Abstract
Epithelial ovarian cancer accounts for more than 90% of ovarian tumours and continues as a leading cause of death from gynaecological malignancies. It is often difficult to differentiate a benign ovarian mass from malignant ones. Invasive histopathological biopsy is used as the gold standard diagnostic tool to diagnose cancer in patients with ovarian mass. A wide spectrum of Biomarkers were tried in various studies to develop a non invasive diagnostic tool, out of which HE4 and CA 125 remain the only clinically useful biomarker. Consequently various Biomarker based algorithms i.e. Risk of Malignancy Index, risk of ovarian cancer algorithm, OVA1, risk of malignancy algorithm were generated that have been developed to assess the risk of a mass being malignant. These algorithms help in timely triage of patients. Recently in 2016 FDA cleared Ova1 test (OVERA) with CA 125-II, HE4, apolipoprotein A-1, FSH, and transferring (Sensitivity 91% and Specificity 69%) as a referral or Triage test in patients presenting with ovarian mass. Combination of protein and circulating Micro RNA analysis in blood, could provide a comprehensive screening and diagnostic panel, in management of patients presenting with ovarian mass in one clinical setting.
Keywords: Epithelial ovarian cancer, Biomarkers, Biomarker based algorithms, Circulating Micro RNA
Introduction
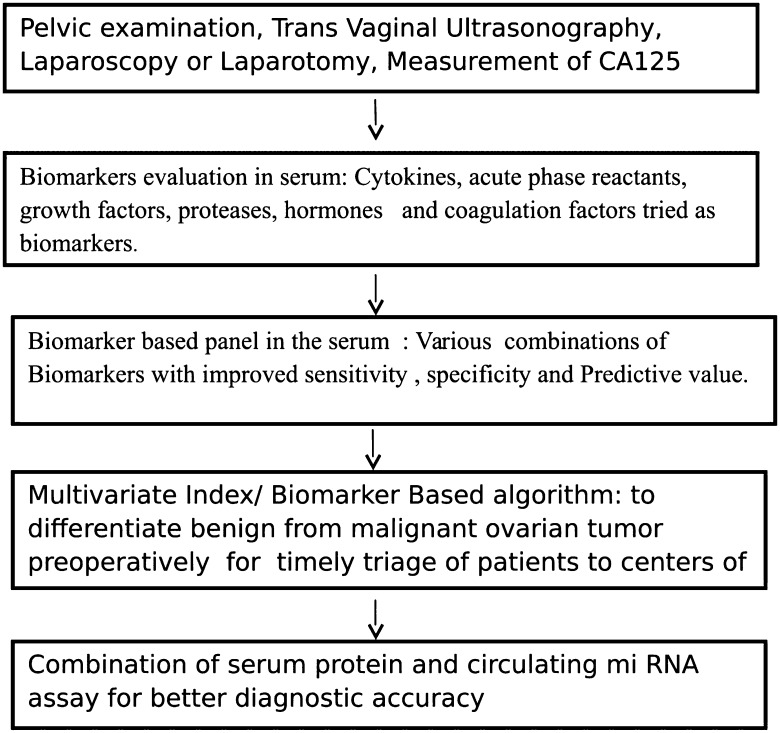
Ovarian cancer (Epithelial cell tumours, Stromal tumours, Germ cell tumours etc.) accounts for approximately 4% of all cancers among women around the world having highest death rate of all gynaecological malignancies. Unfortunately almost 85 percent of women with the common epithelial ovarian cancer are not diagnosed in early stage due to lack of symptoms and its location deep inside the pelvis, that together accounts for its higher mortality rate. If diagnosis would be possible in the initial stage, when cancer is limited to the ovaries, up to 90% of the ovarian cancers can be treated successfully [1]. The Current approach to women with Ovarian mass is based on laparoscopy or laparotomy, pelvic examination, trans-vaginal ultrasonography, in asymptomatic women to establish a diagnosis of ovarian cancer preoperatively [2]. It is often difficult to differentiate a benign ovarian mass from malignant ones. Invasive histopathological biopsy can only discriminate a malignant ovarian mass from a benign, that is used as the gold standard diagnostic tool in patients with ovarian mass [3]. Translational research advancements mostly aim to detect ovarian cancer preoperatively by detecting biomarkers in body fluid i.e. serum or urine.
