Abstract
1. Many UDP-glucuronosyltransferases (UGTs) require phosphorylation by protein kinase C (PKC) for glucuronidation activity. Inhibition of UGT phosphorylation by PKC inhibitor drugs may represent a novel mechanism for drug-drug interactions.
2. The potential for PKC-mediated inhibition of human UGT1A6, an isoform involved in the glucuronidation of drugs such as acetaminophen and endogenous substrates including serotonin, was evaluated using various cell model systems.
3. Of 10 different PKC inhibitors screened for effects on acetaminophen glucuronidation by human LS180 colon cells, only rottlerin (PKC-δ selective inhibitor; IC50 = 9.0 ± 1.2 μM) and the non-selective PKC inhibitors (calphostin-C, curcumin and hypericin), decreased glucuronidation by more than 50%.
4. Using UGT1A6-infected Sf9 insect cells, calphostin-C and hypericin showed three-times more potent inhibition of serotonin glucuronidation in treated whole cells versus cell lysates. However, both curcumin and rottlerin showed significant direct inhibition and so (indirect) PKC effects could not be differentiated in this model system.
5. Of 9 PKC isoforms co-expressed with UGT1A6 in human embryonic kidney 293T cells only PKC δ increased protein-normalized UGT1A6-mediated serotonin glucuronidation significantly (by 63±4%).
6. These results identify an important role for PKC δ in UGT1A6 mediated glucuronidation and suggest that PKC δ inhibitors could interfere with glucuronidation of UGT1A6 substrates.
Keywords: UDP-glucuronosyltransferase (UGT), UGT1A6, glucuronidation, protein kinase C (PKC), drug-drug interaction
1. Introduction
UDP-glucuronosyltransferase (UGT) enzymes are involved in the conjugation of UDP-glucuronic acid (UDPGA) to a wide variety of substrates, both endogenous and exogenous, to form hydrophilic products that can readily be excreted from the body. In 2004, it was reported that approximately 1 in 13 of the top 200 drugs on the market were metabolized at least in part by UGTs (Williams et al. 2004). Today, drug metabolism by UGTs is playing an ever increasing role in pharmaceutical development, yet much is still unknown about the regulation of UGT enzymes. Alterations in UGT activity by for example a co-administered drug can potentially lead to adverse drug reactions. Inhibition of the UGT enzymes can lead to elevated levels of UGT drug substrates and potential toxicity (drug-drug interaction). Therefore, understanding the mechanisms that drugs can affect UGT activity is of substantial importance.
There is accumulating evidence for a novel mechanism of UGT inhibition involving protein kinase C (PKC) (Basu et al. 2008; Basu et al. 2003; Basu et al. 2005; Basu et al. 2004c). PKC enzymes are calcium- and/or diacylglycerol-dependent kinases that phosphorylate serine and threonine residues in a wide variety of cellular proteins, most notably those involved in signal transduction pathways (Mackay and Twelves 2007). Twelve different PKC isoforms have been identified including α, β1, β2, δ, ε, γ, ι, η, μ, θ, τ, and ζ. Putative PKC phosphorylation consensus sequences have been identified on eight UGT1A and two UGT2B enzymes (Basu et al. 2003). Furthermore, mutation of two predicted phosphorylation sites on UGT1A1, 1A7, and 1A10 completely abolishes glucuronidation activity, while calphostin-C, a selective pan-PKC inhibitor, inhibits glucuronidation of bilirubin and anthraflavic acid by UGT1A1, capsaicin by UGT1A7, and eugenol by UGT1A10 expressed in COS-1 (monkey kidney) cells (Basu et al. 2003; Basu et al. 2005). UGT1A6 is predicted to have four PKC phosphorylation sites (S/TXK/R) at positions 41, 50, 74, and 206 and one tyrosine phosphorylation site at position 191 (Basu et al. 2003). Moreover, 100μM curcumin treatment of UGT1A6-transfected COS-1 cells reduced 4-nitrophenol glucuronidation by 40% without significant effects on UGT1A6 expression (Basu et al. 2008), although the effect of calphostin-C or other known selective PKC inhibitors was not reported.
Isoform specific PKC effects on UGT1A isoforms have also been described (Basu et al. 2008; Basu et al. 2005). UGT1A7 and PKC ε were found to co-localize and associate in the endoplasmic reticulum of UGT1A7 expressing COS-1 cells (Basu et al. 2005). A PKC ε specific inhibitor peptide inhibited eugenol glucuronidation by 50% in human LS180 colon cells (Basu et al. 2005). PKC ε-specific siRNA inhibited UGT1A7-mediated glucuronidation of mycophenolic acid in UGT1A7 expressing COS-1 cells (Basu et al. 2008). Overexpression of PKC ε resulted in 30 – 50% increase in UGT1A7 eugenol activity in UGT1A7-transfected COS-1 cells (Basu et al. 2005). Additionally, in that study, UGT1A7 and PKC α and ε co-localized in the endoplasmic reticulum of COS-1 cells. UGT1A10 and PKCs α and δ also were found to co-localize in the endoplasmic reticulum of COS-1 cells, and in UGT1A10-transfected COS-1 cells, curcumin and calphostin-C treatment resulted in a dose-dependent decrease in the phosphorylation of UGT1A10 (Basu et al. 2008).
Although these studies indicate that PKC plays a role in UGT phosphorylation and activity, further studies are needed to validate these results with other UGT enzymes and in other model systems. Furthermore, the role of specific PKC isoforms in UGT phosphorylation would be of importance as several isoform-specific PKC inhibitors are currently in clinical development. For example, ruboxistaurin (a PKC β inhibitor) is being developed for the treatment of diabetic neuropathy and macular oedema, while KAI-9803 (PKC δ inhibitor) is being tested for the treatment of acute myocardial infarction reperfusion injury (Bates et al. 2008; Brooks et al. 2008).
In previous studies, we showed that acetaminophen (APAP) glucuronidation by LS180 cells was inhibited by curcumin and calphostin C much more potently in intact LS180 cells than in cell homogenates (Volak et al. 2008). This suggests a role for PKC in the activation of the UGTs that mediate acetaminophen glucuronidation, although this has not yet been explored. Acetaminophen is predominantly metabolized by UGT1A1, 1A6, and 1A9 in human liver, although the relative contributions of these enzymes can differ depending on acetaminophen concentration (Court et al. 2001). UGT1A6 has the highest affinity for acetaminophen as determined in human liver microsomes (Km = 2.2 ± 0.3 mM). In the human colon, UGT1A6 mRNA is expressed at 2.4 and 41.8-fold higher levels than UGT1A1 and UGT1A9, respectively, suggesting that UGT1A6 could be a major enzyme responsible for APAP glucuronidation in LS180 cells (Nishimura and Naito 2006). PKC phosphorylation and activation of UGT1A6 has yet to be studied in detail. UGT1A6 is expressed in the liver as well as extrahepatic tissues such as the intestine, kidney, and brain and is involved in the glucuronidation of acetaminophen as well as serotonin (Bock and Kohle 2005).
In order to further explore the role of PKC in acetaminophen glucuronidation mediated by UGT1A6, we initially screened a panel of ten PKC inhibitors, including PKC α, β, δ, and θ specific inhibitors, to determine their effect on acetaminophen glucuronidation in LS180 cells. Inhibitors found to be active in this initial screen were further screened for dose-dependent effects and for direct inhibition of acetaminophen glucuronidation in human liver microsomes (HLM). Because acetaminophen glucuronidation could also be mediated by UGT1A1 and UGT1A9 in LS180 cells, we also evaluated the effects of the active PKC inhibitors on glucuronidation of the UGT1A6-specific substrate serotonin in human UGT1A6-infected Sf9 insect cells, cell lysates, and in HLM. Due to the limited selectivity of available PKC inhibitors, the effect of coexpression of nine different PKC isoforms with UGT1A6 on serotonin glucuronidation in human embryonic kidney (HEK293T) cells was also studied.
Ultimately, results from these studies could be used to aid in the prediction of which isoform-specific PKC isoform-specific PKC inhibitors in drug development may interfere with UGT1A6 mediated drug metabolism potentially resulting in a drug-drug interaction.
