Abstract
Nutrient interactions with prescription drugs is a topic of ongoing basic and clinical research. Pomegranate (POM) juice, and a 1-gram capsule containing POM extract, were evaluated in vitro and in vivo as inhibitors of CYP2C9, with flurbiprofen serving as the index substrate. Fluconazole was the positive control inhibitor. The in vitro 50% inhibitory concentrations (IC50) for POM juice and extract were below 1% (v/v), with no evidence of mechanism-based (irreversible) inhibition. In clinical studies, flurbiprofen pharmacokinetics were unchanged by POM juice or extract compared to a low-polyphenol placebo control beverage. However, fluconazole significantly reduced the oral clearance of flurbiprofen. Despite inhibition of CYP2C9 in vitro, POM juice and extract had no effect on CYP2C9 activity in human subjects, and can be consumed by patients taking CYP2C9 substrate drugs with negligible risk of a pharmacokinetic interaction.
Keywords: Pomegranate, CYP2C9, Metabolic inhibition, Drug-nutrient interactions
In the area of prescription drug interactions with nutrients and natural products, much of the attention has focused on citrus and other fruit beverages, in particular grapefruit juice (GFJ).1–6 Natural substances in GFJ, classified as furanocoumarins, have the property of producing irreversible inhibition (also termed “mechanism-based inhibition”) of Cytochrome P450 3A (CYP3A) enzymes in the mucosal cells of the gastrointestinal tract lining. Ingestion of GFJ can cause a decrease in presystemic extraction of drugs ordinarily metabolized by enteric CYP3A. A possible result is an increased extent of absorption and higher systemic plasma levels. Relatively few drugs are significantly affected by this interaction, but in some cases the GFJ interaction effect can be of clinical importance.1–6
Clinical drug-drug interaction (DDI) studies, though costly and time-consuming, are needed to confirm or rule out drug interactions between any given pair of concurrently-administered medications.7–15 Over the last two decades, in vitro models have become widely applied to identify or exclude potential clinical DDIs. While the in vitro paradigms cannot precisely predict the magnitude of clinical DDIs, the in vitro data have in many cases proved to be useful in identifying high-probability DDIs, thereby allowing resources available for clinical studies to be targeted in a more informed way.
However, in vitro models are of limited value in identifying clinical DDIs involving fruit and citrus products. Inhibition of CYP3A by GFJ is predicted by such models,6 but other fruit beverages, including pomegranate juice, are in vitro inhibitors of CYP3A (and other CYPs), though not producing clinically important DDIs in humans.5, 6, 15 A possible explanation is that GFJ and some related fruit juices (e.g., Seville orange juice and pomelo juice) contain furanocoumarins, which are unique among natural substances in producing irreversible inhibition of CYP3A.4,6 We have proposed that a clinical inhibitory interaction caused by a liquid may require an irreversible effect on the enzyme.4–6, 15
Pomegranate-derived products contain antioxidants reported to have health benefits, described in clinical studies.16–22 In vitro data indicate that pomegranate juice inhibits the activity of human cytochrome P450 2C9 (CYP2C9).23 This isoform mediates the clearance of many drugs, including some with potential toxicity (phenytoin, warfarin, oral antidiabetics).24, 25 Anecdotal reports suggest potentiation of the anticoagulant effect of warfarin due to ingestion of pomegranate juice.26, 27 While single case reports do not prove a cause-and-effect relationship, the reports nonetheless raise questions as to whether pomegranate products will interact with drugs metabolized by CYP2C9 in humans.
The present study evaluated the effect of pomegranate coadministration, in the form of juice or extract, on the metabolic activity of CYP2C9 in human volunteers. Flurbiprofen was used as the index (or probe) substrate. We have previously used flurbiprofen as the index substrate for clinical studies of interactions involving cranberry juice28 and Ginkgo biloba.29
RESULTS
Polyphenol content
Two lots of pomegranate juice and the control beverage were utilized in the clinical study. Only one lot of pomegranate extract capsules was used because their expiration date exceeded the study’s duration. The total polyphenol content (as gallic acid equivalents) of the pomegranate juices were 3.2 mg/mL and 3.3 mg/mL. In contrast, the polyphenol content of the control beverage lots were 0.11 and 0.12 mg/mL. The pomegranate extract contained 689 mg per capsule. These values are consistent with what has been reported in the literature.30,31
In vitro inhibition of CYP2C9 by pomegranate juice and pomegranate extract
The placebo control beverage produced no detectable inhibition of CYP2C9 activity in vitro, either without or with preincubation of liver microsomes with candidate inhibitors prior to addition of substrate (Figure 1).
Figure 1.
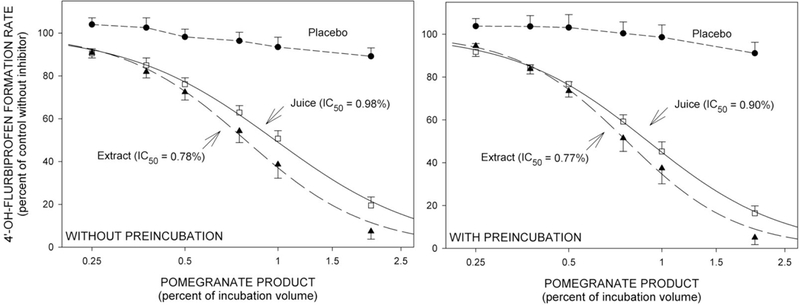
Mean (±SE, n=4) rates of formation of 4’-OH-flurbiprofen from flurbiprofen by human liver microsomes in vitro. Reaction velocities with co-addition of inhibitor are expressed as a percentage ratio versus the control velocity with no inhibitor present. IC50 values were determined by nonlinear regression based on the aggregate data points. Left: Without preincubation -- substrate and inhibitor simultaneously mixed with metabolic enzyme. Right: With preincubation -- inhibitor mixed with metabolic enzyme 20 minutes prior to addition of substrate.