Till date, no single biomarker displays high sensitivity and specificity to detect early Ovarian Cancer and the implementation of a panel of biomarkers is not yet feasible in clinical practice [4]. In the present review, an overview of the development of biomarker research will be highlighted starting from the use of single biomarker to integration of biomarkers to develop a panel and currently the trend of biomarker-based algorithms.
Single Marker Diagnostics
Many cytokines, acute phase reactants, growth factors, proteases, hormones and coagulation factors were explored as non invasive biomarkers to investigate a case of Ovarian cancer [5, 6]. Currently CA 125 and Human Epididymis protein 4 (HE4) are the only two markers that have been approved by the FDA for monitoring treatment and detecting disease recurrence [7].
CA 125
CA 125 a glycoprotein produced by coelomic epithelium, is routinely used as a proteomic marker for the papillary serous adenocarcinoma of ovary. It has a poor diagnostic specificity, as its level is also increased in non malignant conditions like Cirrhosis of liver, endometriosis, Interstitial lung disease, TB abdomen as well as in benign or malignant diseases affecting pleura, pericardium and peritoneum, that derive from coelomic epithelium. Few non-ovarian malignancies including cervix, breast, colon, pancreatic, lung, gastric and liver cancers also documented raised levels of CA 125 [8, 9]. Inflammatory conditions like rheumatoid arthritis, scleroderma, lupus and Sjogren’s syndrome also registered elevated levels of it. CA 125 also has poor sensitivity in premenopausal women presenting with ovarian mass, being elevated in only 50% of ovarian cancer patients in stage-1, rendering itself rather a useful prognostic marker in ovarian cancer [10].
HE4
Human Epididymis secretory protein 4 (HE4) is a member of the Whey acidic protein gene family expressed in normal tissues of the reproductive and respiratory tract. It is among the most frequently up-regulated genes in epithelial ovarian carcinomas based on gene expression profiles [11]. A single peptide and two whey acidic protein (WAP) domains containing a “four disulfide core” with eight cysteine residues constitute its secretory form which is approximately of 25 kDa. Among the series of studies conducted in ovarian cancer, HE4 has emerged as the most promising new biomarkers. Case control studies comparing the diagnostic accuracy of HE4 with clinically routinely used marker CA 125, have demonstrated that HE4 is more useful in the differential diagnosis of ovarian masses [12, 13]. However these biomarkers individually lack high sensitivity and specificity to meet the clinical standards. Hence focus has shifted to use panel of biomarkers with better sensitivity and specificity.
Biomarker Based: Panel Assay
Recent studies used a combination of CA 125, HE4 and menopausal status to predict the presence of a malignant ovarian tumour [14]. When CA 125 was combined with HE4, the prediction rate was higher, showing a sensitivity for detecting malignant disease of 76.4% at a specificity of 95% [15]. Moore and colleagues investigated 9 novel biomarkers alone and in different combinations in patients with ovarian cancer. The combination of HE4 and CA 125 resulted in highest sensitivity and concluded that combination of markers may be useful in the triage of women with ovarian mass to appropriate centre of care [16]. Macuks et al. [17] in their study at Latvian Oncology Centre, Riga, Latvia concluded that Apolipoprotein A1 in combination with CA 125 could improve ovarian cancer detection. Simmons AR analyzed 4-marker panel comprising CA 125, HE4, MMP-7, and CA72-4 and reported that multiplex panel is suitable for the early detection of ovarian cancer and longitudinal algorithm could be developed, as the individual markers have their own baseline. Encouraging findings from these studies revealed that biomarkers can also distinguish benign from malignant ovarian mass. Consequently the development of Biomarker based algorithm started (Fig. 1) that have been developed combining the age, imaging findings and biomarker into a single value to assess the risk of a mass being malignant [18].
Fig. 1.
Development in the field of biomarkers in ovarian cancer
Multivariate Index/Biomarker Based Algorithm
Risk of malignancy index (RMI) was devised utilizing ultrasound, menopausal status and serum CA 125 with a sensitivity from 71 to 88% and specificity from 97 to 74% as reported by various studies [19]. RMI is used throughout the United Kingdom. Advancements in proteomics led to identification of seven biomarkers that distinguished benign from malignant pelvic masses [19]. “Risk of Ovarian Cancer algorithm” (ROCA) was developed few years later [20]. This algorithm compared the CA 125 profile of cases to that of Healthy Control and calculated the risk of developing cancer. Accordingly, in ROCA mathematical model included CA 125 changes over time and the woman’s age. Successive studies were conducted to investigate the performance of ROCA and the reported specificity was 99.8% and the positive predictive value was between 35.1 and 37.5%.
