Abstract
Objectives:
It is important to describe and understand the prevalence and risk factors for the syndrome of delirium in critical illness. Since anticholinergic medication may contribute to the development of delirium in the PICU, we have sought to quantify anticholinergic medication exposure in patients with prolonged admission. We have used Anticholinergic Drug Scale scores to quantify the magnitude or extent of this burden.
Design:
Retrospective cohort study, January 2011 to December 2015.
Setting:
Single academic medical center PICU.
Patients:
Children under 18 years old with a PICU admission of 15 days or longer, requiring mechanical ventilation.
Interventions:
None.
Measurements and Main Results:
Daily Anticholinergic Drug Scale scores for the first 15 days of admission, in each of 88 subjects (total of 1,320 PICU days), were collected and assessed in relation to demographic data, severity of illness, and medication use. Median (interquartile range) of daily Anticholinergic Drug Scale score was 5 (interquartile range, 3–7). Anticholinergic Drug Scale score was not associated with age, sex, medical history, presenting Severity of Illness score, PICU length of stay, ventilator hours, or hospital mortality. Medications most frequently associated with high Anticholinergic Drug Scale score were low potency anticholinergic drugs such as morphine, midazolam, vancomycin, steroids, and furosemide, with the exception of ranitidine (Anticholinergic Drug Scale score 2). Patients receiving high doses of midazolam infusion had significantly higher Anticholinergic Drug Scale scores compared with those receiving lower or no midazolam dosing.
Conclusions:
A high number of medications with anticholinergic effects are administered to PICU patients receiving prolonged mechanical ventilation. These exposures are much higher than those reported in adult intensive care patients. Since anticholinergic drug exposure is associated with delirium, further study of this exposure in PICU patients is needed.
Keywords: anticholinergic, benzodiazepines, critical care, delirium, toxicity
The impact of medications with anticholinergic effects has long been recognized as a significant problem in adult medicine, such that multiple scales exist to measure the degree of “anticholinergic burden” (1–4). One reason for this concern is an association between delirium and medications with anticholinergic properties (1, 5–7), although studies of correlation in the adult ICU (AICU) have produced mixed results (7, 8). In the PICU, treatment with benzodiazepines—a drug class that has anticholinergic properties as well as potentiating the anticholinergic effect of other drugs—is associated with an increased risk of delirium. In one study, subjects who had received an anticholinergic had an odds ratio for developing delirium of 2.2 (p = 0.006) (9).
The anticholinergic toxidrome includes either peripheral (i.e., tachycardia, hypertension, mydriasis, fever, constipation and urinary retention, myoclonus, tremor, and dry skin) or central (i.e., disorientation, confusion, hallucinations, and seizures) features, or both (10, 11). The “Anticholinergic Drug Scale” (ADS) rates individual medications using a variety of characteristics, including any known anticholinergic properties from pharmacologic receptor binding studies, known anticholinergic adverse events, or consensus expert opinion (2). The ADS scores range from 0 (none) to 1 (potential effect), to 2 (observed effect), or to 3 (effect almost always occurs) anticholinergic effects. For example, according to the ADS, diphenhydramine is given a value of 3, ranitidine a value of 2, and common PICU medications such as furosemide, midazolam, hydralazine, hydrocortisone, fentanyl, morphine, clindamycin, and gentamicin a score of 1 (2). Another tool is the Anticholinergic Cognitive Burden (ACB) scale, which is based on expert opinion and a systematic review of the literature (4). A value of 0–3 is assigned to 88 medications based on evidence of likely effect on cognitive function. Both the ADS and ACB scores have been used extensively in the adult population to sum up the total anticholinergic burden from multiple medications (5, 6, 8).
In a recent systematic review, we were not able to identify any pediatric studies that assessed the anticholinergic drug burden in the PICU (12). Because many of the medications assigned a value on the ADS and ACB scores are commonly used in the PICU, we sought to quantify the extent of exposure to anticholinergic medications in a single center because it is a potentially modifiable risk factor for morbidity, including ICU delirium.