Both pomegranate juice and pomegranate extract inhibited flurbiprofen hydroxylation in vitro. IC50 values were less than 1% (v/v) (Figure 1). Inhibitory potency was not enhanced by preincubation.
Fluconazole, the positive control, inhibited 4’-OH-flurbiprofen formation with an IC50 value of 20.3 micromolar (6.2 μg/mL). This was reduced to 14.3 micromolar (4.4 μg/mL) by preincubation (Figure 2).
Figure 2.
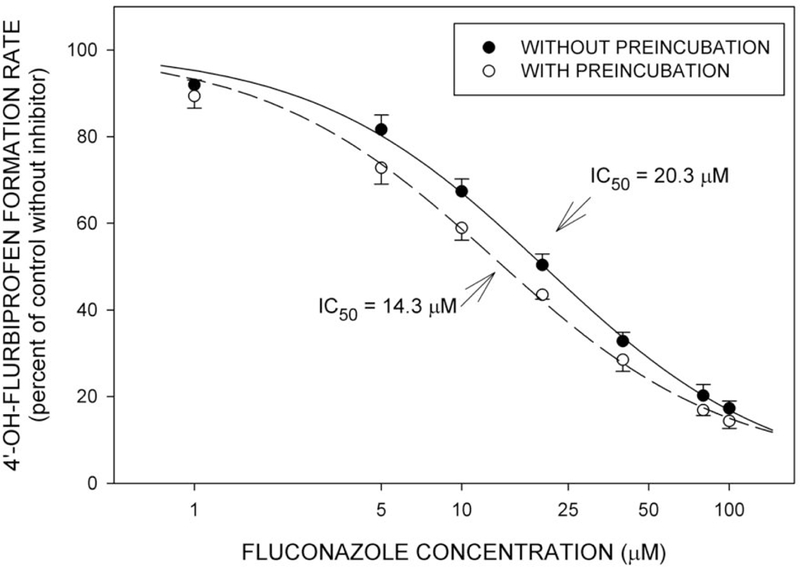
Mean (±SE, n=4) rates of formation of 4’-OH-flurbiprofen from flurbiprofen in relation to concentration of fluconazole. Studies were performed as in Figure 1. IC50 values are based on the aggregate data points.
Clinical study
Subjects
Twelve subjects completed the four trials of the study. There were no adverse reactions.
Effect of pomegranate juice, pomegranate extract, and fluconazole on flurbiprofen pharmacokinetics.
Figure 3 shows mean plasma flurbiprofen concentrations in each of the four treatment conditions. Pomegranate juice and pomegranate extract conditions were very similar to the placebo control. However, flurbiprofen concentrations were elevated by coadministration of fluconazole. Likewise plasma concentrations of 4’-OH-flurbiprofen were similar among placebo, pomegranate juice, and pomegranate extract conditions, but were reduced by coadministration of fluconazole (Figure 4).
Figure 3.
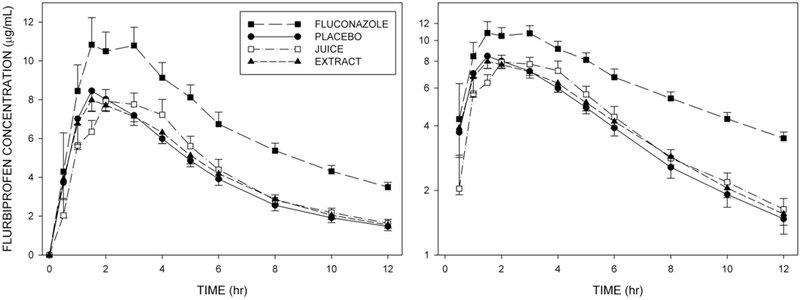
Mean (±SE, n=12) plasma flurbiprofen concentrations at corresponding times in each of the four treatment conditions. Left panel: Linear concentration axis. Right panel: Logarithmic concentration axis.
Figure 4.
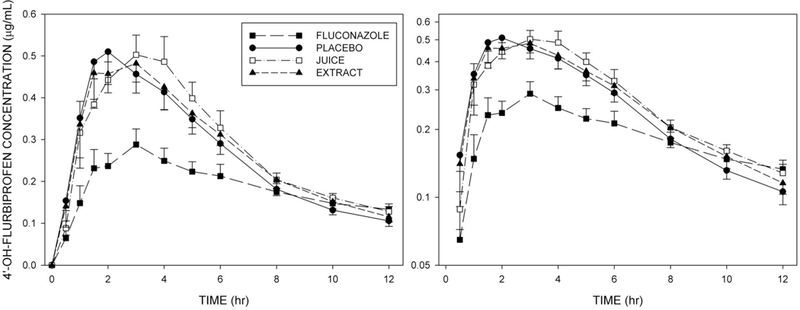
Mean (±SE, n=12) plasma concentrations of 4’-OH-flurbiprofen at corresponding times in each of the four treatment conditions. Left panel: Linear concentration axis. Right panel: Logarithmic concentration axis.
Figure 5 shows plasma concentrations of fluconazole during Trial 2.
Figure 5.