2. Materials and Methods
2.1. Materials.
Curcumin, calphostin-C, valproic acid, Ro 32–0432, CGP-53353, serotonin hydrochloride (5-hydroxytryptamine), phorbol myristate acetate (PMA), 1-oleoyl-2-acetyl-sn-glycerol (OAG), and acetaminophen were purchased from Sigma-Aldrich (St. Louis, MO). Unless otherwise noted, other reagents were also from Sigma-Aldrich. NPC 15437 was purchased from Tocris Bioscience (Ellisville, MO), LY-333531 was purchased from A.G. Scientific (San Diego, CA), PKC-β inhibitor from Calbiochem (Gibbstown, NJ), and rottlerin from Alexis Biochemicals (San Diego, CA). Acetaminophen glucuronide was provided by McNeil Consumer Products Co. (Fort Washington, PA). pHACE mammalian expression vectors containing the catalytic domain (pHACE-PKC-CAT) of human PKC-α, mouse PKC-δ, mouse PKC-ε, mouse PKC-γ, human PKC-ι, mouse PKC-η, and rat PKC-ζ with a C-terminal HA tag and pHANB mammalian expression vectors containing the catalytic domain (pHANB-PKC-CAT) of rat PKC-β1 and mouse PKC β2 with a N-terminal HA tag were kindly provided by Dr. Jae-Won Soh (Inha University, Incheon, Korea). pcDNA-DEST40 was purchased from Invitrogen (Carlsbad, CA). The GST-HA positive immunoblot control was obtained from Pierce / ThermoFisher Scientific (Rockford, IL).
2.2. Cell culture.
LS180 cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in minimal essential medium with 2 mM L-glutamine and Earle’s salts and supplemented with 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, 50 U / mL penicillin, 50 μg / mL streptomycin, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Sf9 insect cells were maintained in Sf900 II serum-free medium with 50 U / mL penicillin and 50 μg / mL streptomycin, and HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium containing 4,500 mg/L D-glucose and L-glutamine supplemented with 50 U / mL penicillin, 50 μg / mL streptomycin, and 10% fetal bovine serum (Invitrogen).
2.3. Human liver microsomes preparation.
Microsomes were prepared by ultracentrifugation as described in detail previously (von Moltke et al. 1993). Human liver tissue was acquired from the International Institute for the Advancement of Medicine (Exton, PA), the Liver Tissue Procurement and Distribution System, University of Minnesota (Minneapolis, MN), or the National Disease Research Interchange (Philadelphia, PA). All livers were either intended for transplantation but failed to tissue match or were normal tissue adjacent to surgical biopsies. Donor characteristics of each of the livers have been reported previously (Hesse et al. 2004). Liver microsomes from 50 different individuals were pooled for UGT activity assays.
2.4. Acetaminophen glucuronidation in LS180 cells.
LS180 cells were seeded onto 24-well plates at 175,000 cells per well. The cells were incubated in 10 mM acetaminophen alone (including DMSO vehicle at 0.5% final concentration) or combined with varying concentrations of PKC inhibitors. After 24 hr, cells and media were collected, and protein precipitated by adding an equal volume (1 mL) of a 5% acetic acid / 95% acetonitrile solution that included sulfaphenazole (200 μM) as an internal standard. After mixing and then centrifuging at 13,000 g for 5 min, the supernatant was evaporated in a vacuum oven set at 40oC, and then re-suspended in mobile phase for LC-MS analysis (see below).
2.5. Acetaminophen and serotonin glucuronidation by pooled HLM.
Glucuronidation activities were measured by methods similar to that used previously unless otherwise noted (Court et al. 2001; Krishnaswamy et al. 2003b). Briefly, incubation buffer containing 50 mM phosphate buffer, pH 7.5, with 5 mM MgCl2, 20 mM UDPGA, and 0.05 mg / mL alamethicin was added to 10 mM acetaminophen or 5 mM serotonin that had been previously evaporated in a vacuum oven set at <40oC. Vehicle alone (0.5% DMSO final concentration) or various concentrations of PKC inhibitors were added directly to the incubation mixture. Microsomes were added for a final concentration of 0.5 mg / mL with a final assay volume of 100 μL. For acetaminophen glucuronidation, incubation time was 3 hr at 37°C with the reaction terminated by the addition of 50 μL of 5% acetic acid in acetonitrile with 200 μM sulfaphenazole as the internal standard. For serotonin glucuronidation, incubation time was 2 hr at 37°C with the reaction terminated by the addition of 50 μL of acetonitrile with 0.2 mg /mL acetaminophen as the internal standard. Under these conditions, product formation was linear with respect to time and protein concentration. Samples were mixed and centrifuged at 13,000 g for 5 min. The resulting supernatant was removed, evaporated by vacuum, and reconstituted with 100 μL of mobile phase for HPLC or LC-MS analysis (see below).
2.6. UGT1A6 expression in Sf9 insect cells and PKC inhibitor/activator treatment.
The coding region of UGT1A6 was subcloned from pcDNA 3.1 (described previously in (Krishnaswamy et al. 2005a)) by PCR using primers Pri341 5’-cac cat ggc ctg cct cct tcg-3’ (forward) and Pri342 5’-atg ggt ctt gga ttt gtg ggc ttt ct-3’ (reverse) with PfuUltra Hotstart DNA polymerase (Stratagene, La Jolla, CA) into pENTR using pENTR directional TOPO cloning (Invitrogen) and the sequence was checked by sequencing using M13 forward and reverse primers. UGT1A6 was then subcloned from the pENTR vector into expression baculovirus using the Baculodirect baculovirus expression system (Invitrogen). After transfection of Sf9 insect cells and selection with ganciclovir, the virus was amplified by infecting at least three subsequent passages of Sf9 cells with the medium from the previous passage containing virus. UGT1A6 protein was expressed in Sf9 cells plated in 12-well culture plates and treated with 30 μL of viral stock for three days. On the third day, the Sf9 cells were treated with curcumin, calphostin-C, hypericin, rottlerin, PMA, or OAG (in 0.5% DMSO final concentration) at various concentrations in growth media for 2 hours. A two hour treatment time was selected based on initial experiments where the 2 hour treatment allowed for maximum inhibition. Cells and media were then collected, centrifuged at 2350 g for 5 min, and washed three times with cold Dulbecco’s phosphate buffered saline. The cells were lysed by the addition of 50 μl of pure water, frozen at −80oC overnight, sonicated briefly three times on ice, mixed with an equal volume of 100 mM potassium phosphate buffer (pH 7.5), and stored at −80°C until assay. Sf9 cell lysates were assayed for acetaminophen or serotonin glucuronidation as described above for HLM using 0.5 mg/mL cell lysate in place of 0.5 mg/mL HLM.
2.7. Subcloning PKC-CAT-HA, TMED7, and UGT1A6 into pcDNA-DEST40.
The catalytic subunit coding region of PKC α (human), δ (mouse), ε (mouse), γ (mouse), ι (human), ε (mouse), and ζ (rat) with C-terminal HA tag was subcloned from pHACE-PKC-CAT by PCR using primers Pri589 5’-cac ccg act cac tat agg gag acc c-3’ (forward) and Pri590 5’-tta ggc gta gtc agg cac g-3’ (reverse) with PfuUltra Hotstart DNA polymerase (Stratagene, La Jolla, CA) into pENTR using pENTR directional TOPO cloning. PKC β1 (rat) and β2 (mouse) with N-terminal HA tag were subcloned in the same manner from pHANB-PKC-CAT using primers Pri642 5’-cac cca ctg ctt act ggc tta tcg a-3’ (forward) and Pri643 5’-gcc gcc agt gtg atg gat at-3’ (reverse). TMED7 (used as a non-specific protein control) was subcloned from MGC clone 3625342 in pOTB7 (FL1002, Invitrogen) using Pri591 5’-cac cgc cgc ctt ctc ggg atg-3’ (forward) and Pri638 5’-tga tcc aac acg agt tgt ggt g-3’ (reverse). The coding region of UGT1A6 was subcloned from the pENTR plasmid (see earlier methods) by PCR using primers Pri341 5’-cac cat ggc ctg cct cct tcg-3’ (forward) and Pri641 5’-tca atg ggt ctt gga ttt gtg ggc ttt ct-3’ (reverse). This subcloning introduced a stop codon at the end of the UGT1A6 sequence to prevent readthrough to the V5/His tag on pcDNA-DEST40. All of the resulting plasmids were checked by sequencing using M13 forward and reverse primers. PKC ε and γ sequences had silent point mutations resulting in no change in the protein sequence and also lacked the native stop codon resulting in readthrough to the V5/His tag on pcDNA-DEST40. The mouse PKC δ catalytic region had one mutation at position 11 (t →a, based upon the PKC δ sequence provided by Dr. Jae-Won Soh) resulting in a predicted glutamate to valine amino acid change; however, this amino acid change was also found in the human PKC δ form based on a sequence provided by Dr. Mitchell Denning at Loyola University Medical Center (Maywood, Illinois). Expression clones of PKC isoforms and UGT1A6 were generated by performing an LR recombination reaction between pENTR and pcDNA-DEST40 Gateway vector using the Gateway LR Clonase II enzyme Mix (Invitrogen). After transformation of Top10 competent cells (Invitrogen) with the resulting pcDNA-DEST40 plasmids and selection with chloramphenicol, plasmid DNA was extracted and purified using the E.Z.N.A endo-free plasmid mini kit (Omega Bio-tek, Norcross, GA) and quantified using a NanoDrop 1000 instrument (Wilmington, DE). The sequences of the isolated plasmids were re-confirmed by sequencing using the BGH reverse primer.
