Abstract
Objectives:
Deep brain stimulation of the internal pallidum (GPi-DBS) is an established therapeutic option in treatment-refractory dystonia, and the identification of factors predicting surgical outcome is needed to optimize patient selection.
Methods:
In this retrospective multicenter study, GPi-DBS outcome of 8 patients with DYT6, 9 with DYT1, and 38 with isolated dystonia without known monogenic cause (non-DYT) was assessed at early (1–16 months) and late (22–92 months) follow-up using Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) scores.
Results:
At early follow-up, mean reduction of dystonia severity was greater in patients with DYT1 (BFMDRS score: −60%) and non-DYT dystonia (−52%) than in patients with DYT6 dystonia (−32%; p = 0.046). Accordingly, the rate of responders was considerably lower in the latter group (57% vs >90%; p = 0.017). At late follow-up, however, GPi-DBS resulted in comparable improvement in all 3 groups (DYT6, −42%; DYT1, −44; non-DYT, −61%). Additional DBS of the same or another brain target was performed in 3 of 8 patients with DYT6 dystonia with varying results. Regardless of the genotype, patients with a shorter duration from onset of dystonia to surgery had better control of dystonia postoperatively.
Conclusions:
Long-term GPi-DBS is effective in patients with DYT6, DYT1, and non-DYT dystonia. However, the effect of DBS appears to be less predictable in patients with DYT6, suggesting that pre-DBS genetic testing and counseling for known dystonia gene mutations may be indicated. GPi-DBS should probably be considered earlier in the disease course.
Classification of evidence:
This study provides Class IV evidence that long-term GPi-DBS improves dystonia in patients with DYT1, DYT6, and non-DYT dystonia.
Deep brain stimulation of the internal pallidum (GPi-DBS) is an established and safe therapeutic option in otherwise treatment-refractory generalized and segmental dystonia.1 A recent prospective follow-up study has confirmed long-term benefit after 5 years.2 However, it is becoming increasingly clear that not all patients have an equally beneficial response to DBS surgery. It is thus crucial to identify factors predicting the surgical outcome. Suggested predictors for a good clinical outcome of GPi-DBS include absence of fixed skeletal deformities, younger age at surgery, short disease duration, and positive DYT1 mutation status.3–6
Aside from DYT1 dystonia, DYT6 dystonia is another cause of monogenic isolated dystonia.7,8 Both forms are autosomal dominantly inherited, the penetrance is considerably reduced (DYT1 30%, DYT6 40%),9 and dystonia is the single clinical manifestation apart from tremor (=isolated dystonia).10 Whereas nearly all DYT1 dystonia cases carry a single 3–base pair deletion in the TOR1A gene,11 the mutational spectrum of DYT6 dystonia is much broader, with more than 50 thanatos-associated domain containing, apoptosis-associated protein 1 (THAP1) gene mutations described to date.12 In both forms, dystonia usually manifests in childhood or early adulthood, with a mean age at onset of 13 (DYT1)13 and 19 (DYT6)9 years, respectively. Both forms tend to generalize and the phenotypic spectrum is broad. However, there are certain clinical symptoms that appear to be more common in one or the other form. Whereas DYT1 dystonia presents with a predominant leg and lower body distribution, DYT6 dystonia often involves the upper half of the body, including laryngeal adductor-type dystonia, which occasionally culminates in aphonia.14
Recently published small case series have reported a modest response of bilateral GPi-DBS in 2 patients14 and an improvement of 15% to 55% in 4 patients with DYT6 dystonia.15 While the motor function showed a moderate to good response, speech involvement responded much less or not at all. A comparison between 3 patients with DYT6 and 23 with DYT1 dystonia suggested less robust results in the first 2 postoperative years in DYT6 dystonia and even some deterioration between the second and third years despite adjustments of stimulation parameters and repositioning of one DBS electrode in one patient.16
Our aim was to systematically study the observation of a possible differential response to GPi-DBS according to dystonia genotype in a larger patient sample; herein, we report on a group of 8 patients with DYT6 dystonia who underwent bilateral GPi-DBS for disabling dystonia refractory to medical treatment. In this retrospective study, we compared their postoperative outcomes with those of 9 patients with DYT1 dystonia and 38 patients with dystonia without TOR1A and THAP1 mutations.
