloud system, and Blackfynn Inc. (https://www.blackfynn.com) were utilized for collecting, viewing, annotating, and analyzing data. The cloud services provide a central location for researchers to review and annotate iEEG data, device information (Fig. 3b), seizure diaries (Fig. 3d), seizure detections, sleep analytics, and real-time video iEEG links to remotely housed canines (Fig. 4). Similar capabilities could be useful in future medical applications for monitoring seizures, determining sleep quality, and optimizing electrical stimulation therapy. In particular, the ability to continuously monitor iEEG and analytics to forecast and detect seizure to alert patients and caregivers of seizures [50] is important for patient safety and could help prevent SUDEP.
The epilepsy management system described here is the first system that integrates an implanted device streaming 24/7 brain electrophysiology data to a local, and distributed computing resource. The off-the-body computational devices run machine learning algorithms to detect and predict electrophysiology anomalies and seizures in a real-time scenario. This research demonstrates the feasibility of a system that creates a link between the brain and computers, and patients and their physicians. While additional data and experimental results are needed to establish the reliability of this technology, the potential for intelligent electrical stimulation informed by physiological (wake/sleep) and pathological (pre-seizure state) brain states is demonstrated. Furthermore, electrical stimulation tuned in an individualized manner to suppress iEEG biomarkers of cortical excitability could prove useful for preventing seizures and rapidly optimizing the management of epilepsy [29].
Acknowledgment
The authors would like to acknowledge Cindy Nelson, Karla Crockett, and Daniel Crepeau for administrative and technical support and Veronika Krakorova for graphics. Brian Litt is founder of Blackfynn Inc. The authors also want to acknowledge Joost Wagenaar from Blackfynn Inc. for project related data management on Blackfynn cloud system. Greg Worrell and Mayo Clinic have equity in Blackfynn Inc. Medtronic provided devices for implants and technical support.
Funding Statement
This work was supported in part by the Mayo Clinic Discovery Translation Grant, National Institutes of Health under Grant R01 NS09288203 and Grant UH2/UH3NS95495, the Institutional Resources for Research by Czech Technical University in Prague, Czech Republic, the Czech Science Foundation under Grant 1720480S, the Temporal Context in Analysis of LongTerm NonStationary Multidimensional Signal, Czech Republic Grant Agency, under Grant P103/11/0933, the European Regional Development Fund through the Project FNUSA-ICRC under Grant CZ.1.05/ 1.1.00/02.0123, and the Mirowski Family Foundation.
Contributor Information
Vaclav Kremen, Email: kremen.vaclav@mayo.edu.
Gregory A. Worrell, Email: worrell.gregory@mayo.edu.
References
- [1].Deeb W.et al. , “Proceedings of the fourth annual deep brain stimulation think tank: A review of emerging issues and technologies,” Front Integr. Neurosci., vol. 10, p. 38, Nov. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ezzyat Y.et al. , “Direct brain stimulation modulates encoding states and memory performance in humans,” Current Biol., vol. 27, no. 9, pp. 1251–1258, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lozano A. M.et al. , “A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease,” J. Alzheimer’s Disease, vol. 54, no. 2, pp. 777–787, 2016, doi: 10.3233/JAD-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Laxton A. W.et al. , “A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease,” Ann. Neurol., vol. 68, no. 4, pp. 521–534, 2010. [DOI] [PubMed] [Google Scholar]
- [5].Mayberg H. S.et al. , “Deep brain stimulation for treatment-resistant depression,” Neuron, vol. 45, no. 5, pp. 651–660, 2005. [DOI] [PubMed] [Google Scholar]
- [6].Raymaekers S., Luyten L., Bervoets C., Gabriëls L., and Nuttin B., “Deep brain stimulation for treatment-resistant major depressive disorder: A comparison of two targets and long-term follow-up,” Transl. Psychiatry, vol. 7, no. 10, p. e1251, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Langevin J.-P.et al. , “Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder,” Biol. Psychiatry, vol. 79, no. 10, pp. e82–e84, 2016. [DOI] [PubMed] [Google Scholar]