2.8. PKC-CAT, TMED7, and UGT1A6 co-expression in HEK293T cells.
HEK293T cells at approximately 90–95% confluence in 12-well culture plates were co-transfected with 1 μg of pcDNA-DEST40-UGT1A6, and either 1.5 μg of pcDNA-DEST40-PKC or 1.5 μg of pcDNA-DEST40 without insert (negative control) or 1.5 μg of pcDNA-DEST40-TMED7 plasmid (non-specific protein control) using 2 μL of Lipofectamine 2000 per well in OptiMEM medium (both from Invitrogen) for 6 hours, after which growth medium was added to the cells. After another 42 hours, cells and media were collected, and cell lysates were prepared and assayed as described previously for UGT1A6-infected Sf9 cells. Well to well differences in transfection efficiency and nonspecific effects of PKC isoforms on gene expression were controlled by cotransfecting 5 ng per well of β-galactosidase expression vector (pMIR-REPORT control vector, Ambion / Applied Biosystems, Austin, TX). β-galactosidase activity in the HEK293T lysates was determined according to the manufacturer’s instructions using the β-galactosidase enzyme assay system kit from Promega (Madison, WI).
2.9. Immunoblotting of PKC-CAT-HA and UGT1A6.
Expression of the various PKC isoform catalytic domains (tagged with a hemagglutinin (HA) epitope) in HEK293T cells was confirmed by immunoblotting. Cell lysate (50 μg protein) was separated by SDS-polyacrylamide gel electrophoresis using a Criterion 4–20% gradient gel and then transferred to a polyvinyl diflouride membrane at 15 V for 35 min in a SD semi-dry electrophoretic transfer cell (Biorad, Hercules, CA). The membranes were blocked with 5% dry milk in TBST (0.15 M NaCl, 0.04 M Tris, pH 7.7, and 0.1% Tween 20) overnight at 4oC and then probed with 1.5 μg/mL of a polyclonal rabbit anti-HA antibody (Invitrogen) in 1% dry milk in TBST for 1 hour. After washing in TBST, the membranes were incubated in a 1:1000 dilution of goat anti-rabbit IgG conjugated with horseradish peroxidase for 1 hour, washed again, and then incubated for 5 min in SuperSignal West Pico chemiluminescent substrate (Pierce/Thermo Fisher Scientific, Rockford, IL). Blots were imaged using Kodak Image Station (Kodak, Rochester, NY).
Relative expression of UGT1A6 was determined according to a previously reported method with slight modifications (Krishnaswamy et al. 2003a). Briefly, protein was separated by SDS-polyacrylamide gel electrophoresis using a Criterion 4–15% gradient gel and then transferred to a polyvinyl diflouride membrane at 15 V for 40 min in a semi-dry electrophoretic transfer cell (all from Biorad, Hercules, CA). The membranes were fixed with water:methanol:glacial acetic (5:5:1) acid for 5 min, washed with TBST, and then blocked with 10% dry milk in TBST overnight at 4oC. The membrane was again washed and then incubated with a 1:1000 dilution of a rabbit anti-human UGT1A peptide antibody in 1% dry milk in TBST for 1 hour. This antibody had been prepared using the common C-terminal end of human UGT1A enzymes as described previously (Court 2001). After washing in TBST, the membranes were incubated in a 1:5000 dilution of goat anti-rabbit IgG conjugated with horseradish peroxidase (Sigma-Aldrich) for 1 hour, washed again, and then incubated for 5 min in SuperSignal West Pico chemiluminescent substrate (both from Pierce/Thermo Fisher Scientific, Rockford, IL). Blots were imaged and quantified using Kodak Image Station and Kodak 1D Image Analysis software (Kodak, Rochester, NY).
2.10. Glucuronide quantitation.
Acetaminophen glucuronide levels were measured as described previously (Volak et al. 2008). Briefly, acetaminophen glucuronide and sulfaphenazole (internal standard) were separated and detected using an HPLC instrument (Surveyor, Thermofinnigan, Somerset, NJ) using a 150 × 3 mm column (Synergi Fusion-RP, Phenomenex) connected serially to an ion-trap mass detector (LCQ Deca XP Max, Thermofinnigan) with an electrospray ionization source. Mobile phase consisted of 10 mM ammonium acetate, pH 4.2, with acetonitrile gradually increased from 0 to 50% over 14 min at a flow rate of 0.5 mL/min and returned to 0% acetonitrile at 14.1 min (total run time of 23 min). Acetaminophen glucuronide was monitored in positive ion mode by MS-MS (m/z ratio: 345.2 → 152.2) while sulfaphenazole was monitored at an m/z ratio of 315.3 with respective retention times of 1.4 and 10.7 min.
Serotonin glucuronide levels were measured as described previously (Krishnaswamy et al. 2003b). Briefly, serotonin glucuronide and the internal standard (acetaminophen) were separated and detected using an HPLC system (Model 1100, Agilent, Palto Alto, CA) with a 250 × 4.6-mm C18 column (Synergi Hydro-RP, Phenomenex, Torrance, CA) serially connected to a UV absorbance diode array detector and a fluorescence detector. Mobile phase consisted of 95% 20 mM potassium phosphate buffer, pH 2.2, with acetonitrile increasing gradually from 0 to 2% at 5 min, 2 to 7% at 10 min, and then held at 7% until 15 min when it was gradually decreased to 0% at 22 min at a flow rate of 1 mL / min (total run time of 25 min). The fluorescence detector was set at 225 nm excitation wavelength and 330 nm emission wavelength for serotonin glucuronide, and the UV absorbance detector was set at 254 nm for acetaminophen. Typical retention times for serotonin glucuronide and acetaminophen were 14 and 20 min, respectively.
2.11. Data analysis.
IC50 values (inhibitor concentration resulting in 50% decrease in enzyme activity) were calculated using Prism Software (San Diego, CA). Results are presented as the mean of triplicate determinations ± standard error (SE) unless described otherwise. Comparisons of relative UGT1A6 activity in PKC / TMED7 co-expression experiments were analyzed by one-way ANOVA followed by a Dunnett’s post hoc test (SigmaStat 3.0, Systat Software, Inc., Chicago, IL).
3. Results
3.1. Screen to identify PKC inhibitors that decrease acetaminophen glucuronidation in intact LS180 cells.
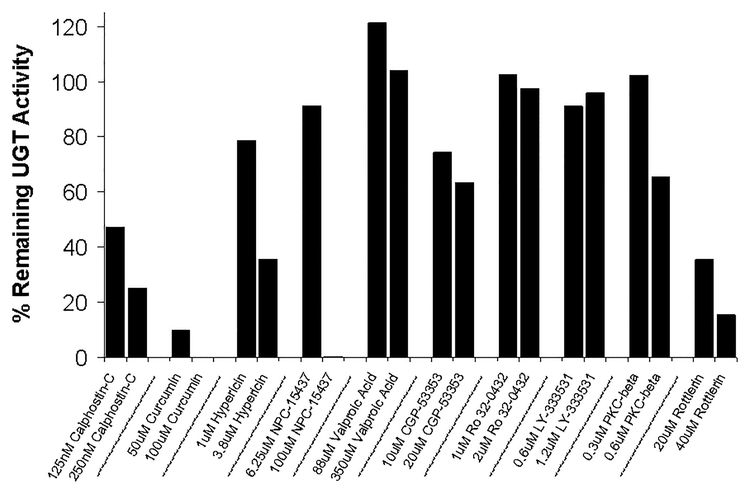
Ten PKC inhibitors with varying PKC-isoform selectivities (based upon IC50 values from the literature or from commercial product information) were initially screened at two concentrations to determine their effect on acetaminophen glucuronidation by LS180 human colon cells. Of these inhibitors, only the pan-PKC inhibitors (calphostin-C, hypericin, and curcumin) and the PKC δ selective inhibitor, rottlerin, were found to inhibit acetaminophen glucuronidation by more than 50% without overt toxicity (Figure 1). Valproic acid, LY-333531, Ro 32–0432, PKC-β inhibitor, and CGP-53353 at the highest concentration tested did not result in substantial (>50%) inhibition of acetaminophen glucuronidation. Although the inhibitors were selected based on previous reports of their limited toxicity in cultured cells, NPC-15437 at 100 μM, but not at 6.3 μM, was found to be toxic to the LS180 cells and so specific effects on PKC θ could not be evaluated. Specifically, after 2 hr of NPC-15437 treatment, the cells detached from the plate and spontaneously lysed after a further 24 hr. Similar results as shown in Figure 1 were observed in a second screen of the PKC inhibitors but with a final DMSO concentration of 1% resulting in reduced acetaminophen glucuronidation in the untreated control (data not shown).