METHODS
The Boston Children’s Hospital (BCH) institutional review board (IRB) approved this retrospective cohort study (IRB-CR00020606-2). Eligible pediatric patients were identified via a computerized search system through the electronic medical record (EMR) programmed to identify subjects meeting the following criteria: age less than 18 years old; admitted to the PICU between January 2011 and December 2015; PICU length of stay of at least 15 days; and receiving ventilatory support (either invasive or noninvasive) during the first 15 days of admission. Exclusion criteria were newborns and patients with severe brain injury progressing to diagnosis of brain death during the admission. Patients with a PICU length of stay less than 15 days were excluded to reduce heterogeneity in the cohort.
All subjects received care in the same PICU, managed by a common group of PICU attendings and trainees, and subject to medication guidelines to the same degree. Our PICU manages sedative infusions using a nurse-implemented, goal-directed sedation protocol on which the Randomized Evaluation of Sedation Titration fOr REspiratory failure clinical trial was based (13).
Data Collection
The EMR data collection for each patient included patient demographics, medical history, clinical values from day of PICU admission, and throughout PICU stay. Severity of illness in the first 24 hours was measured using the Pediatric Risk of Mortality (PRISM)–III score (14). Medical history and reason for admission to PICU were collected from the PICU attending admission documentation. Medications administered during each of the first 15 days of the PICU stay were extracted from the medication administration record, including all changes to sedative infusion rates and intermittent bolus doses administered. Additional daily information included Withdrawal Assessment Tool (WAT)–1 scores (15), recorded by nursing on the patient flowsheet. Patients with any WAT-1 score over 3 at any point during data collection period were considered to have withdrawal.
Anticholinergic scores using the ADS (2) and ACB scale (4) were then calculated based on the value ascribed to each medication, using Microsoft Excel (Microsoft Office Standard 2010, Version 14.0.7190.5000). Dose adjustment for the daily dose of medications has been proposed for the ADS (2); however, based on the methods of Wolters et al (8), who studied ACB and ADS in AICU patients, we did not dose-adjust level 1 medications (which includes all sedative and analgesic medications). These data were managed using the Research Electronic Data Capture database hosted at BCH (16).
Each patient chart was reviewed for diagnosis of “Delirium” by diagnostic codes or referral to the clinical psychology/psychiatry service at BCH. Psychiatry notes were reviewed for diagnosis of delirium and, along with administration of an antipsychotic medication (not administered before PICU admission), were all considered diagnostic for delirium. Widespread screening using bedside delirium assessment tools was not carried out as routine practice during the time period studied, for reasons explained in our previous review, for example, the overlap in case definition with benzodiazepine withdrawal and the anticholinergic toxidrome (12).
Statistical Analyses
Descriptive data are presented as median (interquartile range [IQR]) because of presumed nonnormal distribution. Nonparametric comparison between groups was performed using the Kruskal-Wallis test. All statistical computations were carried out with IBM SPSS Statistics for Windows, Version 23 (IBM, Armonk, NY). Maximum, minimum, mean, median, and IQR of ADS and ACB scores for each patient over the 15 PICU days was determined using SPSS (IBM SPSS Statistics for Windows, Version 23 [IBM]). As the median ADS score reported in an AICU study was 2 (8), we defined an ADS score greater than 3 as “high,” and likewise as the 75th percentile of ADS scores in our cohort was 7, and we further defined a score greater than 7 as “very high.” We also calculated the total sum of all ADS scores per patient over the first 15 days of PICU stay, so-called “cumulative anticholinergic exposure,” to gain a measure of the consistency of high anticholinergic exposure, as we hypothesized that this would be a better measure of the consistency of high anticholinergic exposure throughout the 15 days.
We hypothesized that ADS score would be associated with the primary benzodiazepine exposure, which was midazolam in this cohort, so maximum dose was calculated for each patient in mg/kg, and the subjects were divided into tertiles based on distribution (lower tertile, no midazolam; middle tertile, midazolam < 2.2 mg/kg/d; upper tertile, midazolam ≥ 2.2 mg/kg/d). ADS median score by patient was then compared based on tertile of midazolam dose using the Kruskal-Wallis test.
We also hypothesized that anticholinergic burden would be associated with critical illness severity, so we analyzed the PRISM-III score (14). Since the median PRISM-III for the cohort was 9.5 for the cohort (IQR, 3–13.75), ADS score medians were analyzed for each quartile of the PRISM-III score. Withdrawal was defined per subject as a WAT-1 score of 3 or greater on any of the 15 days of data collection.