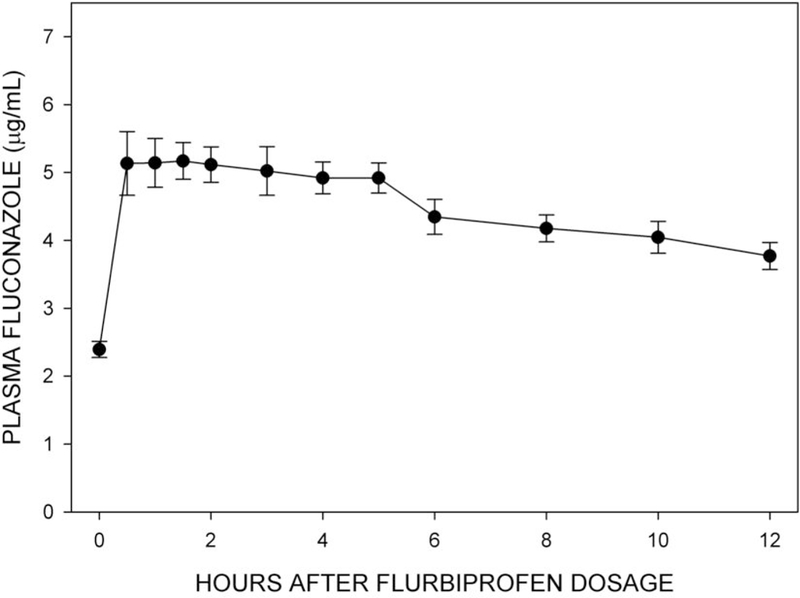
Mean (±SE, n=12) plasma fluconazole concentrations at corresponding times during Trial 2. Note: 1 μg/mL is equal to 3.26 μmol/L.
Analysis of variance for repeated measures showed significant differences among the four treatment conditions in flurbiprofen total AUC, CL/F, Cmax, and T1/2 (Table 1). Evaluation of individual treatment values relative to placebo control indicated that fluconazole differed significantly from control, whereas pomegranate juice and extract did not differ from control. The findings were similar for 4’-OH-flurbiprofen.
Table 1. SUMMARY OF PHARMACOKINETICS FOR FLURBIPROFEN AND 4’-OH-FLURBIPROFEN.
| Arithmetic Mean ± SD (n=12) | |||||
|---|---|---|---|---|---|
| FLURBIPROFEN | Control | Fluconazole | Pomegranate Juice | Pomegranate Extract | F value (ANOVA) |
| AUC0−∞ (μg/mL x hr) | 59.3 ± 18.7 | 113.8 ± 22.9* | 61.6 ± 16.6 | 61.5 ± 13.9 | 72.4 (P < 0.001) |
| CL/F (mL/min) | 30.7 ± 9.7 | 15.2 ± 2.8* | 28.7 ± 6.8 | 28.5 ± 7.1 | 23.3 (P < 0.001) |
| Cmax (μg/mL) | 9.8 ± 4.3 | 13.9 ± 4.0* | 9.7 ± 2.6 | 9.6 ± 1.8 | 6.64 (P = 0.001) |
| Tmax(hr)‡ | 1.75 (1–3) | 1.5 (0.5–4) | 2 (1–4) | 1.75 (0.5–4) | 0.44 (P = N.S.) |
| T1/2 (hr) | 3.9 ± 1.1 | 6.5 ± 1.7* | 4.0 ± 0.7 | 4.2 ± 1.0 | 24.7 (P < 0.001) |
| 4’-OH-FLURBIPROFEN | Control | Fluconazole | Pomegranate Juice | Pomegranate Extract | F value (ANOVA) |
| AUC0–12 (μg/mL * hr) | 3.3 ± 1.3 | 2.3 ± 0.9* | 3.5 ± 1.0 | 3.4 ± 1.1 | 13.3 (P < 0.001) |
| Cmax (μg/mL) | 0.6 ± 0.4 | 0.3 ± 0.1* | 0.6 ± 0.2 | 0.6 ± 0.2 | 8.01 (P < 0.001) |
| Tmax (hr)‡ | 2.5 (1–6) | 2 (1.5–4) | 3 (1–4) | 3 (1–5) | 0.27 (P = N.S.) |
| FLURBIPROFEN | Geometric mean (90% confidence interval) | ||||
| AUC (μg/mL x hr) | 56.8 (48.4–66.6) | 111.8 (101.0–123.7) | 59.7 (52.3–68.2) | 60.0 (53.1–67.9) | |
| Cmax (μg/mL) | 9.1 (7.5–11.0) | 13.4 (11.5–15.5) | 9.4 (8.1–10.8) | 9.4 (8.6–10.4) | |
Median and range
denotes significant difference (P < 0.05) versus control based on Dunnett’s test
Analysis of AUC ratios indicated that the arithmetic mean ratios (vs. placebo control) for flurbiprofen Cmax and total AUC were significantly higher than placebo control for the fluconazole condition (Table 2). Likewise the upper boundary of the geometric mean ratio for Cmax (value with fluconazole divided by value with placebo control) exceeded the 1.25 boundary, and the entire 90% confidence exceeded 1.25 for the geometric mean AUC ratio.
Table 2. ANALYSIS OF RATIOS FOR FLURBIPROFEN.
| Cmax ratio | Total AUC ratio | |||||
|---|---|---|---|---|---|---|
| Fluconazole/ placebo |
Pom juice/ placebo | Pom extract/ placebo | Fluconazole/ placebo |
Pom juice/ placebo | Pom extract/ placebo | |
| Arithmetic | ||||||
| Mean | 1.57* | 1.09 | 1.08 | 2.04* | 1.08 | 1.09 |
| SD | ±0.55 | ±0.38 | ±0.29 | ±0.62 | ±0.30 | ±0.29 |
| Geometric | ||||||
| Mean | 1.47 | 1.03 | 1.03 | 1.97 | 1.05 | 1.06 |
| 90% CI | 1.19–1.80 | 0.85–1.25 | 0.88–1.21 | 1.71–2.27 | 0.93–1.19 | 0.93–1.21 |
Asterisk () indicates mean value significantly larger than 1.0.