- [8].Butson C. R., Tamm G., Jain S., Fogal T., and Krüger J., “Evaluation of interactive visualization on mobile computing platforms for selection of deep brain stimulation parameters,” IEEE Trans. Vis. Comput. Graphics vol. 19, no. 1, pp. 108–117, Jan. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boyden E., Allen B., and Fritz D., “Brain coprocessors: The need for operating systems to help brains and machines work together,” Intell. Mach., Technol. Rev., Sep. 2010. [Online]. Available: https://www.technologyreview.com/s/420884/brain-coprocessors/
- [10].Téllez-Zenteno J. F., Dhar R., and Wiebe S., “Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis,” Brain, vol. 128, pp. 1188–1198, May 2005. [DOI] [PubMed] [Google Scholar]
- [11].Jehi L., Wyllie E., and Devinsky O., “Epileptic encephalopathies: Optimizing seizure control and developmental outcome,” Epilepsia, vol. 56, no. 10, pp. 1486–1489, Oct. 2015, doi: 10.1111/epi.13107. [DOI] [PubMed] [Google Scholar]
- [12].Fisher R. S. and Velasco A. L., “Electrical brain stimulation for epilepsy,” Nature Rev. Neurol., vol. 10, pp. 261–270, 2014. [DOI] [PubMed] [Google Scholar]
- [13].Bourget D.et al. , “An implantable, rechargeable neuromodulation research tool using a distributed interface and algorithm architecture,” in Proc. 7th Int. IEEE/EMBS Conf. Neural Eng. (NER), Montpellier, France, Apr. 2015, pp. 61–65. [Online]. Available: https://ieeexplore.ieee.org/document/7146560/, doi: 10.1109/NER.2015.7146560. [DOI] [Google Scholar]
- [14].Fisher R.et al. , “Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy,” Epilepsia, vol. 51, no. 5, pp. 899–908, 2015. [DOI] [PubMed] [Google Scholar]
- [15].Salanova V.et al. , “Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy,” Neurology, vol. 84, no. 10, pp. 1017–1025, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morrell M. J., “Responsive cortical stimulation for the treatment of medically intractable partial epilepsy,” Neurology, vol. 77, no. 13, pp. 1295–1304, 2011. [DOI] [PubMed] [Google Scholar]
- [17].Heck C. N.et al. , “Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS system pivotal trial,” Epilepsia, vol. 55, no. 3, pp. 432–441, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bergey G. K.et al. , “Long-term treatment with responsive brain stimulation in adults with refractory partial seizures,” Neurology, vol. 84, no. 8, pp. 810–817, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hoppe C., Poepel A., and Elger C. E., “Epilepsy: Accuracy of patient seizure counts,” Arch. Neurol., vol. 64, no. 11, pp. 1595–1599, 2007. [DOI] [PubMed] [Google Scholar]
- [20].Cook M. J.et al. , “Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: A first-in-man study,” Lancet Neurol., vol. 12, no. 6, pp. 563–571, 2013, doi: 10.1016/S14744422(13)700759. [DOI] [PubMed] [Google Scholar]
- [21].Elger C. E. and Mormann F., “Seizure prediction and documentation—Two important problems,” Lancet Neurol., vol. 12, no. 6, pp. 531–532, 2013. [DOI] [PubMed] [Google Scholar]
- [22].Osorio I., “The NeuroPace trial: Missing knowledge and insights,” Epilepsia, vol. 55, no. 9, pp. 1469–1470, 2014. [DOI] [PubMed] [Google Scholar]
- [23].Morrell M. J., “In response: The RNS System multicenter randomized double-blinded controlled trial of responsive cortical stimulation for adjunctive treatment of intractable partial epilepsy: Knowledge and insights gained,” Epilepsia, vol. 55, no. 9, pp. 1470–1471, 2014. [DOI] [PubMed] [Google Scholar]
- [24].Davis K. A.et al. , “A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG,” Epilepsy Res., vol. 96, nos. 1–2, pp. 116–122, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Howbert J. J.et al. , “Forecasting seizures in dogs with naturally occurring epilepsy,” PLoS ONE, vol. 9, no. 1, p. e81920, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brinkmann B. H.et al. , “Forecasting seizures using intracranial EEG measures and SVM in naturally occurring canine epilepsy,” PLoS ONE, vol. 10, no. 8, p. e0133900, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brinkmann B. H.et al. , “Crowdsourcing reproducible seizure forecasting in human and canine epilepsy,” Brain, vol. 139, no. 6, pp. 1713–1722, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kremen V.et al. , “Behavioral state classification in epileptic brain using intracranial electrophysiology,” J. Neural Eng., vol. 14, no. 2, p. 026001, 2017, doi: 10.1088/17412552/aa5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lundstrom B. N., Worrell G. A., Stead M., and Van Gompel J. J., “Chronic subthreshold cortical stimulation: A therapeutic and potentially restorative therapy for focal epilepsy,” Expert Rev. Neurotherapeutics, vol. 17, no. 7, pp. 661–666, 2017, doi: 10.1080/14737175.2017.1331129. [DOI] [PubMed] [Google Scholar]
