Summary
A+β+ ketosis prone diabetes (KPD) is associated with slowly progressive autoimmune beta cell destruction. Plasma unmethylated and methylated insulin DNA (biomarkers of ongoing beta cell damage and systemic inflammation, respectively) were elevated in A+β+ KPD compared to all other KPD subgroups.
Keywords: Ketosis prone diabetes, insulin DNA, beta cell death, islet dysfunction
1. Introduction
A+β+ ketosis prone diabetes (KPD) patients have islet autoantibodies and beta cell functional reserve despite presenting with diabetic ketoacidosis (DKA)1, 2. They are middle-aged and overweight; 50% discontinue insulin therapy within 6–12 months for prolonged periods while maintaining glycemic control1, 2. Slowly progressive autoimmune beta cell destruction underlie A+β+ KPD, hence we measured unmethylated insulin DNA (INS DNA) and methylated INS DNA in A+β+ KPD versus all other subtypes of KPD patients. We have previously shown that circulating unmethylated INS DNA, produced only in beta cells, is increased in newly diagnosed type 1 diabetes (T1D) patients and falls to control levels 8 weeks later, indicating that it is a marker of ongoing beta cell destruction. Methylated INS DNA, in contrast, is not produced exclusively in beta cells, is elevated in new-onset T1D patients compared with “typical” type 2 diabetes adults (T2D), and is higher in the latter than in obese non-diabetic adults - these data suggest that methylated INS DNA may reflect systemic inflammation associated with islet dysfunction3.
2. Methods
The study was approved by the Institutional Review Board for Human Studies of Baylor College of Medicine. KPD patients were identified after presentation with DKA based on our published criteria and treated with standard management protocols2, 4.
All participants were classified by the validated Aβ classification scheme for KPD1, 2, 4, 5. A+β+ KPD was defined by presence of antibodies directed against the 65 kDa glutamic acid decarboxylase, islet antigen-2, or zinc transporter T8, and presence of beta cell functional reserve (defined and validated as a fasting serum C-peptide level ≥1 ng/mL or maximum glucagon stimulated C-peptide level ≥1.5 ng/mL)1, 2, 4.
Sera and DNA were obtained 4–6 months after the index DKA from 45 A+β+ KPD patients and 67 patients randomly selected to represent all other KPD subtypes, once they were without ketosis on stable doses of insulin or oral medications3, 6. Other KPD subtypes included A+β− (N=16), A−β− (N=21), and A−β+ (N=30). All DNA samples underwent bisulfite conversion before analysis by droplet digital PCR, utilizing a QX200 Droplet Reader and QuantaSoft Software (Bio-Rad). Values were normalized for DNA recovery, back-calculated to the volume of blood used for DNA isolation and reported as concentrations of copies/µL3, 6.
For direct group comparisons, two-tailed unpaired t-tests were used. P values < 0.05 were considered significant. Data is reported as means ± SEM.
3. Results
Of the antibody-positive patients in this cohort, GAD-65 was present in 75.0%, IA−2 in 13.6%, and Zn in 26.2%, and 77.3% had only a single antibody.
The A+β+ KPD patients had a shorter history of diabetes (P=0.01) and greater mean BMI (P=0.04) than the patients of all other KPD subtypes, but there were no other significant differences between the groups (Table 1).
