Abstract
Background:
More than half of older adults experience urinary (UI) or fecal incontinence (FI), but the majority have never discussed symptoms with health care providers. Little is known about primary care providers’ (PCPs’) screening for UI and FI.
Methods:
We conducted a cross-sectional electronic survey of PCPs within a Midwest academic institution to ascertain and compare PCPs’ beliefs, attitudes, and behaviors regarding screening and treatment for UI and FI; determine factors associated with screening for FI; and identify potential barriers to and facilitators of FI screening and treatment.
Results:
Among 154 PCPs, the screening rate for UI (75%) was more than double that for FI (35%;P < .001). PCPs believed that both UI and FI screening were important but felt better informed to treat UI (P < .001). Screening for FI was associated with UI screening (OR, 11.27; 95% CI, 4.9–26.0; P < .001); feeling informed to treat FI (OR, 10.21; 95% CI, 1.2–90.0; P =.01); screening verbally (OR, 3.9; 95% CI, 1.9–8.0; P < .001); perceiving screening as important (OR, 3.7; 95% CI, 1.8–7.4; P < .001); using the term, “accidental bowel leakage” (OR, 2.9; 95% CI, 1.2–6.7; P =.02) or “bowel control issues” (OR, 2.2; 95% CI, 1.1–4.5; P =.03); and being a resident (OR, 0.37; 95% CI, 0.16–0.82; P = .02). PCPs reported high interest in patient and provider educational materials about UI and FI.
Conclusions:
Most PCPs screen for UI but not FI. High reported interest in educational materials, coupled with high reported rates of perceived importance of screening for UI and FI, suggests that PCPs welcome informative interventions to streamline diagnosis and treatment. (J Am Board Fam Med 2018; 31:774–782.)
Keywords: Cross Sectional Analysis, Fecal Incontinence, Primary Health Care, Surveys, Questionnaires
More than half of independent adults aged 65 years and older suffer from urinary (UI) and/or fecal incontinence (FI) in the United States (US), and the prevalence among institutionalized adults is even higher.1 UI is associated with increased risk of falls, caregiver burden, and hospitalization,2–4 and both UI and FI are associated with increased care-giver burden and nursing home placement.5 Incontinence is associated with negative impact on quality of life and mental health, and this impact is more pronounced in those with combined UI and FI, also termed dual incontinence.6–9 While many clinicians are aware of the high prevalence of UI, few are aware that FI affects 8% of US adults (both male and female) monthly, or that the prevalence rises to 15% among older adults living independently and even higher among institutionalized adults.1,10 Further, it is predicted that the prevalence of pelvic floor disorders, including UI and FI, will increase by almost 60% in the next 30 years.11
The last 2 decades have seen tremendous advances in treatment for both UI and FI. Current available treatment options for UI in the United States include behavioral modification and bladder training, medication, pelvic floor muscle exercises with or without biofeedback, vaginal pessaries, chemodenervation, neuromodulation, and surgery.12Similar options are available to treat FI. For FI, the American College of Gastroenterology recommends treating FI starting with education, dietary modifications, skin care, and pharmacologic agents to modify stool delivery and consistency, followed by pelvic floor muscle rehabilitation with biofeedback; these interventions will improve or resolve symptoms in 50% to 80% of patients.13,14 For patients who do not respond to these interventions, additional options include the use of a vaginal pessary (Eclipse vaginal bowel control system) or rectal insert, several minimally invasive procedures to the anal canal, neuromodulation, and more invasive surgical options, which improve symptoms in approximately 85% of patients.13,14
Despite the range of effective treatment options available, most people with incontinence do not seek care, and those who do often delay seeking treatment. While as many as 50% of women with UI seek care,15 estimated rates of care seeking for FI are lower and range from 10% to 30% with delays from onset of symptoms of 2 years on average for women and 3 years for men.15–18 Screening by primary care providers (PCPs) has the potential to shorten this delay and improve access to effective treatments, but limited data exist about physician screening for these conditions.19,20 We were able to identify only 1 existing study published in English that queried physicians about screening for FI, and that study included only 11 physicians.20
Given the prevalence and significant negative impact of UI and FI, availability of effective treatment options, and the limited rates of spontaneous care seeking for these conditions, we sought to quantify screening rates, attitudes, beliefs, and behaviors for these conditions among PCPs in our health care system. We particularly emphasized FI in our analyses given lower rates of care seeking by patients and the paucity of information about screening for FI in the existing literature.