Figure 1. PKC inhibitor screen evaluating the effect of ten different PKC isoform selective and non-selective inhibitors on acetaminophen glucuronidation in LS180 cells.
Calphostin-C, curcumin, hypericin, and valproic acid are considered pan PKC inhibitors, while the other inhibitors selected have some selectivity for specific PKC isoforms. NPC-15437 at 100 μM resulted in the detachment of the LS180 cells from the culture plates after 2 hours with complete cell lysis by 26 hours (overt toxicity). Data points correspond to the mean of duplicate determinations performed on the same day.
3.2. IC50 of PKC inhibitors that decrease acetaminophen glucuronidation in intact LS180.
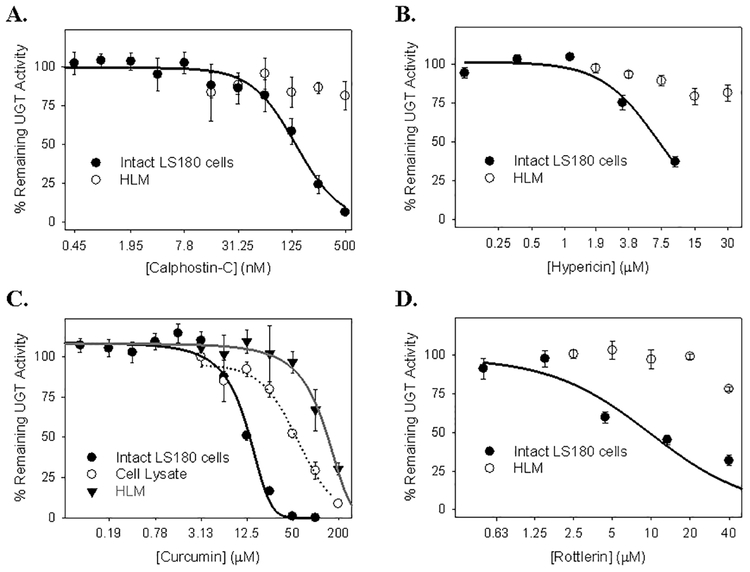
Based on the results from the initial screen, further studies were performed with calphostin-C, hypericin, curcumin, and rottlerin to determine the concentration-dependence of these inhibitors in the LS180 cells. We also determined whether these compounds could directly inhibit acetaminophen glucuronidation by evaluating effects on HLM (and LS180 cell lysates in the case of curcumin) which should lack functional PKC enzymes. As shown in Figures 2A, 2B and 2D, both of the pan-selective PKC inhibitors (calphostin-C and hypericin) as well as the selective PKC δ inhibitor (rottlerin) inhibited acetaminophen glucuronidation in LS180 cells (calphostin-C: IC50 = 140 ± 30 nM; hypericin: IC50 = 7.1 ± 0.6 μM; rottlerin: IC50 = 9.0 ± 1.2 μM) over 3.5 fold more potently than in HLM with IC50 values exceeding the highest evaluated concentration (calphostin-C: IC50 >500 nM; hypericin: IC50 > 30 μM; rottlerin: IC50 > 40 μM). For curcumin, which is a non-selective PKC inhibitor (i.e., it is known to inhibit enzymes other than PKC), it was possible to measure the IC50 value for inhibition of acetaminophen glucuronidation in HLM (IC50 = 133.5 ± 17.9 μM), which was over 10-fold higher (i.e. less potent) than the value determined using intact LS180 cells (IC50 = 12.1 ± 0.4 μM) (Figure 2C). The IC50 value for curcumin inhibition of (untreated) LS180 cell lysates (IC50 = 60.9 ± 12.2 μM) was also more than 5-fold higher than the value for intact LS180 cells.
Figure 2. Concentration-dependent inhibition of acetaminophen glucuronidation by the PKC inhibitors calphostin-C (A), hypericin (B), curcumin (C), and rottlerin (D) in human liver microsomes, LS180 cell lysates, and intact LS180 cells.
In panel A, B, and D, the effect of selective PKC inhibitors, calphostin-C (A) and hypericin (B), and PKC δ selective inhibitor, rottlerin (D) on acetaminophen glucuronidation was evaluated using both intact LS180 cells and human liver microsomes. In panel C, inhibition of acetaminophen glucuronidation by curcumin was compared between intact LS180 cells, homogenates of (washed and untreated) LS180 cells, and human liver microsomes. Data points correspond to the mean ± standard error of three determinations performed on the same day.
3.3. Effect of PKC inhibitors on serotonin glucuronidation by treated UGT1A6-infected Sf9 insect whole cell lysates and HLMs.
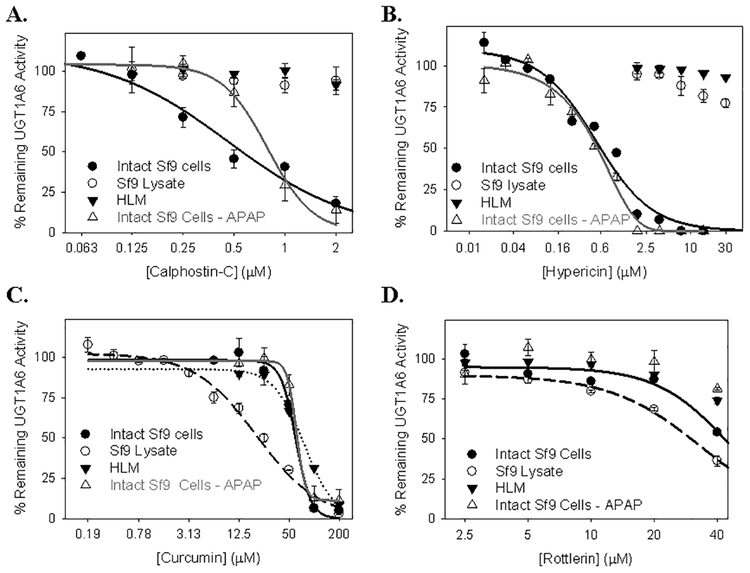
Serotonin glucuronidation was measured using the lysates from insect cells infected with virus expressing UGT1A6 cDNA that were treated with PKC inhibitors either prior to (“treated intact cells”), or following (“treated cell lysates”), cell harvesting and lysis. Both hypericin (IC50 = 0.59 ± 0.05 μM) and calphostin-C (IC50 = 0.5 ± 0.1 μM) were relatively potent inhibitors of serotonin glucuronidation in the treated intact UGT1A6-infected Sf9 cells (Figure 3A and B). Furthermore, this inhibition was more potent in the treated intact UGT1A6-infected Sf9 cells as compared with either UGT1A6-infected Sf9 treated cell lysate or HLM (for each preparation, IC50 >2 μM for calphostin-C and >30 μM for hypericin) (Figure 3A and B). On the other hand, both curcumin and rottlerin were poor inhibitors of serotonin glucuronidation in treated intact UGT1A6-infected Sf9 cells (Figure 3C, D). For rottlerin, IC50 values in all three preparations analyzed (UGT1A6-infected treated Sf9 cells, UGT1A6-infected Sf9 treated lysates, and HLM) were greater than the highest inhibitor concentration that could be tested (40 μM). While curcumin did inhibit serotonin glucuronidation, the potency of inhibition observed in treated intact Sf9 cells (IC50 = 64.9 ± 7.5 μM) was similar to that of HLMs (IC50 = 73.9 ± 1.0 μM) and somewhat less potent than UGT1A6-infected Sf9 treated lysates (IC50 = 31.6 ± 2.4 μM).
Figure 3. Concentration-dependent inhibition of serotonin and acetaminophen (APAP) glucuronidation by the PKC inhibitors calphostin-C (A), hypericin (B), curcumin (C), and rottlerin (D) in human liver microsomes, UGT1A6-expressing Sf9 insect cell lysates, and intact UGT1A6-expressing Sf9 cells.
Sf9 cells were infected with UGT1A6-expressing baculovirus for three days after which the UGT1A6-expressing cells were treated for 2 hours with PKC inhibitor. Cell lysates were then prepared and either serotonin or acetaminophen glucuronidation was monitored ex cellulo. Untreated UGT1A6-expressing cell lysates and pooled HLM were also prepared and subsequently treated with PKC inhibitor to monitor direct inhibition of UGT1A6. Acetaminophen glucuronidation activity was only monitored in intact UGT1A6-expressing Sf9 cells. Data points correspond to the mean ± SE of three independent determinations.
3.4. Effect of PKC inhibitors on acetaminophen glucuronidation by pretreated UGT1A6-infected Sf9 insect cell lysates.
Acetaminophen glucuronidation was also evaluated in UGT1A6-infected Sf9 lysates from cells that had been treated with various PKC inhibitors. In general, the effect of the different PKC inhibitors on acetaminophen glucuronidation was similar to the effect of these agents on serotonin glucuronidation (Figure 3A–D). IC50 values for inhibition of acetaminophen glucuronidation by calphostin-C, hypericin, curcumin, and rottlerin were 0.83 ± 0.10 μM, 0.55 ± 0.04 μM, 58.9 ± 4.5 μM, and > 40 μM, respectively.