METHODS
Recruitment.
Six German (Berlin, Hamburg, Kiel/Würzburg, Lübeck, Rostock, Tübingen) and one Canadian DBS center (Toronto) participated in this study. Patients with dystonia refractory to medical treatment who had undergone bilateral GPi-DBS were contacted by mail. They were informed about the ongoing project and were asked for their participation. If they agreed, either their general practitioner or an investigator of the referring DBS center took an EDTA blood sample and sent it to the Institute of Neurogenetics, University of Lübeck, for genetic analyses. Demographic and DBS-related data were provided by the participating centers.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participating subjects and the study was approved by the local ethics committees.
Genotyping.
DNA was extracted from all blood samples and screened for the known 3-nucleotide (GAG) deletion in the torsin family 1, member A (torsin A) (TOR1A) gene (DYT1). All 3 coding exons and exon-intron boundaries of the THAP1 gene (DYT6) were sequenced as previously described.17 In addition, blood samples were screened for mutations in the GNAL gene (DYT25).18–20 GNAL is the third confirmed gene causing isolated dystonia but all included subjects screened negative for pathogenic GNAL mutations. Patients with a heterozygous GAG deletion in the TOR1A gene and THAP1 mutation were considered to have DYT1 dystonia and DYT6 dystonia, respectively. Patients without mutations in these genes (TOR1A, THAP1, GNAL) were considered to have non-DYT dystonia.
Statistical analysis.
The primary endpoint of the study was the change in the motor score of the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) at early (1–16 months postoperatively) and late (22–92 months postoperatively) follow-up. Furthermore, the BFMDRS disability score was assessed preoperatively to estimate the impact of dystonia on daily activities before surgery. Chi-square tests were done for categorical and analyses of variance (ANOVAs) for continuous variables. No corrections for multiple comparisons were performed. Repeated-measures ANOVA and subsequent Sidak post hoc tests were used to compare pre- and postoperative BFMDRS motor scores. Responder analysis with ≥25% improvement of the BFMDRS motor score was done to assess the number of patients with a clinically relevant DBS effect.2 For the change of BFMDRS motor score at early and late follow-up and its potential predictors, 2 group-spanning, step-wise, multivariate linear regression models were generated with “age at onset,” “years until surgery,” and “preoperative BFMDRS motor score” as independent variables. Values are shown as mean ± SD. Statistics were performed using SPSS version 21.0 (IBM Corp., Armonk, NY); the level of significance was set at p < 0.05.