Bold type indicates 90% confidence interval boundary exceeding 1.25.
In contrast, arithmetic mean ratios for flurbiprofen Cmax and AUC in the pomegranate juice and pomegranate extract conditions did not differ from 1.0, and the entire 90% confidence interval for the geometric mean ratio of Cmax and AUC fell within the 0.80–1.25 boundaries (Table 2).
DISCUSSION
CYP2C9 is the principal enzyme responsible for clearance of S-warfarin, the active enantiomer of racemic warfarin.24,25,32 The possibility of CYP2C9 inhibition by herbal products, nutrients, or other dietary components would raise concerns that warfarin-treated patients who ingest such inhibitors would be at risk for excessive anticoagulation and bleeding due to elevated plasma levels of S-warfarin. However, the validity of risk attribution depends on the sources of biomedical data used to develop risk estimates. Limitations of anecdotal case reports for establishing cause-and-effect are well recognized, particularly when the “effect” event is relatively common (such as excessive anticoagulation with warfarin). Studies in experimental animals are of limited value for predicting human drug-nutrient interactions due to species differences in enteric and hepatic physiology, in the substrate/inhibitor profiles of the CYP enzymes, and in the levels of exposure to the substrate and inhibitors under study. In vitro metabolic models, though useful in screening for drug interactions involving small molecules, are of limited value in predicting in vivo drug interactions involving nutrients and natural products, particularly interactions with fruit and citrus beverages.4, 6,9,15
The dilemma is illustrated by what had been inferred to be potentiation of warfarin anticoagulation by cranberry juice. This interaction was suggested by anecdotal clinical case reports, and by in vitro studies showing impairment of CYP2C9 activity by cranberry juice.5, 33–35 However, a number of controlled studies in humans -- involving both human volunteers and patients receiving warfarin anticoagulation -- showed that customary doses of cranberry juice have minimal or no effect on the pharmacokinetics of S-warfarin and other CYP2C9 substrates, as well as minimal or no effect on warfarin anticoagulation. 28,33, 34, 36, 37 A realistic risk assessment, based on all available data, is that cranberry juice can be ingested by warfarin- treated patients with negligible hazard of excessive anticoagulation or bleeding due to a pharmacokinetic drug interaction.35, 38
The present study evaluated the effect of pomegranate juice and pomegranate extract on the activity of human CYP2C9, both in vitro and in a clinical pharmacokinetic study. The index substrate was flurbiprofen, which is used as a probe compound to profile CYP2C9 activity in vitro and in vivo. 28,29,39–41 Flurbiprofen and S-warfarin share pharmacokinetic similarities, in that both are metabolized by CYP2C9, have low hepatic clearance and minimal presystemic extraction, have small apparent volumes of distribution, and are extensively protein-bound. Pomegranate juice and a suspension made from the encapsulated extract both were in vitro inhibitors of CYP2C9, with IC50 values of less than 1%. The inhibition curve versus 4’-OH- flurbiprofen formation did not shift leftward (increased inhibitory potency) due to preincubation of the pomegranate inhibitor with microsomal enzyme prior to addition of substrate, thereby providing no evidence of mechanism-based (irreversible) inhibition.6, 8 The placebo beverage, containing no pomegranate components and a low polyphenol content, produced no inhibition of CYP2C9 in vitro, either without or with preincubation. The positive control inhibitor, fluconazole, inhibited 4’-OH-flurbiprofen formation with an IC50 of 20.3 micromolar; this was reduced to 14.3 micromolar by preincubation.
The inhibitory effect of pomegranate in vitro did not extend to the clinical study, whereas inhibition by fluconazole did extend to the human study. Compared to the control beverage, fluconazole increased flurbiprofen AUC by a factor of about 2, increased Cmax, prolonged T1/2, and correspondingly reduced plasma levels of 4’-OH-flurbiprofen. In contrast, neither pomegranate juice nor pomegranate extract had a significant effect on any of the pharmacokinetic parameters for flurbiprofen when compared to the low-polyphenol placebo control beverage. The findings indicate that patients taking warfarin, or other CYP2C9 substrate drugs, can consume pomegranate juice or extract, in usual dietary amounts, with negligible risk of a pharmacokinetic interaction. These results parallel previous studies of pomegranate as a potential inhibitor of CYP3A. Although pomegranate juice inhibited CYP3A in vitro,15 clinical trials showed that pomegranate had no effect on the pharmacokinetics of midazolam,15, 42 an index substrate used to profile CYP3A activity. Some limitations of the present study should be noted. Although unlikely, the possibility of a pharmacodynamic interaction between a pomegranate constituent and the anticoagulant effects of warfarin cannot be ruled out. In addition, the present study only addressed the effects of acute consumption of typical amounts of pomegranate juice and extract on CYP2C9. Finally, as natural products are known to vary in their composition, the present results may not extend to all pomegranate products on the market.
As in the case of cranberry juice, the pomegranate-CYP2C9 studies further illustrate the limitations of in vitro models in predicting clinical drug interactions involving fruit beverages.4–6, 9, 15 It is possible that the concentrations of potentially inhibitory substances is not high enough to produce significant inhibition of hepatic CYP2C9. It is also possible that the naturally- occurring inhibitory compounds themselves are degraded or inactivated prior to reaching the liver.43–45
METHODS
Phenolic content of pomegranate products.
