Summary
Some individuals have higher efavirenz plasma concentrations during rifampin-containing tuberculosis (TB) therapy, contrary to the expected induction effect of rifampin. Among HIV-infected patients without (N=38) and with TB on rifampin-containing therapy (N=18), we tested the hypothesis that drug-gene interaction may explain the highly variable drug interactions. Two-way analysis of variance revealed a significant interaction between CYP2B6 516G→T polymorphism and rifampin-containing therapy, suggesting that efavirenz dose-adjustment may need to be individualized based on the patient’s genotype.
Keywords: CYP2B6 polymorphism, drug-gene interaction, rifampin, efavirenz concentration
Efavirenz-based antiretroviral regimen is preferred during rifampin-containing antituberculous therapy. The adult fixed-dose of 600 mg/day is associated with wide inter-individual variability in plasma concentrations as well as clinical outcome [1–3]. The variability in efavirenz concentrations is even greater during coadministration with rifampin or rifampin-containing antituberculous therapy [4–6], suggesting a variable degree of drug-drug interaction. Among healthy volunteers, rifampin caused a significant reduction in efavirenz area under the curve (AUC) in 10 subjects but two subjects had higher AUC on than off rifampin [4]. In HIV/TB co-infected patients, the change in efavirenz AUC with concomitant rifampin administration ranged from −65% to +37% in one study [7]. Efavirenz is primarily metabolized by hepatic CYP2B6, with some contributions from CYP3A4/5 [8], CYP2A6[9] and UGT2B7 enzymes [10]. The lack of induction of efavirenz metabolism by rifampin in some individuals is contrary to the expected effect of rifampin on CYP2B6 activity [11].
A bimodal effect of rifampin-containing therapy on efavirenz plasma concentrations has also been reported, with paradoxically higher concentrations with antituberculous therapy in the patients who appeared to be slow efavirenz metabolizers (based on phenotype only since enzyme genotyping was not done) [5, 12]. Also, some HIV/TB co-infected patients have required decreased efavirenz doses and/or discontinuation during rifampin-containing therapy because of severe toxicities associated with elevated efavirenz concentrations [13, 14].
Elevations of efavirenz plasma concentration with rifampin-containing therapy that occur predominantly in persons with slow metabolizing phenotype suggest the possibility of a genotype-dependent drug-drug interaction. In this study we investigated the potential for such an interaction between rifampin-containing antituberculous therapy and CYP2B6 516 G→T polymorphism using data from a pharmacokinetic study conducted previously. The characteristics of the patients and results of the primary analysis are reported elsewhere [15]. All patients received efavirenz 600 mg/day with didanosine and lamivudine, and pharmacokinetic sampling performed on day-28 of therapy. Antiretroviral therapy was initiated within 2–8 weeks of antituberculous therapy in co-infected patients and only those on concurrent therapy at the time of pharmacokinetic sampling were included in this analysis. The study was approved by the Institutional Review Board of the Nogouchi Memorial Institute for Medical Research, Ghana. Informed written consent was obtained from all patients.
Pharmacokinetic samples were drawn at approximately 12 hours after the efavirenz dose was taken. Efavirenz plasma concentrations were measured using a validated high-performance liquid chromatography (HPLC)/UV method [16]. Subjects were genotyped for CYP2B6 c.516G→T (Q172H, rs3745274 nuclease genotyping assay (Applied Biosystems, Foster City, CA). Two-way analysis of variance (ANOVA), which incorporates an interaction model, was used to investigate effects of CYP2B6 516G→T genotype and rifampin-containing TB therapy. As in previous studies [15, 17], concentration data were not normally distributed and so they were log transformed prior to analysis. In instances where the ANOVA indicated a significant effect (P<0.05), post-hoc multiple pair-wise comparison testing was performed using the Student-Newman-Keuls method to identify groups which were significantly different from each other.