Food and Drug Administration (FDA) approval has been obtained for the OVA1 panel that includes CA 125 and Apolipoprotein A1, Transthyretin, Transferrin and β2-microglobulin. OVA1 provided 96% sensitivity at 28% specificity in post-menopausal women and 85% sensitivity at 40% specificity for pre-menopausal women. The OVA1 multivariate index involved imaging and menopausal status in addition to levels of these five biomarkers. The diagnostic efficacy was investigated in a study in which 53% of participants were enrolled by non-gynecologic oncologists. At surgery there were 363 benign tumors and 161 malignancies of which 151 were ovarian cancers. The OVA1 detected 76% of the malignancies that had been missed by CA 125. The OVA1 algorithm exhibited greater sensitivity, lower specificity than physician assessment. Addition of the OVA1 panel improved the sensitivity from 78 to 98%, but decreased specificity from 75 to 26%. Moore et al. [20] found highest area under a Receiver Operator Characteristic curve (91.4%) with a combination of CA 125 and HE4. Using data from this pilot trial,a risk of malignancy algorithm (ROMA) was developed by Skates and Moore, incorporating CA 125, HE4 and menopausal status, imaging data were not included [21] premenopausal and for postmenopausal women with pelvic masses had separate logistical formulas, assigning them to high and low risk groups. Overall, the ROMA algorithm yielded 93% sensitivity at 75% specificity with a negative predictive value of 93–94%. ROMA was compared to the RMI and was found superior with 76% sensitivity obtained at 75% specificity in premenopausal patients [21]. ROMA achieved 94% sensitivity and the RMI 85% at 75% specificity. This was particularly evident in stage I and II cancers, where ROMA detected 85% and RMI 65% (P < 0.0001).ROMA algorithm was trialed in another study in which algorithm provided 94% sensitivity and 75% specificity overall (Table 1). In premenopausal patients, sensitivity was 100% in this particular study. The negative predictive value was 98%. Several onward studies documented mixed results. ROMA recently achieved approval by the FDA in the United States.
Table 1.
Biomarker and biomarker based algorithms
| Serial number | Proteomic marker/algorithm | Sensitivity/specificity | State of the art |
|---|---|---|---|
| 1 | CA 125 | Not detected in 30–35% cancer ovary | Gold standard proteomic marker |
| Not specific in premenopausal women | |||
| 2 | Risk of malignancy index (RMI) With CA 125, menopausal status and ultrasound | Sensitivity 71–88% | Used in United Kingdom and multiple studies confirmed its value |
| Specificity 74% | |||
| 3 | OVA 1 panel with CA 125, ApoA1, Transthyretin, Transferrin and β2 microglobulin | 96% sensitivity at 28% specificity in post menopausal women 85% sensitivity at 40% specificity in pre menopausal women | USA FDA approval obtained |
| 4 | ROMA (Risk of Malignancy algorithm) with CA 125, HE4 and menopausal status | 93% sensitivity at 75% specificity with a negative predictive value | Recently achieved FDA approval in United states |
Recently in 2016 FDA cleared Ova1 test (OVERA) with CA 125-II, HE4, apolipoprotein A-1, FSH, and transferring (Sensitivity 91% and Specificity 69%) as a referral or Triage test in patients presenting with ovarian mass [22]. Copenhagen Index (CPH-I) comprising HE4, CA 125 and age [23] was generated based on a study by Karlsen et al. CPH-I was then validated in successive international studies showing similar performance of CPH-I to that of ROMA and RMI (AUC: 0.960 vs. 0.954 and 0.959). CPH-I index had the advantage to be independent of ultrasound and menopausal status.
A prospective study with an objective to compare the performance of CA 125, HE4, ROMA, CPH-I and RMI in discriminating benign from malignant ovarian tumor showed Area Under the Curve of 0.920 for CA 125, 0.933 for HE4, 0.946 for ROMA, 0.959 for CPH-I and 0.958 for RMI [24].