Methods
Study Design, Setting, and Population
We conducted an electronic survey of PCPs within our Midwestern academic medical center. All clinicians (physicians, nurse practitioners, and physician assistants) in the Departments of Internal Medicine, Family Medicine, Obstetrics and Gynecology, and Geriatrics were invited to participate. Automated invitational emails were sent every other week during February to March 2015. Up to 3 emails were sent to each provider to maximize participation. This study was deemed exempt by the University of Wisconsin–Madison Health Sciences Minimal Risk Institutional Review Board (2014–1302).
Survey Instrument
The 20-item anonymous online survey assessed clinicians’ knowledge, attitudes, and beliefs related to screening and treatment for UI and FI, as well as information about their level of confidence to treat these conditions, and barriers to and facilitators of screening and treatment. The instrument was pilot tested by 6 physicians and revised before data collection based on their feedback. Minimal demographic data were collected. Most questions were multiple choice, often with a 5-point Likert scale for response options, and some allowed for free-text responses.
Analysis
Descriptive analyses characterized the sample. Providers who reported screening some, most, or every patient for incontinence were considered screeners; those who reported screening few or no patients were considered nonscreeners. χ;2 testing was used to compare providers’ screening rates, beliefs about importance of screening, and confidence to treat UI versus FI. Preferences for screening methods and terminology and populations considered to be at high risk for FI were described. Logistic regression was used to identify factors associated with screening for FI. χ;2 testing was used to compare the proportions of providers who reported various barriers to screening for UI versus FI and to compare their preferences for additional tools to facilitate screening for these conditions. We used STATA 14 (StataCorp, College Station, TX) for all statistical analyses and considered P < .05 as statistically significant.
Results
Among 724 clinicians emailed and 696 verified valid emails, 27% (185/696) responded, of whom 83% (154/185) provided primary care to adult patients at the time and were thus eligible for inclusion (Figure 1). Table 1 describes the sample overall and stratified by screening status. Family medicine providers and attending physicians were more likely to screen for UI. Attending physicians and advanced practice providers were more likely to screen for FI. Information about age and gender were not collected.
Figure 1.
Study sample flow diagram. Describes responses to a 2015 email survey of primary care providers at a Midwest academic medical center.
Table 1.
Characteristics of Respondents to an Electronic Survey of Primary Care Providers at a Midwest Academic Medical Center in 2015, Stratified by Screening for Urinary (UI) and Fecal Incontinence (FI)
| Overall Sample N = 154 |
Screens for UI N = 113 |
Screens for FI N = 53 |
||||
|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n | Percent | |
| Provider Specialty | P = .025 | P = .08 | ||||
| Family medicine | 73 | 47 | 57 | 50 | 23 | 30 |
| Internal medicine | 50 | 33 | 30 | 27 | 16 | 43 |
| Obstetrics & gynecology | 25 | 16 | 20 | 18 | 9 | 17 |
| Geriatrics | 6 | 4 | 6 | 5 | 5 | 9 |
| Provider type | P < .001 | P = .017 | ||||
| Attending | 74 | 48 | 66 | 58 | 30 | 57 |
| Resident or fellow | 55 | 36 | 28 | 25 | 11 | 21 |
| Advanced practitioner | 25 | 16 | 19 | 17 | 12 | 23 |
Table 2 displays differences in opinions and practices regarding screening practices, perceived importance of screening, and confidence to treat UI versus FI among PCPs. Clinicians were twice as likely to screen for UI as they were to screen for FI (P < .001), with 75% screening at least some patients for UI, but only 35% screening at least some patients for FI. Only 10 providers (6%) were aware of online information sources about FI such as the National Institutes of Health Bowel Control Awareness Campaign (https://www.niddk.nih.gov/health-information/digestive-diseases/bowel-control-problems-fecal-incontinence/).