Table 1:
Patient characteristicsa
| Characteristics | A+β+ KPD (N = 45) |
All Other KPD Subtypes (N = 67) |
|---|---|---|
| Male (%) | 52.2 | 59.7 |
| Race | ||
| • Hispanic (%) | 53.3 | 56.7 |
| • African American (%) | 22.2 | 31.3 |
| Family History of Diabetes (%) | 87.8 | 77.4 |
| BMI (kg/m2) | 31.4 ± 1.1b | 28.2 ± 1.0 |
| Age at Diagnosis (years) | 35.7 ± 1.6 | 34.2 ± 1.6 |
| Duration of Diabetes (years) | 2.3 ± 0.7b | 5.7 ± 0.9 |
| Initial HbA1c | ||
| • (%) | 13.0 ± 0.3 | 12.7 ± 0.3 |
| • (mmol/mol) | 118.6 ± 4.3 | 115.3 ± 3.4 |
Data expressed as % or means ± SEM
P < 0.05
Unmethylated INS DNA was elevated in A+β+ KPD (10.2 ± 3.3 vs. 2.8 ± 0.3 copies/µL, p=0.015), as was methylated INS DNA (75.2 ± 34.0 vs. 4.2 ± 0.9 copies/µL, p=0.008) (Figure 1). In a multivariate model adjusting for gender, age at diagnosis of diabetes, duration of diabetes, BMI and initial HbA1c, both unmethylated (P=0.02) and methylated (P=0.03) INS DNA remained significantly elevated in A+β+ KPD compared with other KPD patients. There was no significant difference in unmethylated or methylated INS DNA across the three remaining Aβ categories amongst the 67 patients of all other subtypes of KPD (unmethylated INS DNA: A+β−(2.7 ± 0.8 copies/µL), A−β− (2.6 ± 0.6 copies/µL), A−β+ (3.0 ± 0.4 copies/µL); methylated INS DNA: A+β− (3.0 ± 1.8 copies/µL), A−β− (3.3 ± 1.6 copies/µL), A−β+ (5.0 ± 1.4 copies/µL)).
Figure 1A: Figure 1B:
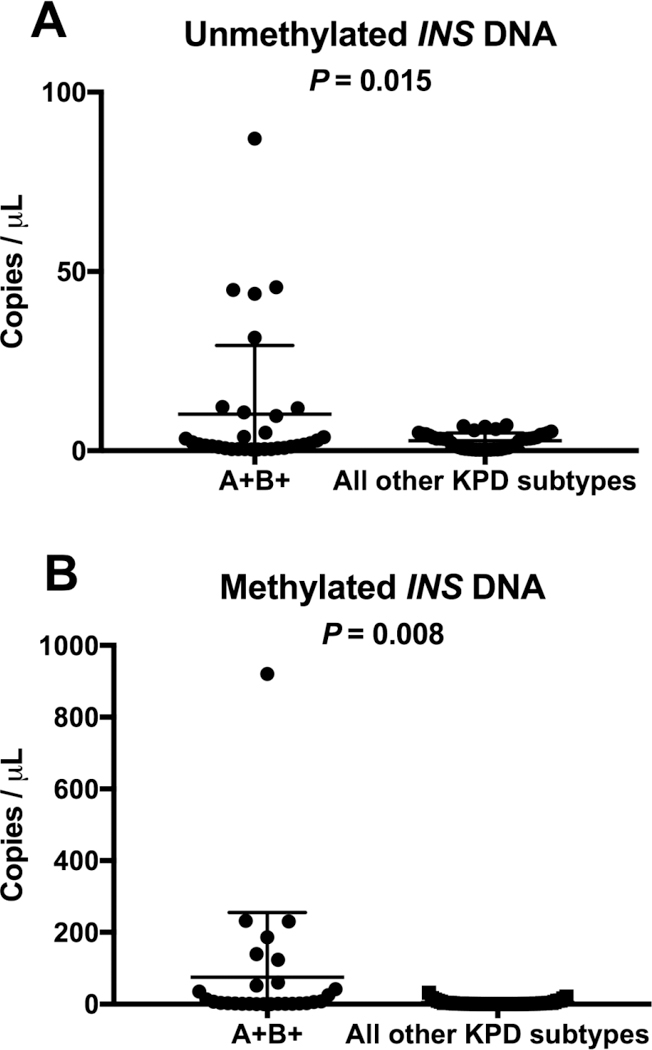
Absolute copies of unmethylated INS DNA normalized to DNA concentration Absolute copies of methylated INS DNA normalized to DNA concentration
4. Discussion
Unmethylated INS DNA levels, indicating ongoing beta cell destruction, were significantly higher in serum samples of metabolically stable A+β+ KPD patients months after their index DKA episode compared to patients of all other KPD subtypes. Unmethylated INS DNA has been reported in children with autoimmune type 1 diabetes, but this is the first report in adult patients with a recent diagnosis of diabetes3, 7, 8. The presence of circulating unmethylated INS DNA many months following the index DKA episode suggests that the underlying pathophysiology of A+β+ KPD involves ongoing, slowly progressive beta cell destruction. The natural history of these patients reflects this process, characterized by achievement of near-normoglycemia (often with insulin-independence) for long periods of time (over 2 years in 50%)1, followed by relapse to ketosis and insulin dependence. On average, A+β+ KPD patients carry a diagnosis of diabetes for 2.3 ± 0.7 years prior to presentation with DKA. We obtained the serum samples for the present analysis an additional 4 – 6 months after these patients became metabolically stable; yet unmethylated DNA is still present at significant levels, suggesting the persistence of functioning beta cells that are nevertheless experiencing chronic inflammatory damage.