RESULTS
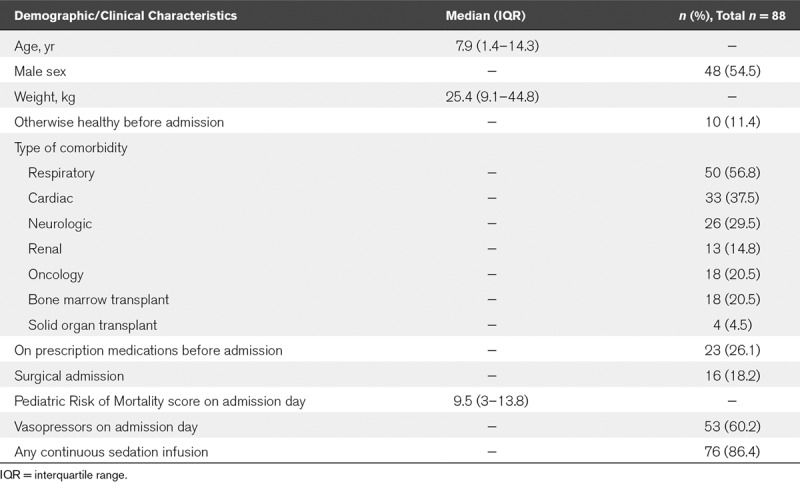
Eighty-eight PICU patients met the inclusion criteria for study (on average 18 cases/yr). The median age was 7.9 years (IQR, 1.4–14.3 yr) (Table 1). The cohort consisted of subjects with a high rate of comorbidities, with only 10 (11%; 95% CI, 6–20%) that were previously well, and only 23 (26%; 17–37%) not receiving any prescription medications before PICU admission. The median raw PRISM-III score was 9 (IQR, 3–13), and 53 subjects (60%; 49–71%) were on vasopressors on the day of PICU admission. During the course of the admission, 76 subjects (86%; 77–93%) were on continuous infusion of sedative agents.
TABLE 1.
Analysis of Patient Characteristics on PICU Admission
ADS Scores
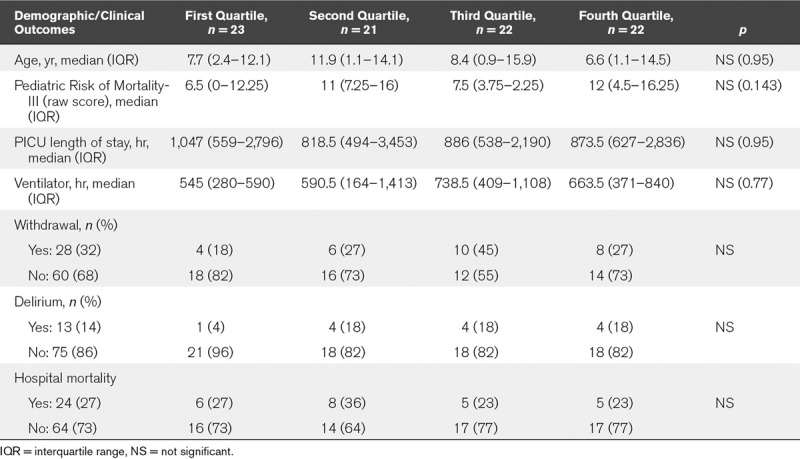
The daily ADS scores are shown in Table 2. The median ADS score across all 1,320 scores for 88 subjects was 5 (IQR, 3–7). When considering individual data for each of the 88 subjects, the median of individual maximum ADS score was 8 (IQR, 6–10), and the median number of days spent with high ADS score (above 3) was 11 (IQR, 8–14) out of the first 15 PICU days. There was no association between age, sex, comorbidities, or admission severity (PRISM-III) and either the median ADS scores for the population or individual maximum ADS scores. None of the PICU outcomes (i.e., mortality, length of stay, duration of mechanical ventilation, and diagnosis of delirium) was associated with ADS scores, with the exception of sedation withdrawal, which was correlated with maximum ADS score over the first 15 days (p = 0.042) (Table 3). Last, on examining the median ADS score for each of the 88 subjects, and then separating the population into quartiles, we found no differences in patient characteristics or PICU outcomes (Table 4).