Table 2.
Differences in Opinions and Practices Regarding Urinary (UI) and Fecal Incontinence (FI) Screening and Treatment Among Primary Care Providers at a Midwest Academic Medical Center in 2015
| UI | FI | P-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| I screen: | |||||
| Every/most patients | 42 | 28 | 13 | 9 | <.001 |
| Some patients | 71 | 47 | 40 | 26 | |
| A few or none | 39 | 25 | 99 | 65 | |
| I perceive screening to be: | |||||
| Very/extremely important | 74 | 48 | 56 | 37 | .04 |
| Somewhat important | 59 | 39 | 61 | 40 | |
| Slightly/not at all important | 20 | 13 | 35 | 23 | |
| Regarding treatment, I feel: | |||||
| Very/extremely informed | 42 | 27 | 6 | 4 | <.001 |
| Somewhat informed | 84 | 55 | 49 | 32 | |
| Slightly/not at all informed | 26 | 17 | 97 | 64 | |
When asked about their preferred method of screening for FI, 49% of PCPs (n = 74) would screen via verbal review of systems, 22% (n = 34) via written review of systems, and 12% (n = 18) via verbal discussion if patient disclosed symptoms on a written review of systems; 17% (n = 25) preferred that the patient bring it up. The most commonly used terms to discuss FI with patients were “bowel control issues” (60%, n = 91), “bowel inconti nence” (36%, n = 55), “fecal incontinence” (25%, n = 38), and “accidental bowel leakage” (18%, n = 27). Thirty participants also mentioned other terms, such as “losing stool,” “having a bowel movement when you do not mean to,” “having your stool come out unexpectedly,” “trouble holding in your stool/poop,” and “fecal incontinence, like pooping your pants.” Four participants bundled bladder and bowel symptoms together and asked about changes/problems/issues with urine or stool.
Participants were asked how important they considered various potential risk factors for FI to be. Older age (86%, = 130), prior surgery or radiation for prostate cancer (83%, n < 125), childbirth (81%, n < 123), diarrhea (83%, n = 125), and constipation (78%, n < 119) were widely perceived to be very or extremely important risk factors. Fewer PCPs recognized diabetes mellitus as a very or extremely important risk factor (38%, n = 58). Female sex was identified as an important risk factor by 46% (n = 69) and hypertension by 11% (n = 16).
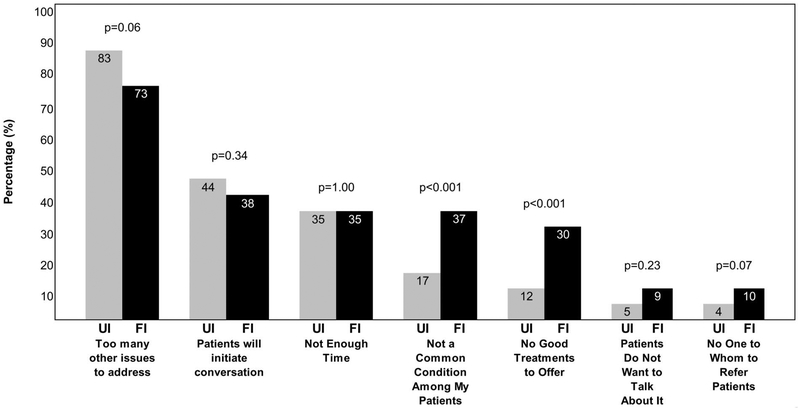
On univariate logistic regression, screening for FI was associated with screening for UI (OR, 11.27; 95% CI, 4.9–26.0; P < .001); feeling somewhat or very informed to treat FI (OR, 10.21; 95% CI,1.2–90.0; P < .01); preferring to screen for FI verbally (OR, 3.9; 95% CI, 1.9–8.0; P <.001); perceiving screening for FI as important (OR, 3.7; 95% CI, 1.8–7.4; P < .001); using the term, “accidental bowel leakage” (OR, 2.9; 95% CI, 1.2–6.7;P =.02) or “bowel control issues” (OR, 2.2; 95% CI, 1.1–4.5; P = .03); and being a resident physician (OR, 0.37; 95% CI, 0.16–0.82; P = .02). Given that only 53 providers screened for FI, multivariate logistic regression was infeasible. PCPs reported similar top barriers in clinical practice to screening for UI and FI (Figure 2). The most commonly cited barrier to screening for both UI and FI was having too many other issues to address during the office visit. However, PCPs were more likely to report that the condition was not common among their patients as a barrier to screening for FI than for UI (P < .001), and were also more likely to endorse the statement that they had no good treatments to offer for FI as compared with UI (P < .001). Of note, 27% (38/139) of providers who did not screen for FI stated their belief that patients would initiate the conversation if they were bothered. Only 5/110 (5%) of providers cited patients’ lack of desire to talk about the condition as a barrier to screening for UI, versus 13/139 (9%) for FI (P = .23). Few reported a lack of specialists or other providers to whom they could refer patients for treatment of UI (4%, n = 4) or FI (10%, n = 14) (P = .07).