- [30].Karoly P. J., Nurse E. S., Freestone D. R., Ung H., Cook M. J., and Boston R., “Bursts of seizures in long-term recordings of human focal epilepsy,” Epilepsia, vol. 58, no. 3, pp. 363–372, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Worrell G. A., Jerbi K., Kobayashi K., Lina J. M., Zelmann R., and Le Van Quyen M., “Recording and analysis techniques for high-frequency oscillations,” Prog. Neurobiol., vol. 98, no. 3, pp. 265–278, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Valentín A.et al. , “Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo,” Brain, vol. 125, no. 8, pp. 1709–1718, 2002. [DOI] [PubMed] [Google Scholar]
- [33].Valentín A.et al. , “Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: A prospective study,” Lancet Neurol., vol. 4, no. 11, pp. 718–726, 2005. [DOI] [PubMed] [Google Scholar]
- [34].Kalitzin S., Velis D., Suffczynski P., Parra J., and da Silva F. L., “Electrical brain-stimulation paradigm for estimating the seizure onset site and the time to ictal transition in temporal lobe epilepsy,” Clin. Neurophysiol., vol. 116, no. 3, pp. 718–728, 2005. [DOI] [PubMed] [Google Scholar]
- [35].Baldassano S. N.et al. , “Crowdsourcing seizure detection: Algorithm development and validation on human implanted device recordings,” Brain, vol. 140, no. 6, pp. 1680–1691, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bower M. R.et al. , “Evidence for consolidation of neuronal assemblies after seizures in humans,” J. Neurosci., vol. 35, no. 3, pp. 999–1010, 2015, doi: 10.1523/JNEUROSCI.301914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bower M. R.et al. , “Reactivation of seizure-related changes to interictal spike shape and synchrony during postseizure sleep in patients,” Epilepsia, vol. 58, no. 1, pp. 94–104, 2017, doi: 10.1111/epi.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sarkisian M. R., “Overview of the current animal models for human seizure and epileptic disorders,” Epilepsy Behav., vol. 2, no. 3, pp. 201–216, 2001. [DOI] [PubMed] [Google Scholar]
- [39].Berendt M. and Gram L., “Epilepsy and seizure classification in 63 dogs: A reappraisal of veterinary epilepsy terminology,” J. Vet. Internal Med., vol. 13, no. 1, pp. 14–20, 1999. [PubMed] [Google Scholar]
- [40].Chandler K., “Canine epilepsy: What can we learn from human seizure disorders?” Vet. J., vol. 172, no. 2, pp. 207–217, 2006. [DOI] [PubMed] [Google Scholar]
- [41].Dewey C. W.et al. , “Zonisamide therapy for refractory idiopathic epilepsy in dogs,” J. Amer. Animal Hospital Assoc., vol. 40, no. 4, pp. 285–291, 2004. [DOI] [PubMed] [Google Scholar]
- [42].Leppik I. E., Patterson E., Hardy B., and Cloyd J. C., “Canine status epilepticus: Proof of principle studies,” Epilepsia, vol. 50, pp. 14–15, Dec. 2009. [DOI] [PubMed] [Google Scholar]
- [43].Lundstrom B. N.et al. , “Chronic subthreshold cortical stimulation to treat focal epilepsy,” JAMA Neurol., vol. 73, no. 11, pp. 1370–1372, Nov. 2016. [DOI] [PubMed] [Google Scholar]
- [44].Volk H. A., Matiasek L. A., Feliu-Pascual A. L., Platt S. R., and Chandler K. E., “The efficacy and tolerability of levetiracetam in pharmacoresistant epileptic dogs,” Vet J., vol. 176, no. 3, pp. 310–319, 2008. [DOI] [PubMed] [Google Scholar]
- [45].Jeserevics J.et al. , “Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: Visual and background quantitative analysis,” J. Vet. Internal Med., vol. 21, no. 6, pp. 1299–1306, 2007. [DOI] [PubMed] [Google Scholar]
- [46].Berendt M., Høgenhaven H., Flagstad A., and Dam M., “Electroencephalography in dogs with epilepsy: Similarities between human and canine findings,” Acta Neurol. Scandinavica, vol. 99, no. 5, pp. 276–283, 1999. [DOI] [PubMed] [Google Scholar]
- [47].Pellegrino F. C. and Sica R. E. P., “Canine electroencephalographic recording technique: Findings in normal and epileptic dogs,” Clin. Neurophysiol., vol. 115, no. 2, pp. 477–487, 2004. [DOI] [PubMed] [Google Scholar]
- [48].Snyder D. E., Echauz J., Grimes D. B., and Litt B., “The statistics of a practical seizure warning system,” J. Neural Eng., vol. 5, no. 4, pp. 392–401, 2008, doi: 10.1088/17412560/5/4/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sun F. T. and Morrell M. J., “The RNS system: Responsive cortical stimulation for the treatment of refractory partial epilepsy,” Expert Rev. Med. Devices, vol. 11, no. 6, pp. 563–572, 2014, doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- [50].Coles L. D.et al. , “Feasibility study of a caregiver seizure alert system in canine epilepsy,” Epilepsy Res., vol. 106, no. 3, pp. 456–460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]