Future Perspective
Nucleic acids are promising as a new group of serum markers including free DNA, mRNA, microRNAs, and circulating tumor DNA (ctDNA) [25]. Circulating miRNAs (miRs) are small non-coding,highly conserved RNA molecules that bind and post-transcriptionally regulate messenger RNAs (mRNAs). miRs play a key role in expression of their target mRNAs that facilitate tumor growth, invasion, angiogenesis, and immune evasion [26]. Measurement of these Circulating mi RNAs in serum is a potential non invasive approach for early diagnosis of ovarian cancer. Being stable in serum and resistant to endogenous endonuclease activity,combination of protein and nucleic acid marker analysis may provide comprehensive screening and diagnostic panel in one setting. Recent studies have demonstrated dysregulation of expression of several miRs involved in Ovarian Cancer pathways. The aberrant miRs expression has been demonstrated in gynecological cancers, in both tissues and serum samples. Though the potential clinical utility has been demonstrated, none of these miRs has been validated in large Ovarian Cancer populations [27]. Recently the expression of circulating miR-200a, miR-200b and miR-200c were found up-regulated (P < 0.05) in ovarian cancer compared to controls, correlated with the stage of disease and reflected tissue expression with no significant increase in the expression of miR-21 and miR-210 [28].
Shapira et al. [29] defined a 22-miRs profile to distinguish between OC and healthy controls and a 6-miRs profile to distinguish benign and OC patients. Whereas another study demonstrated that the combination of miR-200a, miR-200b and miR-200c displayed a sensitivity of 83% and a specificity of 100%, to differentiate malignant from benign ovarian tumors [30].
Conclusion
A wide spectrum of Biomarkers were tried in various studies to explore a stable, highly specific and sensitive diagnostic tool with cost effectiveness. HE4 and CA 125 remain the only biomarkers approved and applied in clinical setting. Despite the potential clinical utility of the circulating miRs, the most of these have not been validated in large population. So, further investigations are needed to verify diagnostic performance with validation in larger populations.
Conflict of interest
Author declares that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by the authors.
Human and Animal Right Statement
For this type of study formal consent is not required. This article does not contain any studies with human participants or animals performed by the authors.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Martina M, Elisa D, Orazio R, Valentina B, Teresita N, Matte G, et al. The ROMA (Risk of ovarian Malignancy algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med. 2011;49(3):521–525. doi: 10.1515/CCLM.2011.075. [DOI] [PubMed] [Google Scholar]
- 3.Perez LFR, Chedraui P, Troyano LJM. Peri and post- menopausal incidental adnexal masses and the risk of sporadic ovarian malignancy, new insight and clinical management. Gynecol Endocrinol. 2010;26:631–643. doi: 10.3109/09513590.2010.487611. [DOI] [PubMed] [Google Scholar]
- 4.Robert CBJ, Steven S, Anna L, Richard GM. Differential diagnosis of a pelvic mass: improved algorithms and novel biomarkers. Int J Gynecol Cancer. 2012;22(1):S5–S8. doi: 10.1097/IGC.0b013e318251c97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, Bast RC, Jr, et al. Serum biomarker panel for the determination of Benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010;117(3):440–445. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van GT, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA 125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of ovarian Malignancy algorithm. Br J Cancer. 2011;104(5):863–870. doi: 10.1038/sj.bjc.6606092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YM, Whang DH, Park J, Kim SH, Lee SW, Park HA, et al. Evaluation of the accuracy of human epididymis protein 4(HE4) in combination with CA 125 for detecting ovarian cancer-a prospective case control study in a Korean population. Clin Chem Med. 2011;49(3):527–534. doi: 10.1515/CCLM.2011.085. [DOI] [PubMed] [Google Scholar]