Figure 2.
Barriers to screening for urinary (UI) (N = 110) and fecal incontinence (FI) (N = 139). Compares responses from primary care providers (PCPs) who screen sometimes, rarely, or never for UI (N = 110) with those who screen sometimes, rarely, or never for FI (N = 139) about barriers to or reasons for not screening in a 2015 email survey of PCPs at a Midwest academic medical center.
When informed that the prevalence of monthly FI is 8% in independent US adults, 75% (n = 114) of PCPs characterized that prevalence as higher than they expected, and 66% (n = 100) reported that they felt screening for FI was more important as a result of learning that prevalence rate. Those who do not screen for FI were more likely to characterize that prevalence as higher than they expected (79%, n < 78/99) than those who do screen for FI (68%, n = 36/53), P = .02.
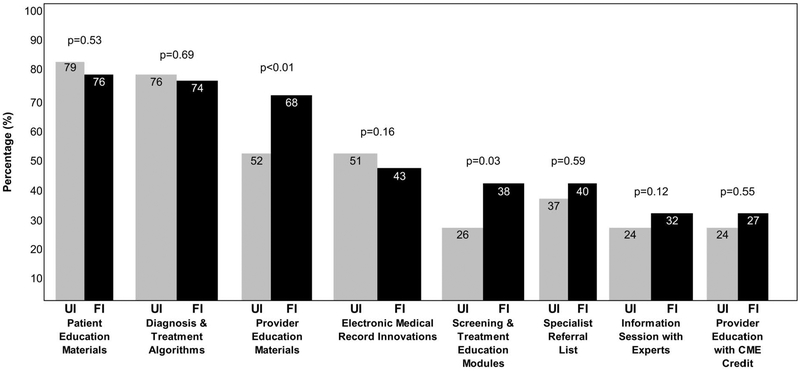
Clinicians reported interest in patient education materials and online or printed algorithms to follow for diagnosis and treatment for both UI and FI (Figure 3). There was significantly higher demand for provider education materials regarding FI (44%) versus UI (34%) (P < 01) and higher demand for screening and treatment education modules for FI (25%) versus UI (17%) (P < .03).
Figure 3.
Recommendations to better inform providers about urinary (UI) and fecal incontinence (FI) (N = 154). Compares responses from primary care providers (PCPs) about preferences for resources relate to UI and FI screening and treatment in a 2015 email survey of PCPs at a Midwest academic medical center.
Discussion
In this survey of 154 PCPs in a Midwestern academic institution, screening rates for UI (75%) were much higher than FI (35%). PCPs were twice as likely to screen for and felt better informed to manage UI versus FI. Those who screen for UI and those who feel informed to treat FI are more likely to screen for FI, suggesting that interventions to improve PCPs’ confidence in treating FI may im prove screening rates. Further, there was high reported interest in educational materials targeting both patients and providers for both UI and FI, with particular interest in diagnosis and treatment algorithms for both conditions and provider-directed education for FI specifically. This high interest, coupled with the common belief that screening for UI and FI is important, suggests that PCPs may welcome interventions that facilitate diagnosis and treatment.