In this study, we aimed to assess the clinical efficacy of GPi-DBS for patients with DYT6 dystonia compared to patients with DYT1 dystonia and non-DYT dystonia. Because of the retrospective design, this clinical therapeutic intervention study will provide Class IV evidence.
RESULTS
Subjects.
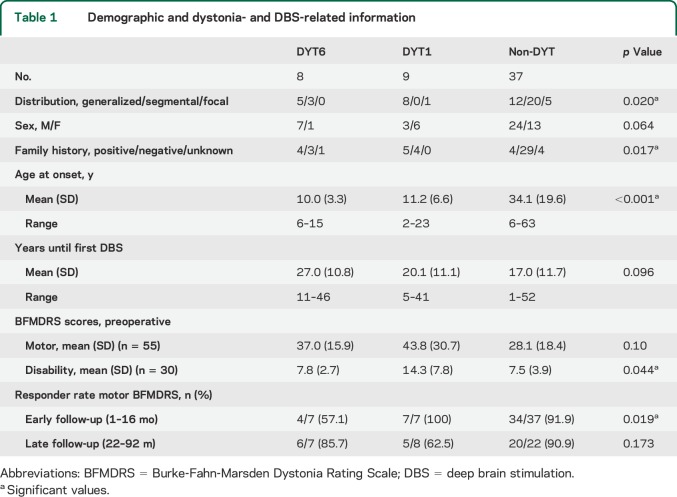
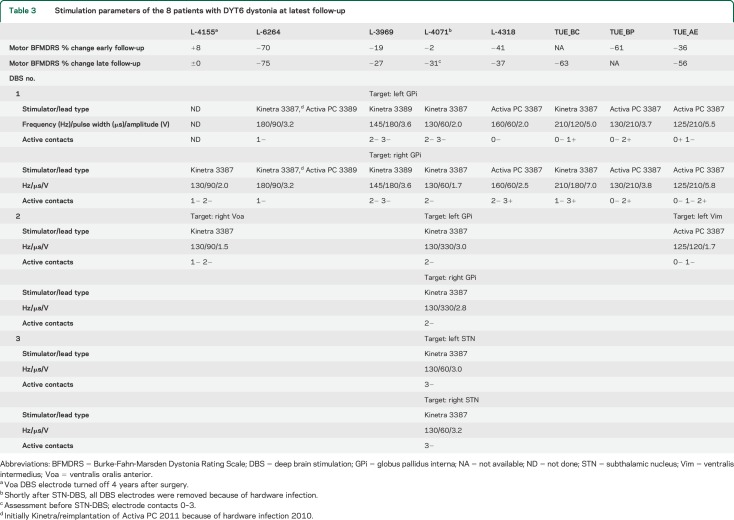
The 54 patients included had an average age at onset of 26.4 ± 19.7 years (range 2–63 years) (see table 1 for detailed demographic data). Patients with complex dystonias were not included. In the monogenic cases, dystonia manifested on average more than 15 years earlier than in patients with non-DYT (F = 11.4, p < 0.001), although there was considerable overlap. The family history was more likely to be positive in patients with DYT1 and DYT6 dystonia (each ≥50%) than in patients with non-DYT dystonia (χ2 = 12.1, p = 0.017). Sex distribution did not differ between groups (χ2 = 5.5, p = 0.064). The average duration until the first DBS surgery (19.2 ± 11.8 years) was not different between groups (F = 2.5, p = 0.096). Regarding the distribution of dystonic signs, all patients with DYT1 dystonia except one with focal leg dystonia presented with generalized dystonia, whereas patients with DYT6 dystonia (table 2) had either generalized or segmental dystonia (χ2 = 11.6, p = 0.020). Among the non-DYT dystonia cases, patients were stimulated because of generalized (n = 12), segmental (n = 20, including Meige syndrome (n = 3), or a focal form of treatment-refractory dystonia, i.e., cervical dystonia (2), truncal dystonia (2), and dystonic voice and head tremor (1). Stimulation settings for patients with DYT6 dystonia at the latest follow-up examination are given in table 3.
Table 1.
Demographic and dystonia- and DBS-related information
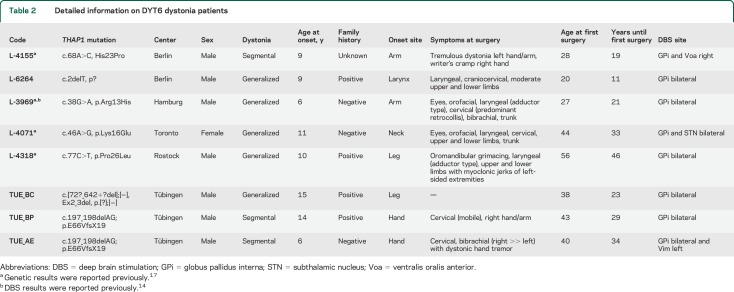
Table 2.
Detailed information on DYT6 dystonia patients
Table 3.
Stimulation parameters of the 8 patients with DYT6 dystonia at latest follow-up
Effect of GPi-DBS on dystonia.
Before surgery, patients with DYT1 dystonia had BFMDRS disability scores almost twice as high as any of the other 2 groups (F = 3.5, p = 0.044) (table 1). The BFMDRS motor score also tended to be higher in the DYT1 group (F = 2.4, p = 0.10).