TABLE 2.
Anticholinergic Exposure in PICU by Several Measures

TABLE 3.
Analysis of PICU Patient Admission Characteristics and Outcomes by Anticholinergic Drug Scale Scores
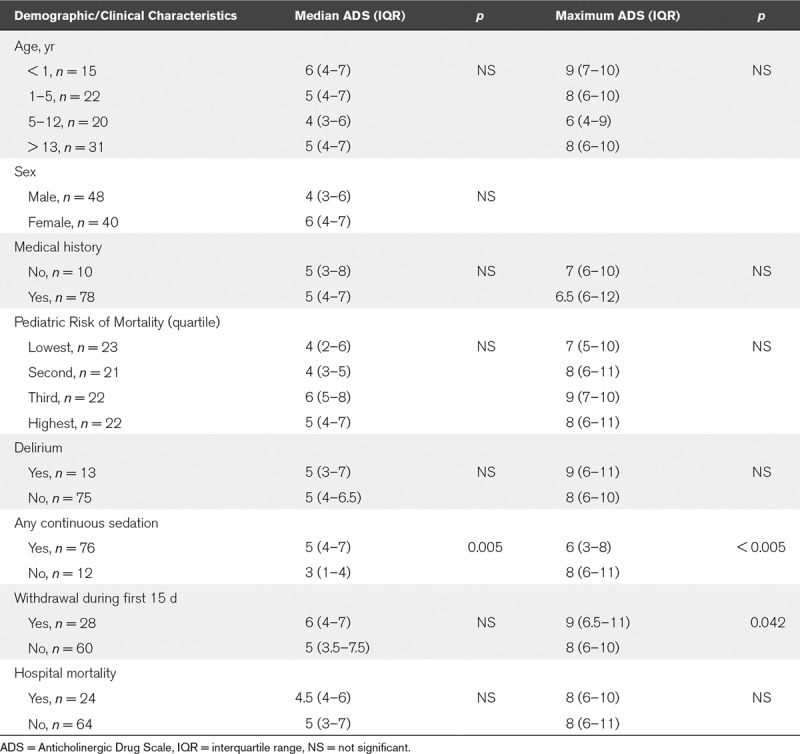
TABLE 4.
Admission Characteristics and PICU Outcomes by Quartile of Cumulative Anticholinergic Exposure, As Measured by Anticholinergic Drug Scale Score
ACB Scores
The ACB scores for the cohort were similar to ADS scores, with the notable difference that midazolam (ADS = 1) carries a score of 0, and ranitidine (ADS = 1) carries a score of 1 on the ACB. The median ACB score was 2 (IQR, 1–4) overall 1,320 PICU days measured. As the results of further analyses conducted using the ACB scores were very similar to the ADS score results, we elected to focus on ADS scores.
Medications and ADS Scores
The four medications that most commonly contributed to the ADS scores were all low-level (i.e., ADS score = 1) anticholinergic drugs (Fig. 1). Midazolam was the most commonly used medication in subjects who had a daily ADS score of 8 or greater (classified as “very high”), received by 94% of subjects on those days. The next most commonly administered medications were morphine, vancomycin, and steroids (including hydrocortisone, prednisone, prednisolone, methylprednisolone, and dexamethasone). Ranitidine (ADS score 2) was the fifth most common medication, contributing to 56% of daily scores greater than or equal to 8, and diphenhydramine (ADS score 3) contributed to 40% of all daily scores greater than or equal to 8.
Figure 1.