The total phenolic content of pomegranate juice, pomegranate extract, and the placebo beverage, was determined using the method of Singleton et al.46 For the pomegranate extract assay, the contents of one capsule (1 gram) were emptied into 250 mL of distilled water to mimic the dosing regimen of the clinical study. After vigorous mixing, an aliquot of the mixture was removed and analyzed. Pomegranate juice and extract samples were diluted 1:10 with water prior to analysis. The placebo beverage was assayed at full strength. All samples were analyzed in duplicate.
A calibration curve was generated using gallic acid (Sigma-Aldrich, St. Louis, MO, USA) as the reference compound. Gallic acid was dissolved in water, at concentrations ranging from 62.5 to 1000 μg/mL. A 50 μL aliquot of each sample -- calibration standard, pomegranate juice, placebo beverage, or extract mixture -- was added to a 2-mL microcentrifuge tube. Water (865 μl) and 75 μl of the Folin-Ciocalteu phenol reagent (Sigma-Aldrich, St. Louis, MO, USA) were added and each tube was vortexed. After 5 minutes, 225 μL of 20% sodium carbonate in water was added to each tube. After mixing, the volume was brought to 1.5 mL by the addition of 285 μl of water. Tubes were vortexed and allowed to stand at room temperature, in the dark, for 2 hours. A 1 mL aliquot of each sample was transferred to a cuvette and the absorbance measured at 765 nm using a UVIKON 860 spectrophotometer.
In vitro inhibition of CYP2C9.
Flurbiprofen and fluconazole were obtained from Sigma- Aldrich (St. Louis, MO, USA). 4’-OH-flurbiprofen was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). Liver samples from individual human donors with no known liver disease were provided by the National Disease Research Interchange (Philadelphia, PA, USA); the International Institute for the Advancement of Medicine (Exton, PA, USA); or, the Liver Tissue Procurement and Distribution System, University of Minnesota (Minneapolis, MN, USA).
The in vitro metabolic model using human liver microsomal preparation has been described previously.5– 9, 41 Fixed concentrations (5 micromolar) of the index substrate (flurbiprofen) were used for inhibition studies. The rates of transformation of flurbiprofen to 4’-OH-flurbiprofen were determined in the control condition (without inhibitor), and with varying concentrations of pomegranate juice, or a suspension prepared by mixing the contents of a 1 gram pomegranate extract capsule in 250 mL distilled water. Also evaluated was the positive control index inhibitor fluconazole.28, 47 Studies were done both without and with preincubation of inhibitors with microsomal protein prior to addition of index substrate. Increased inhibitory potency attributable to preincubation is consistent with mechanism-based (irreversible) inhibition.8, 48
Reaction velocities with coaddition of inhibitor were expressed as a percentage ratio versus the corresponding velocity with no inhibitor present. The relation of reaction velocity to inhibitor concentration was analyzed by nonlinear regression (SAS version 9.2, Cary, NC, USA) to determine the inhibitor concentration reducing the velocity to 50% of inhibitor-free control (IC50).8, 49
Clinical study.
The study protocol and consent form were reviewed and approved by the Institutional Review Board serving Tufts University School of Medicine and Tufts Medical Center. The study was conducted in accordance with the Helsinki Declaration of 1975.
Flurbiprofen tablets were manufactured by Teva Pharmaceuticals. Fluconazole tablets (Diflucan®) were manufactured by Pfizer. The pomegranate placebo beverage, juice, and extract were supplied by POM Wonderful, LLC, Los Angeles CA.
The index substrate in all trials was 100 mg flurbiprofen. The co-treatments for the four trials (in random sequence, as determined by a computer-generated randomization scheme) were: Trial 1, low-polyphenol control beverage (250 mL); Trial 2, fluconazole (200 mg); Trial 3, pomegranate juice (250 mL); and, Trial 4, pomegranate extract (1 gram capsule).
The trials were separated by at least one week. Within each trial, the co-treatments were administered twice: once on the afternoon (between 4 and 6 PM) prior to flurbiprofen dosage, and again 30 minutes prior to flurbiprofen. Fluconazole and the pomegranate extract capsule were given with 250 mL of water. Flurbiprofen itself was given with 8 oz water at 8 AM. Eleven venous blood samples were drawn during 12 hours after flurbiprofen administration.
The principal outcome measure was total AUC for the index substrate. A change in AUC of 35% (Trials 3 or 4 vs. Trial 1) was considered to be clinically important. The standard deviation of the mean difference in AUC was assumed to be less than 40%. A sample size of 12 allowed power of 0.80 with alpha=0.05.
Study participants were 12 healthy volunteers (10 male, 2 female) aged 24 to 55 years. They were active ambulatory adults with no history of significant medical disease and taking no prescription medications. After signing informed consent, subjects underwent medical history screening, physical examination, blood hematology and chemistry testing, and screening for hepatitis and HIV. Potentially childbearing female subjects underwent a pregnancy test. Candidates were accepted as study participants if the screening procedure indicated that they were healthy active adults without significant medical disease or any condition that would make participation unsafe or not appropriate.
Oral clearance of flurbiprofen was used to profile CYP2C9 activity.28,29,39,40 Fluconazole, the positive control, is an azole antifungal agent known to be an inhibitor of CYP2C9.47 Pomegranate juice and pomegranate extract were tested as candidate inhibitors. The extract product was POMx, a capsule containing 1 gram of natural pomegranate polyphenols equivalent to 8 oz of pomegranate juice. The placebo control beverage was a low polyphenol-containing, Gatorade-based product containing high-fructose corn syrup, organic acids, and colorings.