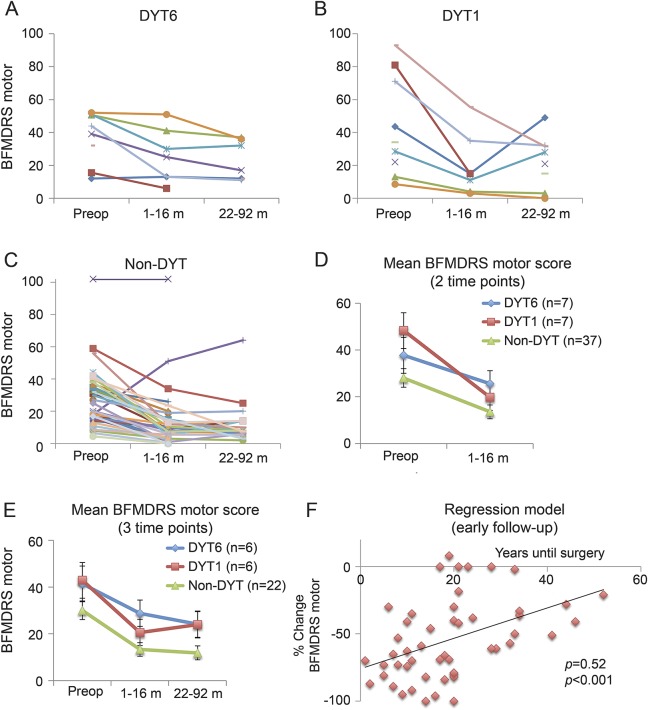
Repeated-measures ANOVA of the BFMDRS motor score for baseline and early follow-up (1–16 months) assessment was performed using datasets from 7 patients with DYT6, 7 with DYT1, and 37 with non-DYT dystonia. Apart from a main effect of time point (F = 48.9, p < 0.001), there was a trend toward an interaction of time point × group (F = 3.2, p = 0.051). At early follow-up, the mean reduction of dystonia severity was stronger in patients with DYT1 (−60%) and patients with non-DYT (−52%) dystonia than in patients with DYT6 dystonia (−32%) (figure, D). According to the results of the ANOVA, the rate of responders was considerably lower in the group of patients with DYT6 dystonia at early follow-up (χ2 = 7.9, p = 0.019; table 1). These data suggest lower efficacy of GPi-DBS in DYT6 at an early postoperative time point.
Figure. Effect of deep brain stimulation of the internal pallidum on dystonia severity in DYT6, DYT1, and non-DYT dystonia.
BFMDRS motor scores in single cases with DYT6 (A), DYT1 (B), and non-DYT (C) dystonia. (D, E) Mean scores for patients in which BFMDRS data were available for 2 and 3 time points, respectively. (F) The correlation of “years until surgery” and “% change of the BFMDRS motor score.” BFMDRS = Burke-Fahn-Marsden Dystonia Rating Scale; preop = preoperative.
For 6 patients with DYT6, 6 with DYT1, and 22 with non-DYT dystonia, BFMDRS motor scores were available for all 3 time points (baseline, early, and late follow-up). Here, repeated-measures ANOVA revealed a main effect of time point (F = 21.5, p < 0.001) whereas there was no interaction of time point × group (F = 0.4, p = 0.77) (figure, E). Likewise, the responder rate was comparable between groups (χ2 = 3.5, p = 0.173; table 1). Thus, GPi-DBS resulted in a sustained improvement in all 3 patient groups without significant differences on a group level (BFMDRS motor score at late follow-up: DYT6, −42%; DYT1, −44%; and non-DYT, −61%).
For multivariate regression analysis (n = 45), one significant outlier (L-4214, non-DYT group) was excluded (Grubbs test, p < 0.05).
“Years until surgery” were positively correlated with the change of the BFMDRS motor score both at early follow-up (n = 45, r = 0.52, p < 0.001) accounting for 27% of the difference in the variances (figure, F) and at late follow-up (n = 30, r = 0.37, p = 0.046) accounting for 14% of the difference in the variances. Thus, patients with early DBS surgery had a more beneficial outcome than patients who underwent DBS later in the disease course. “Age at onset” and “preoperative BFMDRS motor scores” were not associated with a change of the BFMDRS motor score.
DBS of multiple sites in THAP1 mutation carriers.
The primary DBS target in all patients was the GPi. Because of a suboptimal effect of GPi stimulation, 3 patients with DYT6 dystonia subsequently underwent another DBS surgery with a different brain target. No patients with DYT1 and non-DYT dystonia underwent a secondary stereotactic operation.