While several studies to date have examined rates of and reasons for not seeking care for FI in patient populations,15,20 this study is the first large survey to provide information about rates of and reasons for not screening for FI from the health care provider’s perspective. In the only other study in the English literature that queried physicians about screening for FI, 11/56 physicians responded, 9 of whom potentially provided primary care (8 geriatricians and 1 general internist), and the rate of screening for FI among those 9 physicians was 67%, which is significantly higher than the rate in our study of 35%, but somewhat comparable to the screening rate among geriatricians in our study.20 While the sample size of the study by Kunduru and colleagues20 is small, several findings in their study mirror those in ours: nonscreeners perceived screening for FI to be less important than screening for other conditions, 50% noted time constraints as an important barrier to screening, and 100% perceived it to be the patient’s responsibility to initiate the conversation.20 Even among those who screened for FI in that study, 50% reported limited accessibility to subspecialty care and concerns about excessive medical costs as barriers to screening or treatment.20 Similar to our findings, 75% of those who did not screen for FI in that study perceived the prevalence of FI to be low in the general patient population.20
Several of our findings deserve special attention. The association of use of the terms, “accidental bowel leakage” and “bowel control issues,” with screening for FI emphasizes the importance of terminology with this condition. In the Mature Women’s Health Study, an electronic survey of almost 6000 independent women aged 45 years and older, of whom approximately 1000 had FI, the term, “accidental bowel leakage,” was preferred by 71% of women with FI over the terms, “fecal incontinence” (6%) or “bowel incontinence” (23%).21 Of note, very few PCPs in our survey used the term, “accidental bowel leakage,” suggesting that it should be included in clinician education efforts. Because it is impossible to know a given patient’s preferred terminology without asking, it may make sense to include multiple synonyms when verbally inquiring about FI symptoms. For example, when asking about dyspnea, a clinician might say, “Any trouble with breathing, shortness of breath, trouble catching your breath?” Similarly, when asking about FI, a clinician might ask, “Any bowel control issues? Accidental bowel leakage? Incontinence of stool? Not making it to the toilet when you want to?” Since the majority (75%) of PCPs in this survey already screen at least some patients for UI, adding inquiries about FI at the same time may be an easy way to incorporate this screening.
It is important to note that this high screening rate for UI is likely related to its inclusion as a Merit-based Incentive Payment System (MIPS) quality metric (https://qpp.cms.gov/mips/quality-measures). The Centers for Medicare and Medicaid Services MIPS quality metric #048, “the percentage of female patients aged 65 years and older who were assessed for the presence or absence of UI within 12 months,” is based on the rationale that patients may not disclose incontinence symptoms and thus should be asked by a physician about them. If screening for FI in older men and women were similarly recognized as a quality measure, it might similarly increase screening rates.
The strong association of preference to screen verbally with PCP screening for FI also merits mention, since a prior survey of 124 patients with FI reported that over 70% of patients who had not sought care believed that doctors need to speak directly to patients to improve treatment of FI.20 Interestingly, almost 60% of patients who had not sought care for their FI in that study agreed with the statement that patients would prefer to use questionnaires or answer routine questions about FI.20 In prior qualitative studies, women with FI describe written inquiry as a more comfortable way to broach an uncomfortable topic,15 and women with dual incontinence seeking care are more likely to verbally disclose urinary than fecal symptoms.22 Similarly, adults in Ireland are more likely to disclose the use of incontinence aids on an anonymous written survey than they are when asked in face-to-face interviews.23 Use of a previsit electronic pelvic floor health questionnaire, results of which were provided to both patients and their PCPs, improved provider-initiated discussion of incontinence in a randomized trial of women aged 40 years or older presenting to an internal medicine clinic for a well-woman examination.24 It is thus likely that the optimal screening approach for FI should include both written or electronic and verbal in quiry, and we suggest that further research should explore these approaches.
It is striking that only 20% of physicians in training (residents and fellows) in our survey screened for FI, as compared with 41% of attending physicians and 48% of advanced practitioners. Although this finding may be confounded by level of acuity in patient populations, it is important that provider-oriented educational materials target residents and fellows in addition to attending physicians.