This is a different process than in patients with A+β− KPD (“typical” autoimmune type 1 diabetes), who lack beta cell functional reserve, remain insulin-dependent following the index DKA, and have very low levels of circulating unmethylated DNA. Fisher et al previously reported that unmethylated INS DNA levels in children with type 1 diabetes dropped to those of controls within 8 weeks from onset of diabetes3, suggesting that a significant number of beta cells undergoing active destruction are required to produce measurable circulating unmethylated INS DNA. Patients with autoantibody-negative (“A−β−”and “A−β+”) KPD also have very low levels of unmethylated INS DNA, indicating that chronic, ongoing beta cell destruction is not a hallmark of these subtypes.
Levels of methylated INS DNA also were elevated in A+β+ KPD, a pattern we have observed in obesity or inflammatory bowel disease and potentially reflecting systemic inflammation associated with islet dysfunction (Tersey SA, et al, unpublished). Children with new-onset T1D have elevated methylated INS DNA3, but the levels decline to control levels within one year. In contrast, levels of methylated INS DNA in A+β+ KPD remain elevated years after onset of diabetes, suggesting that a more chronic inflammatory process could affect the islets and lead to beta cell dysfunction. This reinforces the concept that A+β+ KPD patients experience slow, prolonged decline in islet mass or function, as opposed to the rapid destruction of islets characteristic of most children with autoimmune type 1 diabetes3.
5. Conclusion
Elevated serum levels of unmethylated INS DNA and methylated INS DNA accurately distinguish A+β+ KPD and suggest a unique pathophysiology of beta cell dysfunction in these patients.
Highlights.
A+β+ ketosis prone diabetes (KPD) patients have islet autoantibodies and substantial β−cell functional reserve.
Using droplet digital PCR to measure insulin DNA, we tested the hypothesis that the coexistence of autoimmunity and preserved β−cell function in A+β+ KPD can be distinguished from other KPD subtypes.
We measured unmethylated and methylated insulin DNA (markers of β−cell death and systemic inflammation, respectively) of 45 A+β+ KPD subjects compared with 67 subjects from all other KPD subtypes.
Both forms of DNA were significantly elevated in A+β+ KPD subjects compared to subjects from all other KPD subtypes.
Acknowledgements
We thank the patients for their time, patience, and contribution.
Funding Source
This work was supported by National Institutes of Health grants RO1-DK1041411 (to AB), R01-DK60581 (to RGM) and UC4-DK104166 (to RGM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
SNM, CSH and AB have no conflicts of interest. SAT and RGM have filed patent applications for assays to measure beta cell dysfunction and death.
References
- 1.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 2008;29(3): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 2003;88(11): 5090–5098. [DOI] [PubMed] [Google Scholar]
- 3.Fisher MM, Watkins RA, Blum J, et al. Elevations in Circulating Methylated and Unmethylated Preproinsulin DNA in New-Onset Type 1 Diabetes. Diabetes 2015;64(11): 3867–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaba R, Gambhire D, Uy N, et al. Factors associated with early relapse to insulin dependence in unprovoked A-beta+ ketosis-prone diabetes. J Diabetes Complications 2015;29(7): 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care 2006;29(12): 2575–2579. [DOI] [PubMed] [Google Scholar]
- 6.Tersey SA, Nelson JB, Fisher MM, Mirmira RG. Measurement of Differentially Methylated INS DNA Species in Human Serum Samples as a Biomarker of Islet beta Cell Death. J Vis Exp 2016(118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher MM, Perez Chumbiauca CN, Mather KJ, Mirmira RG, Tersey SA. Detection of islet beta-cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology 2013;154(9): 3476–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akirav EM, Lebastchi J, Galvan EM, et al. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A 2011;108(47): 19018–19023. [DOI] [PMC free article] [PubMed] [Google Scholar]