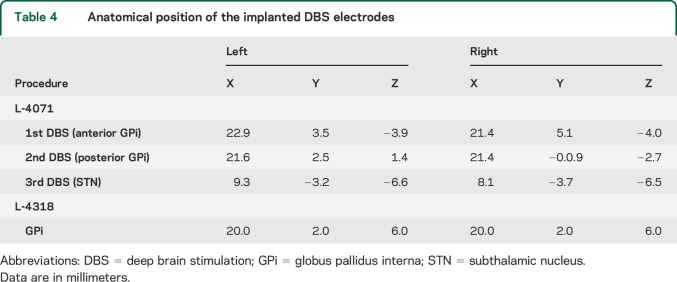
Patient L-4071 (Toronto, video 1 on the Neurology® Web site at Neurology.org) showed first signs of generalized dystonia at the age of 11 years. Her family history was negative. Genetic testing revealed a pathogenic heterozygous mutation in the THAP1 gene (c.46A>G, p.Lys16Glu). Data on functional analysis and probable pathogenicity of this mutation were reported previously.17 At the age of 44 years, she underwent bilateral GPi-DBS (Kinetra, DBS lead model 3387, Medtronic, Minneapolis, MN; for the anatomical position of the implanted leads, see table 4). Because there was no improvement in cervical and truncal dystonia, a second pair of DBS electrodes was implanted into the posterior portions of each GPi 1 year later, but again without benefit on the axial dystonia. Four years later, she additionally received bilateral subthalamic nucleus stimulation (Kinetra, DBS lead model 3387; Medtronic) because of a persistent DBS failure. Shortly thereafter, all GPi and subthalamic nucleus DBS hardware were removed because of infection.
Table 4.
Anatomical position of the implanted DBS electrodes
Patient L-4155 (Berlin, video 2) is a 38-year-old male heterozygous THAP1 mutation carrier (c.68A>C, p.His23Pro17) with tremulous dystonia of the left arm and dystonic ulnar deviation of the left hand since the age of 9 years. He received DBS electrodes for unilateral GPi stimulation at the age of 28 years (Kinetra, DBS lead model 3387; Medtronic). Eighteen months later, unilateral thalamic DBS contralateral to the affected body side was performed in the ventralis oralis anterior nucleus (lead model 3387) because of the persistent disabling arm tremor. High-frequency stimulation as well as low-frequency (60 Hz) stimulation did not improve dystonic symptoms, and the stimulator was turned off 4 years later.
TUE-AE (Tuebingen, video 3), a 44-year-old male carrier of a heterozygous THAP1 mutation (c.[72−?_642+?del];[=], Ex2_3del, p.[?];[=]), experienced first symptoms of a subsequently generalizing dystonia at the age of 6 years. Thirty-four years later, at the age of 40 years, he underwent bilateral GPi-DBS (Activa PC, DBS lead model 3387; Medtronic) with moderate improvement of dystonia. Aged 42 years, bilateral thalamic DBS (ventralis intermedius [Vim] nucleus) was performed to improve control of a progressive dystonic tremor of the head and the upper limbs, more pronounced on the right. In follow-up examinations, the tremor improved significantly but was still moderately disabling. The right Vim DBS electrode was not active.
DISCUSSION
The present report describes the short- and long-term follow-up outcome of bilateral pallidal neurostimulation in 54 patients with genetic and nondetermined forms of isolated treatment-refractory dystonia. At long-term follow-up, GPi-DBS in DYT6 dystonia was as efficient as in DYT1 and genetically nondetermined dystonia, although an early postoperative beneficial response to DBS was less likely in DYT6 dystonia. Additional DBS of another pallidal area (n = 1) or other brain regions (n = 3) was performed in 3 of 8 patients with DYT6 dystonia 1 to 2 years after the initial operation. This reflects the therapeutic challenge in individual patients with DYT6 dystonia who have difficult-to-control dystonia. Revision of the lead position (internal pallidum) was previously reported in another patient with DYT6 dystonia.16 It is remarkable that 2 of the 3 patients studied here were revised for intractable dystonic tremor. To date, no prospective treatment studies are available for tremor associated with dystonia, and there are no data available on the frequency of dystonic tremor in DYT6 dystonia. Several targets including the internal pallidum as well as subthalamic and thalamic areas were attempted with good response to DBS in most refractory cases with dystonic tremor (for review, see reference 21). One retrospective report on 10 patients suggested combined GPi- and Vim-DBS in patients with coexistent severe dystonia and dystonic tremor, whereas patients with only mild dystonia and predominant dystonic tremor may benefit more from Vim-DBS.22 Apart from another DBS surgery in nonresponsive cases, careful adjustments of stimulation parameters over several years may also result in better symptom control in selected cases, e.g., in L-4318 (Dr. Wittstock, personal communication, 2014; video of this patient is shown as video 4).