Provider-oriented educational materials should also target the patient preferred description of FI, including the term, “accidental bowel leakage,” and misconceptions that patients will bring up FI if bothered and should emphasize the existence of effective, minimally invasive treatment options. The high demand for educational materials coupled with our finding that only 6% of clinicians were aware of the National Institutes of Health’s Bowel Control Awareness Campaign,25 whose Web site offers both patient- and provider-oriented information about FI, suggests that efforts to better disseminate this campaign are warranted. Further, demand for evidence-based diagnosis and treatment algorithms for FI is high and should be added to educational materials targeting PCPs.
Our study is limited by the inclusion of PCPs from a single academic medical center, as well as a relatively low response rate of 27%. Physician populations are notoriously difficult to survey, especially via email and without significant incentive for responders.26 Response rates below 20% are not uncommon in Internet surveys of physicians.27 In general, response rates have fallen among health care industry surveys recent years and our experience proved no different.28 We acknowledge that the attitudes, practices, and experiences of survey respondents may not be generalizable to nonresponders or to providers in other health care systems, though Dykema and colleagues29 suggest that nonresponse bias may be lower among physician populations than others because they are relatively homogeneous. Further, the proportion of respondents who reported screening most or all patients for UI in this study (27%), is comparable to that found in a recent survey of primary care providers (n =391) across the country (39%).19 Given that very little information exists regarding PCP screening for UI, and even less exists about screening for FI, this survey of over 150 clinicians who provide primary care in diverse specialties offers valuable insights into differences between UI and FI that may be used to guide improvements in screening for FI.
Based on our findings, we propose that future research should explore whether screening rates can be improved through educational interventions, as well as which formats of screening are optimal for both patients and primary care providers. We hypothesize that patient- and provider-oriented educational materials tailored to primary care providers, complete with evidence-based algorithms for diagnosis and treatment, will promote better screening for FI. We recognize the need to understand the practices and preferences of primary care providers in other locations, as well as in nonacademic practice settings, to facilitate adaptation and dissemination of interventions likely to improve screening and treatment rates across diverse patient populations.
Acknowledgments
Funding: This work was financially supported by the K12 Urologic Research Career Development Program (NIH K12DK100022).
We would also like to acknowledge Sara Vrabec and Kelly Brezoczky for their assistance with this project.
Footnotes
Conflict of interest: HWB, consultant for Grand Rounds, Inc., principal investigator for LIBERATE trial funded by Pelvalon, Inc.; PDS, Principal investigator for as unrestricted educational grant related to shared-decision making for patients with post-menopausal vaginal atrophy funded by Pfizer, co-investigator for grant related to shared-decision making for patients with chronic pain treated by opioid medications funded by Pfizer, consultant on a grant to assess the impact of a health literacy tailored patient education/medication counseling program to improve clinical outcomes in patients with diabetes funded by Merck; RGR, Data Safety Monitoring Board Chair for the TRANSFORM trial sponsored by American Medical Systems, Royalties from Up-to-Date; stipend and travel from American Board of Obstetrics and Gynecology; Stipend and travel from International Urogynecological Association; WW received,NIH Grants on fecal incontinence (U34DI109191 and R21DK096545), Salix grant to investigate epidemiologic characteristics of people with fecal incontinence.