Subjects avoided alcohol, caffeine-containing beverages, and grapefruit juice for 24 hours prior to each trial and continuing until the end of the second day of that trial. On the day prior to flurbiprofen administration, subjects received the appropriate co-treatment (control beverage, fluconazole, pomegranate juice, or extract) between 4 and 6 PM. Subjects reported to the study unit at 7 AM the next day and were given a light breakfast. An intravenous catheter was placed and a pre-dose blood sample taken. At 7:30 AM, subjects received another dose of the appropriate co-treatment (same as the one given the previous afternoon). At 8 AM, 100 mg flurbiprofen was administrated with 8 ounces of water. Venous blood samples (7 mL each) were then collected at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 hours after flurbiprofen administration. Subjects were discharged from the study unit after the last blood sample was taken.
Venous blood samples were centrifuged, and the plasma separated and stored at −18°C until the time of assay. All samples from a given subject’s set of four trials were extracted and analyzed on the same day using one set of calibration standards. Plasma concentrations of flurbiprofen and 4’-OH-flurbiprofen were determined by high-pressure liquid chromatography (HPLC) with fluorescence detection.28 Separate calibration curves for flurbiprofen and 4’-OH- flurbiprofen were prepared using reference standards for each analyte. Quality control (QC) samples were analyzed with each analytical run: 0.75 and 7.5 μg/mL. flurbiprofen; and, 0.08 and 0.8 μg/mL, 4’-OH-flurbiprofen. Across 12 analytical runs, the mean measured flurbiprofen concentrations were 0.76 and 7.42 μg/mL, with coefficients of variation < 11%. For 4’-OH-flurbiprofen the analogous values were 0.08 and 0.83 μg/mL, with coefficients of variation < 21%. For Trial 2, plasma concentrations of fluconazole were determined by HPLC with ultraviolet detection.50 QC samples contained 0.75 and 7.5 μg/mL of fluconazole. The mean measured concentrations across the two analytical runs were 0.72 and 7.53 μg/mL, with coefficients of variation < 2%.
Pharmacokinetic and statistical analysis.
The terminal log-linear phase of each flurbiprofen plasma concentration curve was identified visually, and the terminal slope determined by linear regression analysis using at least four data points. The slope was used to calculate the elimination half-life (T1/2). Area under the plasma concentration curve from time zero until the last non-zero concentration was calculated by the linear trapezoidal method, and extrapolated to infinity by addition of the final concentration divided by the terminal slope. This yields the total area under the curve from time zero to infinity (AUC). Apparent oral clearance (CL/F) was calculated as the administered dose divided by AUC. Observed maximum plasma concentration (Cmax) and time of maximum concentration (Tmax) were used as indicators of the rate of absorption.
For 4’-OH-flurbiprofen, the principal outcome measure was the AUC from time zero until 12 hours after dosage. Also evaluated were Cmax and Tmax.
The principal statistical procedure was analysis of variance (ANOVA) for repeated measures, with subsequent comparison of Trials 2, 3, and 4 each versus Trial 1 using Dunnett’s test. Also calculated were ratios (values for Trial 2, 3, and 4 each divided by the value for Trial 1) for Cmax and AUC. The arithmetic mean, SD, geometric mean, and 90% confidence intervals were determined for these ratios. All calculations were performed using SAS version 9.2 (Cary, NC, USA).
STUDY HIGHLIGHTS
What is the current knowledge on the topic?
Drug-fruit juice interactions are a concern for clinicians and patients. Much of the focus has been on interactions mediated by grapefruit juice. However, several case reports have noted enhanced warfarin effects in patients consuming pomegranate juice. These observations might be explained by inhibition of S-warfarin metabolism by pomegranate juice.
What question this study addressed?
This study investigated the ability of pomegranate juice and pomegranate extract to inhibit CYP2C9 activity in vitro and in vivo, using flurbiprofen as the index substrate.
What this study adds to our knowledge?
The results indicate that pomegranate juice and pomegranate extract are in vitro, but not in vivo, inhibitors of CYP2C9 activity. This in vitro-in vivo disconnect illustrates the need for clinical pharmacokinetic studies to determine the interaction potential of a natural product.
How this might change clinical pharmacology and therapeutics?
Pomegranate juice and extract had no effect on CYP2C9 activity in human subjects, and can be consumed in usual dietary amounts by patients taking CYP2C9 substrate drugs with negligible risk of a pharmacokinetic interaction.
ACKNOWLEDGEMENTS
The work was supported by a grant from PomWonderful, LLC. Dr. Hanley is the recipient of a National Research Service Award (F31 AT 006068) from the National Center for Complementary and Alternative Medicines, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Alternative Medicine or the National Institutes of Health. Dr. Court was supported by grant R01 GM 061834 from the National Institute of General Medical Sciences, National Institutes of Health.
Footnotes
DISCLOSURES
Dr. Greenblatt is a consultant to the Florida Department of Citrus, Lake Alfred, FL. The authors have no other disclosures to report.