Even though DYT1 mutation carrier status was described to be predictive of a beneficial response to bilateral GPi-DBS up to 10 years postoperatively,3,23,24 our results highlight that dystonia may also worsen in several patients who were initially responsive to DBS (figure, B). In our study cohort, the mean improvement of the motor BFMDRS score was less striking in patients with DYT1 dystonia (early follow-up, −44%; late follow-up, −60%) than in most other studies. One important factor is the rather low number of patients with DYT1 dystonia included into this study, which may give rise to higher variability of data. In accordance with other reports, however, the current study confirms the predictive value of years until surgery for DBS outcome, i.e., patients with a shorter duration from onset of dystonia to surgery had a higher likelihood of having a better control of dystonia postoperatively.3 It can be assumed that an earlier time point of DBS may to some extent prevent secondary complications such as fixed musculoskeletal abnormalities (e.g., scoliosis) and cervical myelopathy, which cannot be sufficiently influenced by pallidal neurostimulation.
This study has several limitations. Although this is the largest sample of patients with DYT6 dystonia reported to date, the number of analyzed DBS cases is still relatively small. There is a high temporal variability in both the early and late follow-up time point because of the retrospective design and the involvement of multiple DBS centers using different postoperative protocols. Another weakness is the lack of data on quality of life. Improvement on a dystonia motor scale does not automatically imply an improvement of quality of life as was previously shown in stimulated patients with generalized dystonia.25 Thus, prospective multicenter studies including data on quality of life and nonmotor aspects of dystonia are warranted.
Long-term bilateral pallidal stimulation reduces dystonic signs in otherwise intractable DYT6, DYT1, and non-DYT dystonia. The beneficial effect of GPi-DBS appears to be less predictable in DYT6 dystonia than in other forms of isolated dystonia. Of note, patients with DYT6 dystonia are probably more likely to receive subsequent DBS at the same or another brain target because of an insufficient control of dystonia or dystonic tremor. The high likelihood of subsequent additional neurosurgical procedures in THAP1 mutation carriers suggests that eligible patients with severe dystonia should be screened for mutations in the THAP1 gene before GPi-DBS. Identified THAP1 mutation carriers who are referred for GPi-DBS should be informed about the risk of primary treatment failure and possible secondary interventions. Given the data of the present study, GPi-DBS should nevertheless not be withheld from patients with disabling DYT6 dystonia. Because GPi-DBS appears to be more beneficial in patients with a shorter duration from onset, this treatment option should probably be considered earlier in the disease course of patients with severe dystonia of any kind. Finally, prospective studies on DBS in patients with different clinical types of dystonia are warranted to systematically address not only the genotype but also the phenotype as a predictor for the postoperative outcome.
ACKNOWLEDGMENT
The authors gratefully thank all participants for their invaluable collaboration.
GLOSSARY
- ANOVA
analysis of variance
- BFMDRS
Burke-Fahn-Marsden Dystonia Rating Scale
- DBS
deep brain stimulation
- GPi
globus pallidus interna
- THAP1
THAP domain containing, apoptosis associated protein 1
- TOR1A
torsin family 1, member A (torsin A)
- Vim
ventralis intermedius
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Brüggemann: study concept and design, acquisition of data, analysis and interpretation, wrote the draft of the paper, study supervision. Dr. Kühn: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Dr. Schneider: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Kamm: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Wolters: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Krause: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Moro: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Steigerwald: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Wittstock: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Tronnier: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Lozano: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Hamani: acquisition of data, critical revision of the manuscript for important intellectual content. Ms. Poon: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Zittel: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Wächter: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Deuschl: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Krüger: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Kupsch: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Münchau: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Lohmann: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Volkmann: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Klein: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This study was supported by funding from NS065701 of the ORDR in NCATS and National Institute of Neurological Disorders and Stroke as part of the NIH RDCRN and the German Research Foundation (LO 1555/3-2). C. Klein is the recipient of a career development award from the Hermann and Lilly Schilling Foundation.