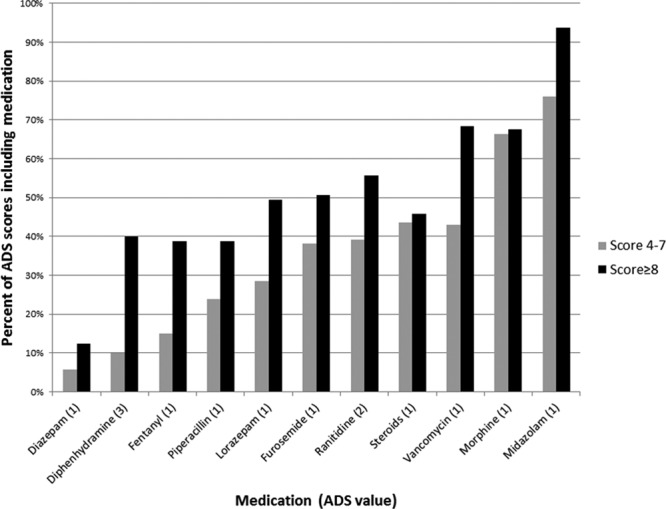
Medications contributing to high Anticholinergic Drug Scale (ADS) score. The medications (x-axis) contributing to the percentage of ADS scores above 8 (black bar) and 4–7 (gray bar). Individual contributions from named medication represented in parentheses on x-axis legend.
Midazolam
Midazolam was administered to 73 of 88 (83; 73–90%) of the cohort at some point during the PICU stay. ADS scores were also computed after excluding all benzodiazepines, and the median score over the whole 15-day period for all subjects was 4 (IQR, 2–5). After excluding all sedative medications, the median ADS score for the whole population data dropped to 3 (IQR, 1–4). When midazolam dose per day in the whole population was divided into tertiles (lower tertile, no midazolam; middle tertile, midazolam < 2.2 mg/kg/d; upper tertile, midazolam ≥ 2.2 mg/kg/d), there were 460 of 1,320 days (34.8%) without midazolam exposure. The median ADS score was higher in the upper tertile compared with middle and lower tertiles (7 [IQR, 6–8] vs 4.5 [IQR, 4–6] vs 4 [IQR, 2–5]; p < 0.001) (Fig. 2).
Figure 2.
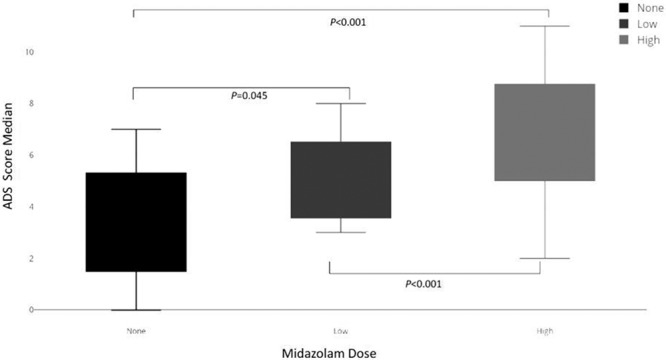
The Anticholinergic Drug Scale (ADS) score based on dose of midazolam administration. Group 1 received no midazolam (median, 4; interquartile range [IQR], 2–5), group 2 less than 2.2 mg/kg/d (median, 4.5; IQR, 4–6), and group 3 greater than or equal to 2.2 mg/kg/d (median, 7; IQR, 6–8). p values represent Kruskal-Wallis comparison between groups.
DISCUSSION
In this report, we have demonstrated a high level of exposure to anticholinergic medications in patients with respiratory failure requiring prolonged mechanical ventilation and sedation in the PICU. Compared with an AICU population, in which the median anticholinergic score was 2 (8), the median of 5 in our PICU study is cause for concern and further study. Additionally, in this cohort of children, a median of 8 of the first 15 days of admission was characterized by a high burden of anticholinergic medications. The medications associated with these elevated scores are commonly used in PICU. In fact, the two most prevalent—midazolam, followed by morphine—are mainstays of PICU sedation and analgesic practice (13). With the exception of ranitidine (ADS = 2) and diphenhydramine (ADS = 3), high ADS scores were attributed to medications with low anticholinergic toxicity (ADS = 1); however, toxicity is likely additive (2).
In a recent review of the literature, we did not identify any studies that had evaluated and characterized anticholinergic burden in the pediatric population (12). However, studies have examined the incidence of delirium in the PICU as it relates to anticholinergic medication as a dichotomous variable, and reported exposure of 68–74% of subjects to this medication class (9, 17). In a recent study examining medications administered to AICU patients, the median number of “low potency” anticholinergic medications was 1.5 in those patients who were never delirious, versus 2.4 in those patients developing delirium (< 0.0001) (7). Another AICU study demonstrated a median daily ADS score of 2 (IQR, 1–3), with 90% of the scores being accounted for by lowest level mediations (8). Hence, in comparison, it appears that the burden of anticholinergic medications in our PICU cohort is considerably higher. It is certainly feasible that the impact of medications with anticholinergic properties is manifested differently in younger age groups due to varying parasympathetic activity; however, the evidence linking anticholinergic medications to delirium in children (9) warrants further study of this relationship.