3.5. Effect of PKC activators on serotonin glucuronidation by pretreated UGT1A6-infected Sf9 insect cell lysates.
Sf9 cells were treated with the PKC activators, phorbol myristate acetate (PMA) and 1-oleoyl-2-acetyl-sn-glycerol (OAG), previously shown to stimulate PKC activity in other cultured cell lines (Cockerill et al. 2007). No increase in serotonin glucuronidation was observed with 2 hr PMA or OAG treatment relative to untreated UGT1A6-expressing Sf9 cells (data not shown).
3.6. Effect of coexpressing PKC on UGT1A6 enzyme protein expression and activity in HEK293T cells.
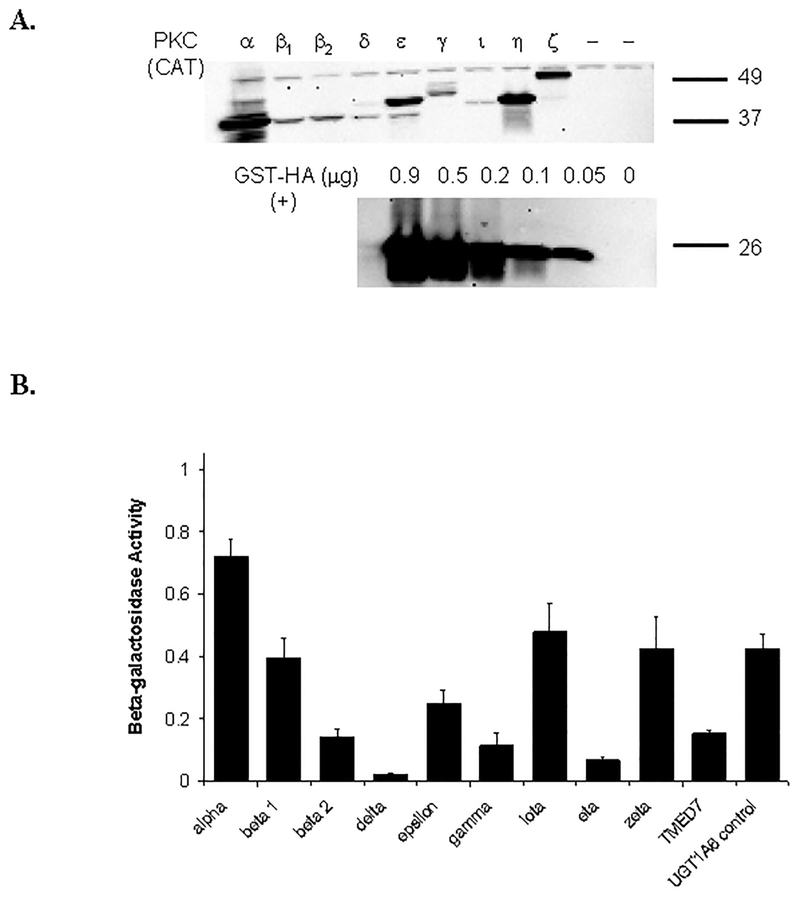
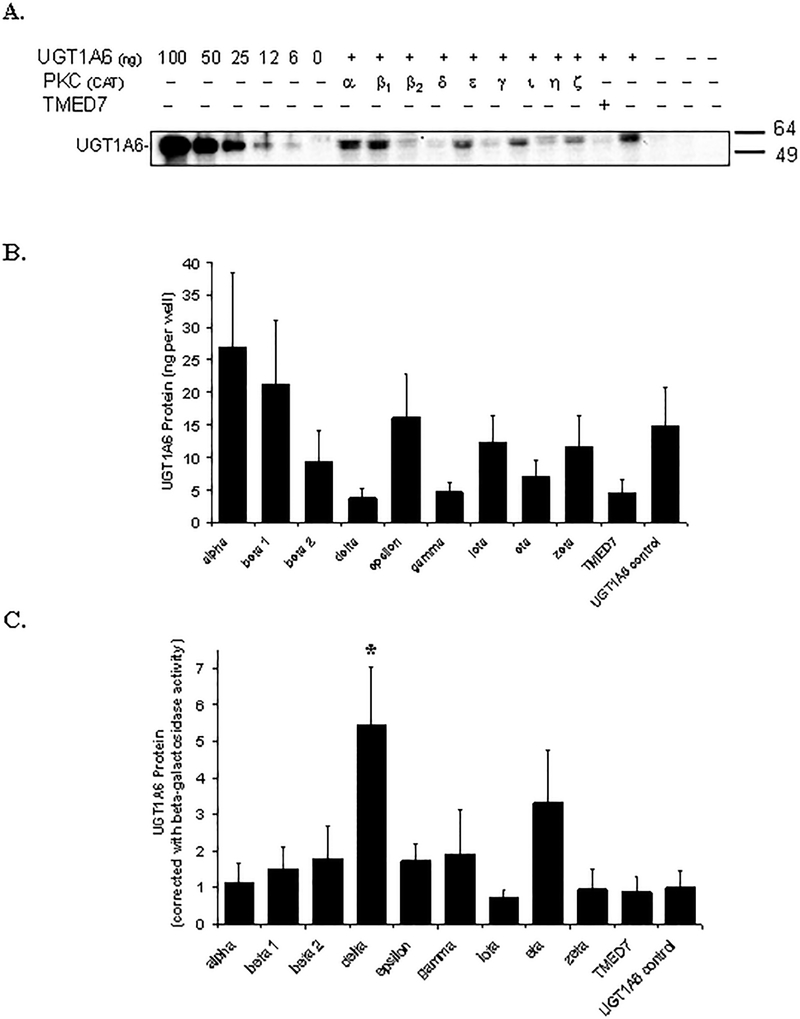
The catalytic domains of nine different PKC isoforms (α, β1, β2, δ, ε, γ, ι, η, and ζ) were transiently coexpressed with UGT1A6 in HEK293T cells to directly investigate their involvement in phosphorylation-dependent activation of UGT1A6 in a mammalian cell culture model. TMED7, a microsomal protein without known PKC activity, was also cotransfected instead of PKC with UGT1A6 as a negative control. As shown in Figure 4A, all nine PKC isoforms (and TMED7) were successfully co-expressed with UGT1A6 in the HEK293T cells, although the relative protein levels of each PKC form varied somewhat. PKC α, ε, η, and ζ had the highest relative expression in the HEK293T cells, followed by PKC β1, β2, and γ. PKC δ and ι were expressed at the lowest levels in all three sets of experiments performed. A fixed amount of β-galactosidase expressing plasmid was also cotransfected along with each PKC isoform, and with UGT1A6 to allow normalization for transfection efficiency differences. Resultant β-galactosidase activities are shown in Figure 4B.
Figure 4. Co-expression of nine different PKC isoforms with UGT1A6 in HEK293T cells.
A) Immunoblot of the catalytic domain of different PKC isoforms (α, β1, β2, δ, ε, γ, ι, η, and ζ) labeled with a hemagglutinin (HA) epitope tag and co-expressed with UGT1A6 (and β-galactosidase as a transfection control) in HEK293T cells. Cell lysates were prepared 48 hours post-transfection and subjected to immunoblot analysis with an anti-HA antibody. Similar results were observed in two other independent experiments. Shown below the PKC blot are the results of blotting different amounts of a positive control protein (glutathione-S-transferase fused with an HA tag). B) β-galactosidase activities measured in the lysates of cells (same as in A) that were cotransfected with plasmids expressing UGT1A6, β-galactosidase, and 9 different PKC isoforms. Instead of PKC, some cells were cotransfected with the PKC vector control (labeled UGT1A6 control) or with a non-specific protein control (labeled TMED7).
A representative UGT1A6 protein blot is shown in Fig. 5A and the relative quantitation data (mean ± SE) from 3 independent experiments is shown in Fig. 5B. The amount of expressed UGT1A6 protein appeared to differ depending on the cotransfected protein (PKC isoforms or TMED7) (Fig. 5A and 5B). Specifically, low UGT1A6 protein was observed when PKC δ, PKC γ, and TMED7 were cotransfected (Figure 5B). However, when results were normalized to the control β-galactosidase activities (Figure 5C), PKC δ and η were associated with increased UGT1A6 protein levels by 5.4- and 3.3-fold, although only the increase in the PKC δ cotransfected cells was statistically significant (P<0.05 vs control, Dunnett’s test).
Figure 5. Effect of overexpression of nine different PKC isoforms on UGT1A6 protein levels in HEK293T cells.