REFERENCES
- 1.Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H and Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol 2006;46:1390–1416. [DOI] [PubMed] [Google Scholar]
- 2.Kirby BJ and Unadkat JD. Grapefruit juice, a glass full of drug interactions? Clin Pharmacol Ther 2007;81:631–633. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DG, Malcolm JAO and Spence JD. Grapefruit juice - drug interactions. British Journal of Clinical Pharmacology 1998;46:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenblatt DJ. Update on drug interactions with grapefruit juice: an evidence-based review. Pharmacy Times 2010;76 (Jan):95–104. [Google Scholar]
- 5.Farkas D and Greenblatt DJ. Influence of fruit juices on drug disposition: Discrepancies between in vitro and clinical studies. Expert Opin Drug Metab Toxicol 2008;4:381–393. [DOI] [PubMed] [Google Scholar]
- 6.Hanley MJ, Cancalon P, Widmer WW and Greenblatt DJ. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol 2011;7:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Moltke LL, Greenblatt DJ, Schmider J, Wright CE, Harmatz JS and Shader RI. In vitro approaches to predicting drug interactions in vivo. Biochemical Pharmacology 1998;55:113–122. [DOI] [PubMed] [Google Scholar]
- 8.Volak LP, Greenblatt DJ and von Moltke LL. In vitro approaches to anticipating clinical drug interactions In, Drug-Drug Interactions in Pharmaceutical Development. Edited by Li Albert P.. Hoboken, NJ, John Wiley & Sons; 2008: p. 75–93. [Google Scholar]
- 9.Farkas D, Shader RI, von Moltke LL and Greenblatt DJ (2008). Mechanisms and consequences of drug-drug interactions In, Preclinical Development Handbook: ADME and Biopharmaceutical Properties. Edited by Gad SC. Philadelphia, Wiley-Interscience; 879–917. [Google Scholar]
- 10.Greenblatt DJ, He P, von Moltke LL and Court MH (2008). The CYP3 family. In, Cytochrome P450: Role in the Metabolism and Toxicology of Drugs and Other Xenobiotics Edited by Ioannides C. Cambridge (UK), Royal Society of Chemistry; 354–383. [Google Scholar]
- 11.Greenblatt DJ and von Moltke LL (2010). Clinical studies of drug-drug interactions: design and interpretation In, Enzyme and Transporter-Based Drug-Drug Interactions: Progress and Future Challenges. Edited by Pang KS, Rodrigues AD and Peter RM. New York, Springer; 625–649. [Google Scholar]
- 12.Obach RS, Walsky RL, Venkatakrishnan K, Houston JB and Tremaine LM. In vitro cytochrome P450 inhibition data and the prediction of drug-drug interactions: qualitative relationships, quantitative predictions, and the rank-order approach. Clin Pharmacol Ther 2005;78:582–592. [DOI] [PubMed] [Google Scholar]
- 13.Brown HS, Galetin A, Hallifax D and Houston JB. Prediction of in vivo drug-drug interactions from in vitro data: factors affecting prototypic drug-drug interactions involving CYP2C9, CYP2D6 and CYP3A4. Clin Pharmacokinet 2006;45:1035–1050. [DOI] [PubMed] [Google Scholar]
- 14.Obach RS, Walsky RL, Venkatakrishnan K, Gaman EA, Houston JB and Tremaine LM. The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J Pharmacol Exp Ther 2006;316:336–348. [DOI] [PubMed] [Google Scholar]
- 15.Farkas D, Oleson LE, Zhou Y, Harmatz JS, Zinny MA, Court MH and Greenblatt DJ. Pomegranate juice does not impair clearance of oral or intravenous midazolam, a probe for cytochrome P450–3A activity: comparison with grapefruit juice. Journal of Clinical Pharmacology 2007;47:286–294. [DOI] [PubMed] [Google Scholar]
- 16.Heber D Multitargeted therapy of cancer by ellagitannins. Cancer Lett 2008;269:262– 268. [DOI] [PubMed] [Google Scholar]
- 17.Basu A and Penugonda K. Pomegranate juice: a heart-healthy fruit juice. Nutr Rev 2009;67:49–56. [DOI] [PubMed] [Google Scholar]
- 18.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev 2008;13:128–144. [PubMed] [Google Scholar]
- 19.Haber SL, Joy JK and Largent R. Antioxidant and antiatherogenic effects of pomegranate. Am J Health Syst Pharm 2011;68:1302–1305. [DOI] [PubMed] [Google Scholar]
- 20.Faria A and Calhau C. The bioactivity of pomegranate: impact on health and disease. Crit Rev Food Sci Nutr 2011;51:626–634. [DOI] [PubMed] [Google Scholar]
- 21.Chong MF, Macdonald R and Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr 2010;104 Suppl 3:S28–39. [DOI] [PubMed] [Google Scholar]
- 22.Adhami VM, Khan N and Mukhtar H. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr Cancer 2009;61:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata M, Hidaka M, Sekiya H, Kawano Y, Yamasaki K, Okumura M and Arimori K. Effects of pomegranate juice on human cytochrome P450 2C9 and tolbutamide pharmacokinetics in rats. Drug Metab Dispos 2007;35:302–305. [DOI] [PubMed] [Google Scholar]
- 24.Miners JO and Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. British Journal of Clinical Pharmacology 1998;45:525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rettie AE and Jones JP. Clinical and toxicological relevance of CYP2C9: drug-drug interactions and pharmacogenetics. Annu Rev Pharmacol Toxicol 2005;45:477–494. [DOI] [PubMed] [Google Scholar]
- 26.Komperda KE. Potential interaction between pomegranate juice and warfarin. Pharmacotherapy 2009;29:1002–1006. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis S, Li C and Bogle RG. Possible interaction between pomegranate juice and warfarin. Emerg Med J 2010;27:74–75. [DOI] [PubMed] [Google Scholar]