References
- 1.Gorina Y, Schappert S, Bercovitz A, et al. Prevalence of incontinence among older americans. Vital Health Stat 3 2014;36:1–33. [PubMed] [Google Scholar]
- 2.Foley AL, Loharuka S, Barrett JA, et al. Association between the Geriatric Giants of urinary incontinence and falls in older people using data from the Leicestershire MRC Incontinence Study. Age Ageing 2012;41:35–40. [DOI] [PubMed] [Google Scholar]
- 3.Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing 1997;26:367–74. [DOI] [PubMed] [Google Scholar]
- 4.Tamanini JT, Santos JL, Lebrão ML, et al. Association between urinary incontinence in elderly patients and caregiver burden in the city of Sao Paulo/Brazil: Health, Wellbeing, and Ageing Study. Neurourol Urodyn 2011;30:1281–5. [DOI] [PubMed] [Google Scholar]
- 5.Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the effect of fecal incontinence on nursing home referral. J Am Geriatr Soc 2010;58:1058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avery JC, Stocks NP, Duggan P, et al. Identifying the quality of life effects of urinary incontinence with depression in an Australian population. BMC Urol 2013;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int J Clin Pract 2012;66:1109–16. [DOI] [PubMed] [Google Scholar]
- 8.Miner PB Jr. Economic and personal impact of fecal and urinary incontinence. Gastroenterology 2004; 126(1 Suppl 1):S8–S13. [DOI] [PubMed] [Google Scholar]
- 9.Shaw C A review of the psychosocial predictors of help-seeking behaviour and impact on quality of life in people with urinary incontinence. J Clin Nurs 2001;10:15–24. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology 2009;137:512–517, 517.e511–e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol 2014;123:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki Y, Brown HW, Brubaker L, et al. Urinary incontinence in women. Nat Rev Dis Primers 2017; 3:17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: Management of benign anorectal disorders. Am J Gastroenterol 2014;109:1141–1157; (Quiz) 1058. [DOI] [PubMed] [Google Scholar]
- 14.Wald A Update on the management of fecal incontinence for the gastroenterologist. Gastroenterol Hepatol (N Y) 2016;12:155–64. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown HW, Wise ME, Rogers RG. Barriers to seeking care for accidental bowel leakage: A qualitative study. Int Urogynecol J 2017;28:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johanson JF, Lafferty J. Epidemiology of fecal incontinence: The silent affliction. Am J Gastroenterol 1996;91:33–6. [PubMed] [Google Scholar]
- 17.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: A population-based study in women. Gastroenterology 2005;129:42–9. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz-Yagüe T, Solís-Muñoz P, Ciriza de los Ríos C, et al. Fecal incontinence in men: Causes and clinical and manometric features. World J Gastroenterol 2014;20:7933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazloomdoost D, Crisp CC, Kleeman SD, et al. Primary care providers’ experience, management, and referral patterns regarding pelvic floor disorders: A national survey. Int Urogynecol J 2018;29: 109–18. [DOI] [PubMed] [Google Scholar]
- 20.Kunduru L, Kim SM, Heymen S, Whitehead WE. Factors that affect consultation and screening for fecal incontinence. Clin Gastroenterol Hepatol 2015;13:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: Prevalence and predictors. Int J Clin Pract 2012;66:1101–8. [DOI] [PubMed] [Google Scholar]
- 22.Cichowski SB, Komesu YM, Dunivan GC, et al. Written versus oral disclosure of fecal and urinary incontinence in women with dual incontinence. Int Urogynecol J 2014;25:1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley B It’s the way you ask that matters: Comparison of data relating to prevalence of incontinence aid use from two surveys of people with multiple sclerosis. J Wound Ostomy Continence Nurs 2006; 33:26–9. [DOI] [PubMed] [Google Scholar]
- 24.Schussler-Fiorenza Rose SM, Gangnon RE, Chewning B, et al. Increasing discussion rates of incontinence in primary care: A randomized controlled trial. J Womens Health (Larchmt) 2015;24:940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowel Control Problems (Fecal Incontinence). National Institute of Diabetes and Digestive and Kidney Diseases. Available from: https://www.niddk.nih.gov/health-information/digestive-diseases/bowel-control-problems-fecal-incontinence. Accessed April 16, 2018.
- 26.Pit SW, Vo T, Pyakurel S. The effectiveness of recruitment strategies on general practitioner’s survey response rates—A systematic review. BMC Med Res Methodol 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dykema J, Stevenson J, Day B, et al. Effects of incentives and prenotification on response rates and costs in a national web survey of physicians. Eval Health Prof 2011;34:434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dykema J, Jones NR, Piché T, et al. Surveying clinicians by web: Current issues in design and administration. Eval Health Prof 2013;36:352–81. [DOI] [PubMed] [Google Scholar]