- 8.Bandiera E, Romani C, Specchia C, Zanoti L, Galli C, Ruggeri G, et al. Serum human epididymis protein 4(HE4) and risk of ovarian malignancy algorithm (ROMA) as new diagnostic and prognostic tool for epithelial ovarian cancer management. Cancer Epidemiol Biomark Prev. 2011;20(12):2496–2506. doi: 10.1158/1055-9965.EPI-11-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su F, Kozak KR, Imaizumi S, Satoshi I, Gaoa F, Malaika WA, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Nat Acad Sci USA. 2010;107(46):19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takaishi S, Wang TC. Gene expression profiling in a mouse model of helicobacter-induced gastric cancer. Cancer Sci. 2007;98:284–293. doi: 10.1111/j.1349-7006.2007.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosov V, Su F, Amneus M, Birrer M, Robins T, Kotlerman J, et al. Validation of serum biomarkers for detection of early stage ovarian cancer. Am J Obstet Gynecol. 2009;200(6):639.e1–639.e5. doi: 10.1016/j.ajog.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Tuft SH, Nymoen DA, TE Hetland F, Kaern J, Trope CG, Davidson B. APOA1 mRNA expression in ovarian serous carcinoma effusions is a marker of longer survival. Am J Clin Pathol. 2014;142(1):51–57. doi: 10.1309/AJCPD8NBSHXRXQL7. [DOI] [PubMed] [Google Scholar]
- 13.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumour biomarkers for the detection of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Barekati Z, Kohler C. Proteomics and biomarkers for ovarian cancer diagnosis. Ann Clin Lab Sci. 2010;40:218–225. [PubMed] [Google Scholar]
- 15.Frederick RU. A perspective on ovarian cancer biomarkers: past, present and yet-to-come. Diagnostics (Basel) 2017;7(1):14. doi: 10.3390/diagnostics7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011;118(2 Pt 1):280–288. doi: 10.1097/AOG.0b013e318224fce2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macuks R, Baidekalna I, Gritcina J, Avdejeva A, Donina S. Apolipoprotein A1 and transferrin as biomarker in ovarian cancer diagnostics. Acta Chirurgica Latviensis. 2010;10(2):16–20. doi: 10.2478/v10163-011-0003-3. [DOI] [Google Scholar]
- 18.Brian MN, Anna EL. Biomarker testing for ovarian cancer: clinical utility of multiplex assays. Mol Diagn Ther. 2013;17(3):139–146. doi: 10.1007/s40291-013-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang WL, Zhen L, Robert CB. The role of biomarkers in the management of epithelial ovarian cancer. Expert Rev Mol Diagn. 2017;17(6):577–591. doi: 10.1080/14737159.2017.1326820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meredith CH, Michael S, Sherri B, Quynh T, Elias L, Malpuri R, et al. A combinatorial proteomic biomarker assay to detect ovarian cancer in women. Biomark Cancer. 2018;10:1–16. doi: 10.1177/1179299X18756646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meenal R, Sameer G, Manisha S. Biomarkers towards ovarian cancer diagnostics: present and future prospects. Braz Arch Biol Technol. 2016;59:1–15. doi: 10.1590/1678-4324-2016160070. [DOI] [Google Scholar]
- 22.Ann RH, Gunnar K, Andy E, Cybil A, Maria PBG, Philip B, et al. Evaluation of prognostic and predictive significance of circulating micrornas in ovarian cancer patients. Dis Mark. 2017;3:1–9. doi: 10.1155/2017/3098542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hentze JL, Hogdall C, Kjaer SK, Blaakaer J, Hogdall E. Searching for new biomarkers in ovarian cancer patients: rationale and design of a retrospective study under the Mermaid III project. Contemp Clin Trials Commun. 2017;13(8):167–174. doi: 10.1016/j.conctc.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marta L, Rui P, Susanne B, Ulla E, Sonja G, Christina B. Platelet protein biomarker panel for ovarian cancer diagnosis. Biomark Res. 2018;6:2. doi: 10.1186/s40364-018-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karen HL. Screening for ovarian cancer in asymptomatic women. JAMA. 2018;319(6):557–558. doi: 10.1001/jama.2017.21894. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359(6378):926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the us preventive services task force. JAMA. 2018;319(6):595–606. doi: 10.1001/jama.2017.21421. [DOI] [PubMed] [Google Scholar]
- 28.Montagnana M, Benati M, Danese E. Circulating biomarkers in epithelial ovarian cancer diagnosis: from present to future perspective. Ann Transl Med. 2017;5(13):276. doi: 10.21037/atm.2017.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pendlebury A, Hannan NJ, Binder N, Beard S, Mcgauran M, Grant P, et al. The circulating microRNA-200 family in whole blood are potential biomarkers for high-grade serous epithelial ovarian cancer. Biomed Rep. 2017;6(3):319–322. doi: 10.3892/br.2017.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kafshdooz L, Pourfathi H, Akbarzadeh A, Kafshdooz T, Razban Z, Sheervalilou R, et al. The role of microRNAs and nanoparticles in ovarian cancer: a review. Artif Cells Nanomed Biotechnol. 2018;23:1–7. doi: 10.1080/21691401.2018.1454931. [DOI] [PubMed] [Google Scholar]