- 28.Greenblatt DJ, von Moltke LL, Perloff ES, Luo Y, Harmatz JS and Zinny MA. Interaction of flurbiprofen with cranberry juice, grape juice, tea, and fluconazole: in vitro and clinical studies. Clin Pharmacol Ther 2006;79:125–133. [DOI] [PubMed] [Google Scholar]
- 29.Greenblatt DJ, von Moltke LL, Luo Y, Perloff ES, Horan KA, Bruce A, Reynolds RC, Harmatz JS, Avula B, Khan IA and Goldman P. Ginkgo biloba does not alter clearance of flurbiprofen, a Cytochrome P450–2C9 substrate. Journal of Clinical Pharmacology 2006;46:214–221. [DOI] [PubMed] [Google Scholar]
- 30.Heber D, Seeram NP, Wyatt H, Henning SM, Zhang Y, Ogden LG, Dreher M and Hill JO. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J Agric Food Chem 2007;55:10050–10054. [DOI] [PubMed] [Google Scholar]
- 31.Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M and Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem 2008;56:1415–1422. [DOI] [PubMed] [Google Scholar]
- 32.Kaminsky LS and Zhang Z-Y. Human P450 metabolism of warfarin. Pharmacology and Therapeutics 1997;73:67–74. [DOI] [PubMed] [Google Scholar]
- 33.Ushijima K, Tsuruoka S, Tsuda H, Hasegawa G, Obi Y, Kaneda T, Takahashi M, Maekawa T, Sasaki T, Koshimizu TA and Fujimura A. Cranberry juice suppressed the diclofenac metabolism by human liver microsomes, but not in healthy human subjects. Br J Clin Pharmacol 2009;68:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngo N, Brantley SJ, Carrizosa DR, Kashuba AD, Dees EC, Kroll DJ, Oberlies NH and Paine MF. The warfarin-cranberry juice interaction revisited: A systematic in vitro-in vivo evaluation. J Exp Pharmacol 2010;2010:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenblatt DJ and von Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol 2005;45:127–132. [DOI] [PubMed] [Google Scholar]
- 36.Mellen CK, Ford M and Rindone JP. Effect of high-dose cranberry juice on the pharmacodynamics of warfarin in patients. Br J Clin Pharmacol 2010;70:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansell J, McDonough M, Zhao Y, Harmatz JS and Greenblatt DJ. The absence of an interaction between warfarin and cranberry juice: A randomized, double-blind trial. Journal of Clinical Pharmacology 2009;49:824–830. [DOI] [PubMed] [Google Scholar]
- 38.Zikria J, Goldman R and Ansell J. Cranberry juice and warfarin: when bad publicity trumps science. Am J Med 2010;123:384–392. [DOI] [PubMed] [Google Scholar]
- 39.Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA and Goldstein JA. Tolbutamide, flurbiprofen, and losartan as probes of CYP2C9 activity in humans. J Clin Pharmacol 2003;43:84–91. [DOI] [PubMed] [Google Scholar]
- 40.Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA and Goldstein JA. Differences in flurbiprofen pharmacokinetics between CYP2C9*1/*1, *1/*2, and *1/*3 genotypes. Eur J Clin Pharmacol 2003;58:791–794. [DOI] [PubMed] [Google Scholar]
- 41.Giancarlo GM, Venkatakrishnan K, Granda BW, von Moltke LL and Greenblatt DJ. Relative contributions of CYP2C9 and 2C19 to phenytoin 4-hydroxylation in vitro: inhibition by sulfaphenazole, omeprazole and ticlopidine. European Journal of Clinical Pharmacology 2001;57:31–36. [DOI] [PubMed] [Google Scholar]
- 42.Misaka S, Nakamura R, Uchida S, Takeuchi K, Takahashi N, Inui N, Kosuge K, Yamada S and Watanabe H. Effect of 2 weeks’ consumption of pomegranate juice on the pharmacokinetics of a single dose of midazolam: an open-label, randomized, single center, 2-period crossover study in healthy Japanese volunteers. Clin Ther 2011;33:246–252. [DOI] [PubMed] [Google Scholar]
- 43.Scalbert A and Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000;130:2073S–2085S. [DOI] [PubMed] [Google Scholar]
- 44.Manach C, Scalbert A, Morand C, Remesy C and Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–747. [DOI] [PubMed] [Google Scholar]
- 45.Spencer JP, Abd-el-Mohsen MM and Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch Biochem Biophys 2004;423:148–161. [DOI] [PubMed] [Google Scholar]
- 46.Singleton VL, Orthofer R, Lamuela-Ravent RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent In: Methods in Enzymology Vol.299: Oxidants and Antioxidants Part A (Packer L, ed.), p. 152–178, Academic Press, New York. [Google Scholar]
- 47.Venkatakrishnan K, von Moltke LL and Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism in humans: clinical relevance. Clinical Pharmacokinetics 2000;38:111–180. [DOI] [PubMed] [Google Scholar]
- 48.Greenblatt DJ, von Moltke LL, Harmatz JS, Chen G, Weemhoff JL, Jen C, Kelley CJ, LeDuc BW and Zinny MA. Time-course of recovery of cytochrome P450 3A function after single doses of grapefruit juice. Clinical Pharmacology and Therapeutics 2003;74:121–129. [DOI] [PubMed] [Google Scholar]
- 49.Greenblatt DJ, Zhao Y, Venkatakrishnan K, Duan SX, Harmatz JS, Parent SJ, Court MH and von Moltke LL. Mechanism of cytochrome P450–3A inhibition by ketoconazole. J Pharm Pharmacol 2011;63:214–221. [DOI] [PubMed] [Google Scholar]
- 50.Greenblatt DJ, von Moltke LL, Harmatz JS, Mertzanis P, Graf JA, Durol ALB, Counihan M, Roth-Schechter B and Shader RI. Kinetic and dynamic interaction study of zolpidem with ketoconazole, itraconazole, and fluconazole. Clinical Pharmacology and Therapeutics 1998;64:661–671. [DOI] [PubMed] [Google Scholar]