Benzodiazepines have also long been associated with delirium in critically ill AICU (7, 18–20) and PICU patients (9, 17, 21). The biologic mechanisms underlying this association are multifactorial. Benzodiazepines activate γ-aminobutyric acid receptors in the CNS and alter levels of neurotransmitters including dopamine, serotonin, acetylcholine, norepinephrine, and glutamate (18, 22, 23). The medication most commonly associated with high anticholinergic burden in our cohort was midazolam, one of several benzodiazepines judged to have anticholinergic properties by the ADS (2, 24). The properties of benzodiazepines have been long discussed in the context of anticholinergic activity (2, 24). A study of adult palliative care patients showed that increased levels of midazolam are associated with decreased levels of serum cholinesterase activity (25), which may be another mechanism accounting for this association. We found that midazolam not only contributed to the total anticholinergic score but was also associated with higher exposure, greater than its individual contribution (Fig. 2). It follows that midazolam exposure would track with other common medications administered in sedated patients, including morphine and fentanyl, which also contribute to the ADS, so this association is not unexpected.
Aside from the relationship between benzodiazepines and delirium, anticholinergic medication burden has been associated with delirium in several studies, although the results are mixed. A large AICU study did not find an association between increasing ADS score and onset of delirium (8). However, another large AICU study did find both low and high potency anticholinergic medications to be associated with delirium (7). One PICU study has also found an association between anticholinergic medication and delirium occurrence (9). Our retrospective cohort was not powered to detect a difference in rate of psychiatrist-diagnosed delirium, based on anticholinergic exposure. Our goal was to demonstrate the degree of exposure to these medications in PICU practice and raise awareness about the possibility of contributing to an anticholinergic toxidrome or delirium.
One likely impact of increased awareness and investigation of the role of anticholinergic medication burden in the PICU would be discussion of alternative medication choices to those with anticholinergic effects. Alternatives to ranitidine (ADS 2), diphenhydramine (ADS 3), and sedative agents with low-level anticholinergic effects (midazolam, morphine, and fentanyl) exist. Several studies have demonstrated a lower incidence of delirium with dexmedetomidine infusion compared with other common agents (26, 27).
This study is limited by the lack of systematic screening for delirium using available scales, limiting our ability to draw any conclusion about an association with delirium prevalence (4). The relatively small sample size analyzed limits our power to rule out statistical association; however, the sample was limited to enhance homogeneity of the cohort with a prolonged PICU stay. The retrospective design also does not allow us to determine a cause and effect relationship for anticholinergic medication exposure and scale scores. However, we have established a high rate of anticholinergic medication exposure in these patients, which is likely to be a potentially modifiable contributor to PICU morbidity.
CONCLUSIONS
PICU patients receive a large number of medications during sedation for mechanical ventilation, many of which also have low-level anticholinergic effects, which may be additive. Whether we are seeing discrete syndromes of anticholinergic toxicity, sedative effects and withdrawal, or delirium remains to be further explored (4). This exposure represents a modifiable risk factor that is, in our experience, not frequently considered by PICU clinicians. Alternative medications should be considered in patients who require a high number of medications with anticholinergic burden. Therefore, we propose further, prospective study of anticholinergic exposure using these validated scales, and its relationship with clinical signs and symptoms of anticholinergic toxicity, delirium and agitation, and other PICU morbidities.