DISCLOSURE
N. Brüggemann has served as faculty at the annual meeting of the American Academy of Neurology 2013. He was funded by the German Research Foundation and received an honorarium from Springer and support for travel to a conference by St. Jude Medical. A. Kühn received honoraries from Medtronic, St. Jude Medical, and Boston Scientific, and support for travel to conferences by Ipsen Pharma. S. Schneider was supported by the Else Kröner-Fresenius-Stiftung, the Bosch Foundation, and the Eva Luise und Horst Köhler Stiftung, and receives intramural funding from the university. C. Kamm received speakers honoraria from Ipsen Pharmaceuticals and GlaxoSmithKline and unrestricted travel grants from Merz Pharmaceuticals and Ipsen Pharmaceuticals. A. Wolters reports no disclosures relevant to the manuscript. P. Krause received funding for a trip to MDS 2012 and DPG 2013 from Allergan and honoraria for a talk in 2013 from GlaxoSmithKline. E. Moro has received honoraria for lecturing from Medtronic, St. Jude Medical, and Merz. F. Steigerwald received project funding from UCB, Medtronic, Boston Scientific, and SIMI-Motion. He served on the advisory board and as consultant for Archimedes Pharma, Boston Scientific, and St. Jude Medical. He received travel grants from Cephalon, Orion, and Medtronic. He served as speaker for Medtronic ECMT, Medtronic, Licher, Abbott, UCB, and Orion. M. Wittstock received speakers honoraria from Allergan, BMS, Ipsen, Lundbeck, Medtronic, Merz, and UCB, as well as travel grants from Ipsen and Merz. V. Tronnier received honoraria for serving on the scientific advisory board from EISAI and honoraria for lectures from EISAI, St. Jude, and Medtronic. A. Lozano reports no disclosures relevant to the manuscript. C. Hamani served as a consultant for St. Jude Medical. Y. Poon reports no disclosures relevant to the manuscript. S. Zittel was supported by the Wegener Stiftung and by an intramural grant of the University of Lübeck (E36-2014). She received research support from St. Jude Medical and Merz Pharmaceuticals. T. Wächter serves as a consultant for Medtronic. He also received speakers honoraria and travel reimbursement for scientific meetings from Medtronic, Solvay, Abbott Pharma, Cephalon, Merz Pharmaceuticals, Ipsen Pharma, and Schwarz Pharma. He has also received financial support for research from and conducted commissioned research for Medtronic, Abbott Pharma, Merz Pharmaceuticals, Ipsen Pharma, and Pharm-Allergan, and worked on advisory boards for Ipsen Pharma, Merz Pharmaceuticals, and Medtronic. G. Deuschl has received lecture fees from UCB, Medtronic, and Desitin and has been serving as a consultant for Medtronic, Sapiens, Boston Scientific, and Britannica. He received royalties from Thieme publishers. He is a government employee and he receives through his institution funding for his research from the German Research Council, the German Ministry of Education and Health, and Medtronic. R. Krüger has received research grants from the German Research Council (DFG; KR2119/3-2 and KR2119/8-1), The Michael J. Fox Foundation, the Fritz Thyssen Foundation (10.11.2.153), the Federal Ministry for Education and Research (BMBF; MitoPD and COURAGE-PD), and the Fonds National de Recherche (FNR; PEARL), as well as speakers honoraria from Medtronic GmbH, St. Jude Medical, and AbbVie. A. Kupsch is member of the advisory board of Medtronic USA. He received honoraria for speaking from Allergan, Boehringer Ingelheim, Ipsen Pharma, Lundbeck, Medtronic, Merck, Merz Pharmaceuticals, Orion, and St. Jude UCB, and research grants from the German Research Council and the German Ministry of Education and Research. A. Münchau received grants from Pharm Allergan, Ipsen, Merz Pharmaceuticals, and Actelion. He received honoraria for lectures from Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion, GlaxoSmithKline, and Desitin. He was supported by nonprofit foundations or societies (Possehl-Stiftung, Lübeck, Dystonia Coalition [USA], Tourette Syndrome Association [Germany], European Huntington Disease Network, and N.E.MO. Charity supporting the research of pediatric movement disorders). He received academic research support from Deutsche Forschungsgemeinschaft (MU 1692/3-1; SFB 936) and the University of Lübeck. K. Lohmann received funding from the German Research Foundation and the Dystonia Coalition. J. Volkmann has received consulting fees from Boston Scientific