A) Immunoblot of UGT1A6 coexpressed with the catalytic domains of different PKC isoforms (α, β1, β2, δ, ε, γ, ι, η, and ζ) and β-galactosidase (as a transfection control) in HEK293T cells. Instead of PKC, some cells were cotransfected with the PKC vector control (labeled UGT1A6 control) or with a non-specific protein control (labeled TMED7). Cell lysates were prepared 48 hours post-transfection and subjected to immunoblot analysis with an anti-UGT1A peptide antibody. A UGT1A6 standard (6 to 100 ng per lane) provided with the WB-UGT1A6 kit (BD Gentest) was used as a standard for quantitation purposes. Shown is a representative blot from 3 independent experiments. B) Quantification of UGT1A6 protein expression by immunoblot analysis (representative blot shown in A). Data points correspond to the mean ± standard error of three independent experiments performed on separate days. C) UGT1A6 protein data (from B) normalized to β-galactosidase activities (shown in Figure 4B) Data points on B and C correspond to the mean ± SE of three independent experiments performed on separate days. Statistical comparisons were made using one-way ANOVA followed by a Dunnett’s post hoc test. * indicates that the UGT1A6 protein level is significantly different (P<0.05) from the UGT1A6 control value.
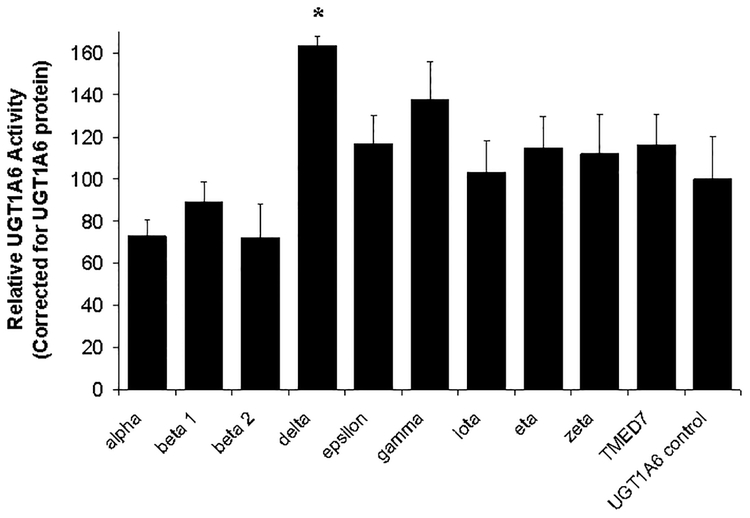
Finally, the effect of cotransfected PKC isoform on the specific activity of UGT1A6 (i.e., serotonin glucuronidation rate normalized to UGT1A6 protein) were evaluated. As shown in Figure 6, only PKC δ cotransfection resulted in a statistically significant effect on serotonin with a 63 ± 4 % increase in specific activity as compared with UGT1A6 expressed alone. The other seven PKC isoforms evaluated as well as TMED7 (negative protein control) had no statistically significant effect on UGT1A6-mediated serotonin glucuronidation. No overt toxicity (such as cell detachment) was observed with the HEK293T cells transiently co-expressing the various PKC isoforms and UGT1A6.
Figure 6. Effect of overexpression of nine different PKC isoforms on UGT1A6 specific activity in HEK293T cells.
The catalytic domains of nine different PKC isoforms (α, β1, β2, δ, ε, γ, ι, η, and ζ) were transiently co-expressed with UGT1A6 in HEK293T cells. Instead of PKC, some cells were cotransfected with the PKC vector control (labeled UGT1A6 control) or with a non-specific protein control (labeled TMED7). Cell lysates were prepared 48 hours post-transfection and assayed for serotonin glucuronidation. Serotonin glucuronidation activities were standardized to UGT1A6 protein (shown in Figure 5) and reported as a percentage of UGT1A6 control (without PKC) activity. Data points correspond to the mean ± standard error of three independent experiments performed on separate days. Statistical comparisons were made using one-way ANOVA followed by a Dunnett’s post hoc test. * indicates that UGT1A6 specific activity is significantly different from the UGT1A6 control value.
4. Discussion
Taken together, the results of the present study identify an important role for PKC δ in the activation of UGT1A6 mediated glucuronidation. Of 10 different PKC inhibitors that we screened, only those predicted to have significant effects on PKC δ, including the PKC δ selective inhibitor, rottlerin as well as three pan-PKC inhibitors, substantially decreased acetaminophen glucuronidation in LS180 cells. All other compounds tested had minimal effect despite using concentrations that were more than 20 times their reported PKC selective IC50 values. The non-selective PKC inhibitor, valproic acid, was not inhibitory and perhaps (retrospectively) should have been tested at higher concentrations than we used here (80 μM and 350 μM) since relatively recent work showed only 40% inhibition of PKC in human astrocytoma cells at 600 μM valproic acid concentration (Kurita et al. 2007). Regardless, three other non-selective PKC inhibitors were also found to have significant inhibitory effects without any overt signs of toxicity. Although a specific toxicity assay was not performed in this study, other reports have demonstrated that calphostin-C up to 1μM, curcumin up to 200μM, and hypericin up to 10μM are not toxic in LS180 cells (Basu et al. 2003; Gutmann et al. 2006). Although effects on LS180 human colon cancer cells have not been reported for rottlerin, in SW480 colon adenocarcinoma cells, 50 μM rottlerin had no effect on the viability of cells after 12 hr treatment as measured by flow cytometry after propidium iodide treatment (Masur et al. 2001). Also, in HT29, HCT8, and HCT116 human colon carcinoma cell lines, 24 hr rottlerin treatment at concentrations up to 10μM did not induce apoptosis as evaluated by Annexin-V-phycoerythrin and 17-aminoactinomycin-D staining (Tillman et al. 2003).
The remaining four compounds tested (CGP-53353, Ro 32–0432, LY-333531, and PKC β inhibitor) did not show significant inhibitory activity at concentrations more than 20 times their reported selective PKC IC50 values. Ro 32–0432 previously was shown to inhibit glucuronidation activity by 30% at 250 nM and between 50 and 62 % at 10 μM in LS180 cells, although it was not clear from the report what glucuronidation substrate was being tested (Basu et al. 2008). The discrepancy between these two studies may be due to both UGT isoform being tested and overall protocol differences; however, considering the IC50 of Ro 32–0432 is 21 nM for PKC α and β1, any effect observed above 250 nM is not likely to be the result of a specific effect on PKC α and β1.
We cannot rule out that the apparently “inactive” inhibitors were ineffective because of extensive metabolism in the LS180 cells, poor penetration to the PKC enzymes within the cells, or lack of expression of the specific PKC isoforms that they affect in LS180 cells. Metabolism of these compounds in LS180 cells has not been evaluated and would require further exploration. CGP-53353, Ro 32–0432, and LY-333531 all have PKC inhibitory activity in cellular assays (Birchall et al. 1994; Buchdunger et al. 1995; Jirousek et al. 1996). The PKC β inhibitor has, to the best of our knowledge, not been tested for PKC inhibition in whole cell assays. Several reports provide evidence that PKC α, β, δ, ε, η, and ζ, but not γ, θ, or μ are expressed in both normal colonic tissue and colon cancer cell lines (Caco-2 and LIM1215) (Abraham et al. 1998; Davidson et al. 1994; Doi et al. 1994; Pongracz et al. 1995; Rickard et al. 2000). However, we were not able to locate selective small molecule inhibitors of ε and η, so effects of these PKC isoforms on acetaminophen glucuronidation activity were not evaluated. Other studies have indicated that PKC ε phosphorylates UGT enzymes in LS180 cells, although the specific UGT isoforms phosphorylated were not evaluated (Basu et al. 2008; Basu et al. 2005).
The PKC δ inhibitor rottlerin demonstrated an IC50 value in intact LS180 cells (9.0 ± 1.2 μM) that was similar to IC50 values established for this compound using recombinant PKC δ (3–6 μM) (Gschwendt et al. 1994). PKC δ is a member of the novel PKC group of isoforms and is activated by diacylglycerol in a calcium-independent manner. Based on expressed sequence tag information in the NCBI database dbEST, PKC δ is expressed in most human tissues including liver, intestine, kidney, and brain, all sites known to express UGT enzymes (http://www.ncbi.nlm.nih.gov/UniGene accessed 7/23/2008). PKC δ protein is expressed in normal human colonic mucosa, bladder, kidney, heart, aorta, and saphenous vein and often at altered levels compared to normal tissues in various cancerous tissues (colonic adenomas, colorectal cancer, bladder transitional cell carcinoma, and pancreatic ductal carcinoma) (Assert et al. 1999; Erdbrugger et al. 1997; Koren et al. 2000; Kuranami et al. 1995; Ozpolat et al. 2007). Furthermore, PKC δ and another UGT enzyme, UGT1A10, were found to co-localize and associate in UGT1A10-transfected COS-1 cells, and phosphorylated PKC δ levels correlate with phosphorylated UGT1A10 levels with curcumin treatment (Basu et al. 2008).