Footnotes
The authors acknowledge that a nonspecific funding source has been used for this article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Salahudeen MS, Duffull SB, Nishtala PS.Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: A systematic review. BMC Geriatr 2015; 15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J Clin Pharmacol 2006; 46:1481–1486. [DOI] [PubMed] [Google Scholar]
- 3.Lertxundi U, Domingo-Echaburu S, Hernandez R, et al. Expert-based drug lists to measure anticholinergic burden: Similar names, different results. Psychogeriatrics 2013; 13:17–24. [DOI] [PubMed] [Google Scholar]
- 4.Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health 2008; 4:311–320. [Google Scholar]
- 5.Naja M, Zmudka J, Hannat S, et al. In geriatric patients, delirium symptoms are related to the anticholinergic burden. Geriatr Gerontol Int 2016; 16:424–431. [DOI] [PubMed] [Google Scholar]
- 6.Pasina L, Djade CD, Lucca U, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: Comparison of the Anticholinergic Cognitive Burden scale and Anticholinergic Risk Scale: Results from the REPOSI study. Drugs Aging 2013; 30:103–112. [DOI] [PubMed] [Google Scholar]
- 7.Burry LD, Williamson DR, Mehta S, et al. Delirium and exposure to psychoactive medications in critically ill adults: A multi-centre observational study. J Crit Care 2017; 42:268–274. [DOI] [PubMed] [Google Scholar]
- 8.Wolters AE, Zaal IJ, Veldhuijzen DS, et al. Anticholinergic medication use and transition to delirium in critically ill patients: A prospective cohort study. Crit Care Med 2015; 43:1846–1852. [DOI] [PubMed] [Google Scholar]
- 9.Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: Epidemiology and outcomes of pediatric delirium. Crit Care Med 2017; 45:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holstege CP, Borek HA.Toxidromes. Crit Care Clin 2012; 28:479–498. [DOI] [PubMed] [Google Scholar]
- 11.Burns MJ, Linden CH, Graudins A, et al. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med 2000; 35:374–381. [DOI] [PubMed] [Google Scholar]
- 12.Madden K, Burns MM, Tasker RC.Differentiating delirium from sedative/hypnotic-related iatrogenic withdrawal syndrome: Lack of specificity in pediatric critical care assessment tools. Pediatr Crit Care Med 2017; 18:580–588. [DOI] [PubMed] [Google Scholar]
- 13.Curley MA, Wypij D, Watson RS, et al. RESTORE Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators Network: Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA 2015; 313:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack MM, Patel KM, Ruttimann UE.PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24:743–752. [DOI] [PubMed] [Google Scholar]
- 15.Franck LS, Harris SK, Soetenga DJ, et al. The Withdrawal Assessment Tool-1 (WAT-1): An assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med 2008; 9:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traube C, Silver G, Reeder RW, et al. Delirium in critically ill children: An international point prevalence study. Crit Care Med 2017; 45:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA 2007; 298:2644–2653. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Cook D, Devlin JW, et al. SLEAP Investigators; Canadian Critical Care Trials Group: Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med 2015; 43:557–566. [DOI] [PubMed] [Google Scholar]
- 20.Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med 2015; 41:2130–2137. [DOI] [PubMed] [Google Scholar]
- 21.Smith HAB, Gangopadhyay M, Goben CM, et al. Delirium and benzodiazepines associated with prolonged ICU stay in critically ill infants and young children. Crit Care Med 2017; 45:1427–1435. [DOI] [PubMed] [Google Scholar]
- 22.Pandharipande P, Ely EW.Sedative and analgesic medications: Risk factors for delirium and sleep disturbances in the critically ill. Crit Care Clin 2006; 22:313–327, vii. [DOI] [PubMed] [Google Scholar]
- 23.van der Mast RC.Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998; 11:138–145.; discussion 157–158 [DOI] [PubMed] [Google Scholar]
- 24.Tune LE.Anticholinergic effects of medication in elderly patients. J Clin Psychiatry 2001; 62(Suppl 21):11–14. [PubMed] [Google Scholar]
- 25.Plaschke K, Petersen KA, Frankenhauser S, et al. The impact of plasma cholinergic enzyme activity and other risk factors for the development of delirium in patients receiving palliative care. J Pain Symptom Manage 2016; 52:525–532. [DOI] [PubMed] [Google Scholar]
- 26.Constantin JM, Momon A, Mantz J, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: A meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med 2016; 35:7–15. [DOI] [PubMed] [Google Scholar]
- 27.Jiang L, Ding S, Yan H, et al. A retrospective comparison of dexmedetomidine versus midazolam for pediatric patients with congenital heart disease requiring postoperative sedation. Pediatr Cardiol 2015; 36:993–999. [DOI] [PubMed] [Google Scholar]