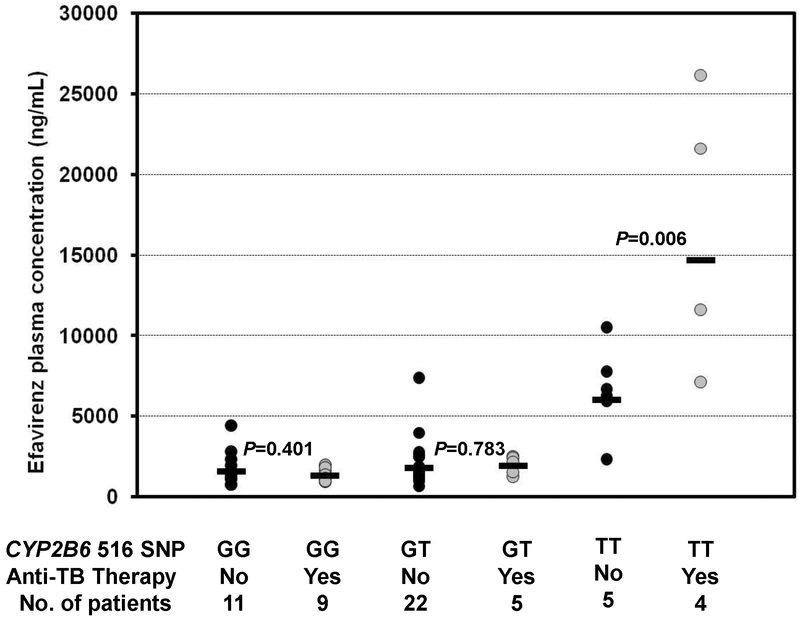
The analysis included 38 HIV-infected patients on efavirenz-based therapy and 18 HIV/TB co-infected patients on efavirenz-based and rifampin-containing antituberculous therapies. Concentration data from individual subjects categorized by CYP2B6 516G→T genotype and type of therapy are shown in Fig. 1. The two-way ANOVA revealed a statistically significant effect of CYP2B6 516 genotype on efavirenz plasma concentrations as well as an interaction between CYP2B6 516G→T genotype and the antituberculous therapy (P<0.001 for CYP2B6 516G→T genotype; P=0.089 for rifampin-containing therapy; P=0.022 for interaction). Post hoc pairwise analysis showed that patients with CYP2B6 516TT genotype who were receiving concurrent therapy had a significantly higher geometric mean efavirenz concentration than those on only antiretrpviral therapy (14689 vs. 6012 ng/mL, respectively; P = 0.006) (Fig. 1). Patients with 516GT genotype on concurrent antituberculous therapy had a slightly higher geometric mean efavirenz concentration than those on HAART only (1901 vs. 1778 ng/mL) and the patients with the 516GG genotype on antituberculous therapy had a lower geometric mean efavirenz concentrations than those with the 516GG genotype on HAART only (1291 vs. 1552 ng/mL) but these latter differences between therapeutic groups did not achieve statistical significance (P>0.05).
Fig. 1.
Influence of CYP2B6 c.516G>T genotypes and rifampin-containing antituberculous therapy on efavirenz plasma mid-dose concentrations. There was a significant interaction between CYP2B6 G→T polymorphism and rifampin-containing therapy (P = 0.022; 2-way ANOVA). Shown are the P values from the post-hoc multiple pair-wise comparisons (Student-Newman-Kuels test) within each genotype group. The horizontal bars represent the geometric mean efavirenz concentration of each genotype group.
As a CYP2B6 enzyme inducer, rifampin co-administration would be expected to enhance efavirenz clearance and reduce plasma levels. However, in this study we found that the patients with CYP2B6 516TT genotype and receiving concomitant antituberculous therapy had higher efavirenz plasma concentrations than those subjects with the same genotype but not receiving antituberculous therapy. These findings are consistent with previous reports of elevated efavirenz concentration with rifampin-containing antituberculous therapy in HIV/TB co-infected patients who appeared to have slow metabolizing phenotype as genotyping was not done [5, 12, 14]. The paradoxical effect of antituberculous therapy in this study may be due to increased susceptibility of the CYP2B6 (172-histidine) variant allozyme (resulting from the c.516G→T polymorphism) to inhibition by one (or more) of the antituberculous drugs as compared with the reference CYP2B6 (172-glutamine) enzyme. Alternately, efavirenz metabolism may be inhibited by effects on the (non-CYP2B6) accessory pathways leading to higher efavirenz concentrations. CYP2A6 mediated 7-hydroxylation is identified as an important alternate pathway in the metabolism of efavirenz [18] and may be the major pathway for efavirenz clearance in CYP2B6 slow metabolizers [19]. Thus, inhibition of CYP2A6 by one (or more) of the antituberculous drugs could explain the higher concentrations. Studies are needed to determine the mechanism by which addition of the standard four-drug antituberculous therapy causes higher efavirenz concentrations in CYP2B6 slow metabolizers.