Acetaminophen glucuronidation in LS180 cells is most likely mediated by UGT1A6, although a significant contribution from other UGT isoforms cannot be excluded at this time (Court et al. 2001; Nishimura and Naito 2006). Consequently, we also attempted to evaluate the effects of PKC inhibitors on glucuronidation of serotonin, a validated specific glucuronidation substrate of UGT1A6 (Krishnaswamy et al. 2003a). However, our preliminary evaluation of serotonin glucuronidation by LS180 cells was unsuccessful in that there was rapid disappearance of serotonin and no formation for serotonin glucuronide presumably because of utilization of alternate metabolic pathways such as oxidation by monoamine oxidase A in these cells.
Instead, we next evaluated Sf9 insect cells infected with a baculovirus construct expressing human UGT1A6 cDNA, which is commonly used to produce recombinant UGTs in large quantities for commercial sale. Using this system, serotonin glucuronidation was readily measurable in intact UGT1A6-infected Sf9 cells. Furthermore, we observed concentration dependent decreases in serotonin glucuronidation when cells were treated with the prototypical PKC inhibitor calphostin-C and also hypericin. This is consistent with results from a previous study that showed Sf9 insect cells have low levels of PKC-like enzymes (Geiges et al. 1997). We also found similar inhibition potency (IC50 values) of both calphostin-C and hypericin on acetaminophen glucuronidation by UGT1A6-infected Sf9 cells suggesting shared inhibition mechanism. However, comparing the data for acetaminophen glucuronidation in insect cells and LS180 cells suggests that the insect cell PKC isoform is less susceptible to inhibition by calphostin C with about 4-fold higher IC50 values in insect cells versus LS180 cells. On the other hand, hypericin was a much more potent inhibitor (10-fold lower IC50value) of acetaminophen glucuronidation Sf9 cells compared with LS180 cells, suggesting a sensitivity of insect cell PKC to this compound. Alternately, we cannot exclude the possibility that cell differences in apparent inhibitor potency may be a consequence of differing cell penetration of inhibitors.
Sf9 cells were treated with the PKC activators, phorbol myristate acetate (PMA) and 1-oleoyl-2-acetyl-sn-glycerol (OAG), previously shown to stimulate PKC activity in other cultured cell lines (Cockerill et al. 2007). However no effect was observed perhaps because insect cell expressed UGT1A6 is maximally phosphorylated, or there may be differences in the sensitivity of insect cell PKC enzymes to these compounds.
Recent work suggests that rottlerin may not be as selective a PKC- δ inhibitor as was once assumed (Soltoff 2007). Consequently we sought to confirm a role for PKC- δ in UGT1A6 activation via other approaches. We transiently coexpressed UGT1A6 with the catalytic domains of nine different PKC isoforms (α, β1, β2, δ, ε, γ, ι, η, and ζ) in HEK293T cells. Differences in the protein levels of each PKC isoform were observed similar to that previously reported by Soh and Weinstein (Soh and Weinstein 2003). Possible reasons for these differences include differences in transfection efficiency, nonspecific effects of PKC overexpression on gene expression, and differences in PKC protein stability. Qualitative comparisons with cotransfected β-galactosidase activity values indicated that these differences might result from differences in transfection efficiency or possibly nonspecific inhibitory effects on gene expression. For example, PKC α had the highest, while PKC δ had the lowest of protein content and β-galactosidase activities (Fig. 4). Various PKC isoforms are known to be mediators of gene expression in a variety of tissues ultimately resulting in cell growth and differentiation (Mackay and Twelves 2007; Takai et al. 1979). Consequently, overexpression of some PKC isoforms may have had nonspecific effects on both β-galactosidase and UGT1A6 expression accounting for parallel effects on UGT1A6 protein levels and β-galactosidase activity. On the other hand, expression of some PKC isoforms clearly did not covary with β-galactosidase activity in that PKC η had high expression with low β-galactosidase activity, while PKC ι had low expression with high β-galactosidase activity. These latter differences could be the result of differences in protein stability of the different PKC isoforms, especially since we are expressing a part of the protein containing the catalytic domain rather than the entire protein, which also contains a regulatory domain. Although we did not test the catalytic activity of each PKC isoform in our laboratory, all of these protein domain constructs have been previously verified as active using an in vitro kinase activity assay (Soh and Weinstein 2003).
Coexpression of PKC δ resulted in over 5-fold higher UGT1A6 protein levels (normalized to β-galactosidase activity) compared with the UGT1A6 control (Fig. 5C). We speculate that this result could be explained by protein-protein interaction and/or phosphorylation of UGT1A6 by PKC δ resulting in stabilization of UGT1A6 protein, retardation of protein degradation and subsequently higher levels measured by immunoblotting. No other PKC isoform (or the nonspecific protein TMED7) affected normalized UGT1A6 protein levels suggesting that the effect was specific to PKC δ. A significant enhancement (65% increase) of UGT1A6 specific activity (i.e., serotonin glucuronidation rate normalized to UGT1A6 protein level) was also observed for the PKC δ cotransfected samples, without significant effect of any other PKC isoform (or the nonspecific protein TMED7). In a previous study, PKC δ was shown to co-localize and associate with UGT1A10 (Basu et al. 2008). Although we do not as yet have evidence for direct interaction (such as through immunoprecipitation or colocalization experiments), the present study suggests that UGT1A6 is an important modulator of UGT1A6 function.
When interpreting the PKC-UGT1A6 coexpression data, the constitutive levels of the various PKC isoforms expressed in the HEK293T cells also must be considered. PKC α, β1, β2, δ, ε, and ζ (PKC ι not studied) have been shown to be expressed in HEK293T cells (Kuriyama et al. 2004). Consequently, it is possible that there is already sufficient constitutive activity of these PKC isoforms in the HEK293T cell lysates such that any additional increase in PKC with overexpression would not affect UGT1A6 phosphorylation. Consequently, a role for other PKC isoforms in UGT1A6 activation cannot be excluded. A cell line without significant PKC activity might be of better utility in this type of overexpression study, or alternatively, siRNA knockdown of specific PKC isoforms or perhaps coexpression of dominant negative mutant PKC isoforms could be performed to investigate these possibilities. Another potential limitation in relating these in vitro results to the in vivo situation is that the mouse form of the PKC δ catalytic domain we used in this study has 89% homology to the human form as opposed to the other rodent PKC isoforms we used that all have more than 98% amino acid sequence homology. Consequently, future studies are needed to evaluate the putative role of the human PKC δ isoform in UGT1A6 phosphorylation and activity.
This work has several implications to the field of drug metabolism if found to extrapolate to humans. Firstly, in vitro drug-drug interaction studies examining inhibition of UGT enzymes by a new chemical entity may need to be carried out in intact cells (such as hepatocytes) as well as isolated membrane fractions (i.e. HLM) otherwise inhibition of UGT enzymes via PKC or other kinase inhibition may be missed. Secondly, compounds with PKC δ inhibitory activity such as KAI-9803, which is being evaluated for the treatment of reperfusion injury following acute myocardial infarction, may potentially impair the metabolism of drugs requiring UGT1A6-mediated glucuronidation (Bates et al. 2008). Finally, PKC modulation of UGT activity may be just one part of a complex kinase mediated regulation of drug-metabolizing enzymes possibly explaining variations observed in not only UGT but also cytochrome P450 mediated metabolism between individuals.
In conclusion, the results of this study are the first to show that glucuronidation by UGT1A6 can be modulated by PKC inhibitors as well as by overexpression of PKC δ in various mammalian and insect cell model systems thereby implicating a role for PKC in UGT1A6 mediated metabolism. Further work will be needed to substantiate the relevance of these in vitro findings to drug-drug interactions in vivo.
5. Acknowledgments
The authors would like to thank Dr. Jae-Won Soh, Inha University (Incheon, Korea) for providing the pHACE and pHANB vectors containing the catalytic domain of the nine different PKC isoforms used in this study. This publication was made possible by predoctoral training grant F31AT003973 from the National Center for Complementary and Alternative Medicine (NCCAM), National Institutes of Health (Bethesda, MD) to L.P.V. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health. Other support was also provided by grant R01GM061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD) to M.H.C.