and Medtronic, honoraria for speaking from Boston Scientific, St. Jude, Medtronic, Novartis, UCB, and TEVA, and grant support from Boston Scientific and Medtronic. C. Klein is a member of the editorial board of Neurology® and has served as editor of the “Continuum Issue Neurogenetics 2008” and as faculty at the annual meetings of the American Academy of Neurology since 2004. She serves as a medical advisor to Centogene. She is the recipient of a career development award from the Hermann and Lilly Schilling Foundation. She is funded by the Deutsche Forschungsgemeinschaft and the Possehl Foundation and received institutional support from the University of Lübeck for genetics research. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006;355:1978–1990. [DOI] [PubMed] [Google Scholar]
- 2.Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol 2012;11:1029–1038. [DOI] [PubMed] [Google Scholar]
- 3.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry 2010;81:1383–1389. [DOI] [PubMed] [Google Scholar]
- 4.Isaias IU, Volkmann J, Kupsch A, et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol 2011;258:1469–1476. [DOI] [PubMed] [Google Scholar]
- 5.Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain 2008;131:1895–1902. [DOI] [PubMed] [Google Scholar]
- 6.Witt JL, Moro E, Ash RS, et al. Predictive factors of outcome in primary cervical dystonia following pallidal deep brain stimulation. Mov Disord 2013;28:1451–1455. [DOI] [PubMed] [Google Scholar]
- 7.Djarmati A, Schneider SA, Lohmann K, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol 2009;8:447–452. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs T, Gavarini S, Saunders-Pullman R, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet 2009;41:286–288. [DOI] [PubMed] [Google Scholar]
- 9.Klein C. Genetics in dystonia. Parkinsonism Relat Disord 2014;20(suppl 1):S137–S142. [DOI] [PubMed] [Google Scholar]
- 10.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet 1997;17:40–48. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard A, Ea V, Roubertie A, et al. DYT6 dystonia: review of the literature and creation of the UMD Locus Specific DataBase (LSDB) for mutations in the THAP1 gene. Hum Mutat 2011;32:1213–1224. [DOI] [PubMed] [Google Scholar]
- 13.Bressman SB, Sabatti C, Raymond D, et al. The DYT1 phenotype and guidelines for diagnostic testing. Neurology 2000;54:1746–1752. [DOI] [PubMed] [Google Scholar]
- 14.Zittel S, Moll CK, Bruggemann N, et al. Clinical neuroimaging and electrophysiological assessment of three DYT6 dystonia families. Mov Disord 2010;25:2405–2412. [DOI] [PubMed] [Google Scholar]
- 15.Groen JL, Ritz K, Contarino MF, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord 2010;25:2420–2427. [DOI] [PubMed] [Google Scholar]
- 16.Panov F, Tagliati M, Ozelius LJ, et al. Pallidal deep brain stimulation for DYT6 dystonia. J Neurol Neurosurg Psychiatry 2011;83:182–187. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann K, Uflacker N, Erogullari A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet 2012;20:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet 2013;45:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vemula SR, Puschmann A, Xiao J, et al. Role of Gα(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet 2013;22:2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar KR, Lohmann K, Masuho I, et al. Mutations in GNAL: a novel cause of craniocervical dystonia. JAMA Neurol 2014;71:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasano A, Bove F, Lang AE. The treatment of dystonic tremor: a systematic review. J Neurol Neurosurg Psychiatry 2014;85:759–769. [DOI] [PubMed] [Google Scholar]
- 22.Hedera P, Phibbs FT, Dolhun R, et al. Surgical targets for dystonic tremor: considerations between the globus pallidus and ventral intermediate thalamic nucleus. Parkinsonism Relat Disord 2013;19:684–686. [DOI] [PubMed] [Google Scholar]
- 23.Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery 2013;73:86–93. [DOI] [PubMed] [Google Scholar]
- 24.Cif L, Vasques X, Gonzalez V, et al. Long-term follow-up of DYT1 dystonia patients treated by deep brain stimulation: an open-label study. Mov Disord 2010;25:289–299. [DOI] [PubMed] [Google Scholar]
- 25.Jahanshahi M, Torkamani M, Beigi M, et al. Pallidal stimulation for primary generalised dystonia: effect on cognition, mood and quality of life. J Neurol 2014;261:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]