Our study has some limitations. Firstly, the sample size is small and the lack of significant effect of antituberculous therapy on efavirenz concentrations in the 516GG and GT genotype groups should be interpreted with caution. Secondly, the study compares two different patient populations and the differences seen in the 516TT genotype group could be due to population differences. However, our observation is supported by previous studies that showed paradoxically higher efavirenz concentrations during antituberculous therapy in the patients who appeared to be slow efavirenz metabolizers [5, 12]. If our results are confirmed in larger studies, CYP2B6 genotyping could inform rational decisions about efavirenz dosing or alternate therapy in HIV/TB co-infected patients.
Acknowledgements
We thank the study participants, the Medical Officers at the Fever’s Unit, staff of Clinical Virology, the Study Coordinators as well as the study nurse for all their valuable assistance in recruitment, evaluation of patients as well as obtaining and handling the samples. This research was supported in part by a K23 developmental award (NIH K23 AI071760) to Dr Kwara and ACRiA grant from Doris Duke Foundation to Dr Lartey. The University of North Carolina at Chapel Hill, Center for AIDS research #9P30 AI50410, Clinical Pharmacology and Analytical Chemistry Laboratory (CPACL) performed the efavirenz concentrations. Dr Court was supported by grant R01GM061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD).
Sources of support: This work was supported in part by NIH K23 Developmental award (AI071760) from NIAID, ACRiA grant from Doris Duke Foundation and R01GM061834 from NIGMS.
Footnotes
Conflict of Interest:
Dr Kwara has previously received a research grant not related to this study from Bristol Myer-Squibb. Drs Lartey, Court, and Mr Sagoe report no conflict of interest.
REFERENCES
- 1.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther 2003,73:20–30. [DOI] [PubMed] [Google Scholar]
- 2.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. Aids 2001,15:71–75. [DOI] [PubMed] [Google Scholar]
- 3.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit 2004,26:267–270. [DOI] [PubMed] [Google Scholar]
- 4.Benedeck IH JA, Fiske WD, et al. Pharmacokinetic interaction between efavirenz and rifampin in healthy volunteers . 12th World AIDS Conference Geneva, Switzerland, June 28 - July 3 1998 1998. [Google Scholar]
- 5.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother 2006,58:1299–1302. [DOI] [PubMed] [Google Scholar]
- 6.Matteelli A, Regazzi M, Villani P, De Iaco G, Cusato M, Carvalho AC, et al. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr HIV Res 2007,5:349–353. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet 2002,41:681–690. [DOI] [PubMed] [Google Scholar]
- 8.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 2003,306:287–300. [DOI] [PubMed] [Google Scholar]
- 9.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 (CYP) 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz (EFV) by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine (AZT). Drug Metab Dispos 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, et al. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther 2006,80:75–84. [DOI] [PubMed] [Google Scholar]
- 12.Ren Y, Nuttall JJ, Eley BS, Meyers TM, Smith PJ, Maartens G, McIlleron HM. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 2009,50:439–443. [DOI] [PubMed] [Google Scholar]
- 13.van Luin M, Brouwer AM, van der Ven A, de Lange W, van Schaik RH, Burger DM. Efavirenz dose reduction to 200 mg once daily in a patient treated with rifampicin. AIDS 2009,23:742–744. [DOI] [PubMed] [Google Scholar]
- 14.Brennan-Benson P, Lyus R, Harrison T, Pakianathan M, Macallan D. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS 2005,19:1541–1543. [DOI] [PubMed] [Google Scholar]
- 15.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol 2009,67:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezk NL, Crutchley RD, Yeh RF, Kashuba AD. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther Drug Monit 2006,28:517–525. [DOI] [PubMed] [Google Scholar]
- 17.Kwara A, Lartey M, Sagoe KW, Kenu E, Court MH. CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS 2009,23:2101–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos 2010,38:1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 2009,19:300–309. [DOI] [PubMed] [Google Scholar]