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
References
- Abraham C, Scaglione-Sewell B, Skarosi SF, Qin W, Bissonnette M, Brasitus TA. (1998). Protein kinase C alpha modulates growth and differentiation in Caco-2 cells. Gastroenterology 114:503–509. [DOI] [PubMed] [Google Scholar]
- Assert R, Kotter R, Bisping G, Scheppach W, Stahlnecker E, Muller KM, Dusel G, Schatz H, Pfeiffer A. (1999). Anti-proliferative activity of protein kinase C in apical compartments of human colonic crypts: evidence for a less activated protein kinase C in small adenomas. Int J Cancer 80:47–53. [DOI] [PubMed] [Google Scholar]
- Basu NK, Kole L, Basu M, Chakraborty K, Mitra PS, Owens IS. (2008). The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J Biol Chem 283:23048–23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu NK, Kole L, Owens IS. (2003). Evidence for phosphorylation requirement for human bilirubin UDP-glucuronosyltransferase (UGT1A1) activity. Biochem Biophys Res Commun 303:98–104. [DOI] [PubMed] [Google Scholar]
- Basu NK, Kovarova M, Garza A, Kubota S, Saha T, Mitra PS, Banerjee R, Rivera J, Owens IS. (2005). Phosphorylation of a UDP-glucuronosyltransferase regulates substrate specificity. Proc Natl Acad Sci U S A 102:6285–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu NK, Kubota S, Meselhy MR, Ciotti M, Chowdhury B, Hartori M, Owens IS. (2004c). Gastrointestinally distributed UDP-glucuronosyltransferase 1A10, which metabolizes estrogens and nonsteroidal anti-inflammatory drugs, depends upon phosphorylation. J Biol Chem 279:28320–28329. [DOI] [PubMed] [Google Scholar]
- Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, Grimes K, Harrington R, Huber K, Kleiman N and others. (2008). Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation 117:886–896. [DOI] [PubMed] [Google Scholar]
- Birchall AM, Bishop J, Bradshaw D, Cline A, Coffey J, Elliott LH, Gibson VM, Greenham A, Hallam TJ, Harris W and others. (1994). Ro 32–0432, a selective and orally active inhibitor of protein kinase C prevents T-cell activation. J Pharmacol Exp Ther 268:922–929. [PubMed] [Google Scholar]
- Bock KW, Kohle C. (2005). UDP-glucuronosyltransferase 1A6: structural, functional, and regulatory aspects. Methods Enzymol 400:57–75. [DOI] [PubMed] [Google Scholar]
- Brooks B, Delaney-Robinson C, Molyneaux L, Yue DK. (2008). Endothelial and neural regulation of skin microvascular blood flow in patients with diabetic peripheral neuropathy: effect of treatment with the isoform-specific protein kinase C beta inhibitor, ruboxistaurin. J Diabetes Complications 22:88–95. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Mett H, Trinks U, Regenass U, Muller M, Meyer T, Beilstein P, Wirz B, Schneider P, Traxler P and others. (1995). 4,5-bis(4-fluoroanilino)phthalimide: A selective inhibitor of the epidermal growth factor receptor signal transduction pathway with potent in vivo antitumor activity. Clin Cancer Res 1:813–821. [PubMed] [Google Scholar]
- Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. (2007). Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol 581:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court MH. (2001). Acetaminophen UDP-glucuronosyltransferase in ferrets: species and gender differences, and sequence analysis of ferret UGT1A6. J Vet Pharmacol Ther 24:415–422. [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI. (2001). Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299:998–1006. [PubMed] [Google Scholar]
- Davidson LA, Jiang YH, Derr JN, Aukema HM, Lupton JR, Chapkin RS. (1994). Protein kinase C isoforms in human and rat colonic mucosa. Arch Biochem Biophys 312:547–553. [DOI] [PubMed] [Google Scholar]
- Doi S, Goldstein D, Hug H, Weinstein IB. (1994). Expression of multiple isoforms of protein kinase C in normal human colon mucosa and colon tumors and decreased levels of protein kinase C beta and eta mRNAs in the tumors. Mol Carcinog 11:197–203. [DOI] [PubMed] [Google Scholar]
- Erdbrugger W, Keffel J, Knocks M, Otto T, Philipp T, Michel MC. (1997). Protein kinase C isoenzymes in rat and human cardiovascular tissues. Br J Pharmacol 120:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiges D, Meyer T, Marte B, Vanek M, Weissgerber G, Stabel S, Pfeilschifter J, Fabbro D, Huwiler A. (1997). Activation of protein kinase C subtypes alpha, gamma, delta, epsilon, zeta, and eta by tumor-promoting and nontumor-promoting agents. Biochem Pharmacol 53:865–875. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. (1994). Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199:93–98. [DOI] [PubMed] [Google Scholar]
- Gutmann H, Poller B, Buter KB, Pfrunder A, Schaffner W, Drewe J. (2006). Hypericum perforatum: which constituents may induce intestinal MDR1 and CYP3A4 mRNA expression? Planta Med 72:685–690. [DOI] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. (2004). Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14:225–238. [DOI] [PubMed] [Google Scholar]
- Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH 3rd, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A and others. (1996). (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-d ione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem 39:2664–2671. [DOI] [PubMed] [Google Scholar]
- Koren R, Langzam L, Paz A, Livne PM, Gal R, Sampson SR. (2000). Protein kinase C (PKC) isoenzymes immunohistochemistry in lymph node revealing solution-fixed, paraffin-embedded bladder tumors. Appl Immunohistochem Mol Morphol 8:166–171. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Duan SX, Von Moltke LL, Greenblatt DJ, Court MH. (2003a). Validation of serotonin (5-hydroxtryptamine) as an in vitro substrate probe for human UDP-glucuronosyltransferase (UGT) 1A6. Drug Metab Dispos 31:133–139. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Duan SX, Von Moltke LL, Greenblatt DJ, Sudmeier JL, Bachovchin WW, Court MH. (2003b). Serotonin (5-hydroxytryptamine) glucuronidation in vitro: assay development, human liver microsome activities and species differences. Xenobiotica 33:169–180. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Hao Q, Al-Rohaimi A, Hesse LM, von Moltke LL, Greenblatt DJ, Court MH. (2005a). UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S). J Pharmacol Exp Ther 313:1340–1346. [DOI] [PubMed] [Google Scholar]
- Kuranami M, Powell CT, Hug H, Zeng Z, Cohen AM, Guillem JG. (1995). Differential expression of protein kinase C isoforms in human colorectal cancers. J Surg Res 58:233–239. [DOI] [PubMed] [Google Scholar]
- Kurita M, Nishino S, Ohtomo K, Rai M, Shirakawa H, Mashiko H, Niwa S, Nakahata N. (2007). Sodium valproate at therapeutic concentrations changes Ca2+ response accompanied with its weak inhibition of protein kinase C in human astrocytoma cells. Prog Neuropsychopharmacol Biol Psychiatry 31:600–604. [DOI] [PubMed] [Google Scholar]
- Kuriyama M, Taniguchi T, Shirai Y, Sasaki A, Yoshimura A, Saito N. (2004). Activation and translocation of PKCdelta is necessary for VEGF-induced ERK activation through KDR in HEK293T cells. Biochem Biophys Res Commun 325:843–851. [DOI] [PubMed] [Google Scholar]
- Mackay HJ, Twelves CJ. (2007). Targeting the protein kinase C family: are we there yet? Nat Rev Cancer 7:554–562. [DOI] [PubMed] [Google Scholar]
- Masur K, Lang K, Niggemann B, Zanker KS, Entschladen F. (2001). High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol Biol Cell 12:1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Naito S. (2006). Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet 21:357–374. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Akar U, Mehta K, Lopez-Berestein G. (2007). PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy 3:480–483. [DOI] [PubMed] [Google Scholar]
- Pongracz J, Clark P, Neoptolemos JP, Lord JM. (1995). Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int J Cancer 61:35–39. [DOI] [PubMed] [Google Scholar]
- Rickard KL, Gibson PR, Wilson NJ, Mariadason JM, Phillips WA. (2000). Short-chain fatty acids reduce expression of specific protein kinase C isoforms in human colonic epithelial cells. J Cell Physiol 182:222–231. [DOI] [PubMed] [Google Scholar]
- Soh JW, Weinstein IB. (2003). Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem 278:34709–34716. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. (2007). Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci 28:453–458. [DOI] [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. (1979). Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem 254:3692–3695. [PubMed] [Google Scholar]
- Tillman DM, Izeradjene K, Szucs KS, Douglas L, Houghton JA. (2003). Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res 63:5118–5125. [PubMed] [Google Scholar]
- Volak LP, Ghirmai S, Cashman JR, Court MH. (2008). Curcuminoids inhibit multiple human cytochromes P450 (CYP), UDP-glucuronosyltransferase (UGT), and sulfotransferase (SULT) enzymes, while piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos 36:1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke LL, Greenblatt DJ, Harmatz JS, Shader RI. (1993). Alprazolam metabolism in vitro: studies of human, monkey, mouse, and rat liver microsomes. Pharmacology 47:268–276. [DOI] [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. (2004). Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos 32:1201–1208. [DOI] [PubMed] [Google Scholar]