Abstract
Hepcidin is a 25-amino acid peptide hormone produced by hepatocytes and plays a key role in body iron metabolism. Hepcidin deficiency is the cause of iron overload in hereditary hemochromatosis, iron-loading anemia, and its excess is associated with anemia of inflammation, chronic disease and iron deficiency anemia (IDA). The aims of this study was to evaluate HAMP gene mutation, namely IVS2 + 1(–G) (c.148–150 + 1del) and Gly71 Asp (c.212G > A (rs104894696) association with iron status in IDA conditions. Our study participants were 500 IDA patients and 550 age and sex-matched healthy controls. Hepcidin, ferritin and CRP analysis was done by ELISA method while ESR analysis was done according to Wintrobe method. CBC analysis was done by auto-analyzer. Two mutations in the HAMP genes were analysed by PCR RFLP method. Among the IDA patients, 7 were heterozygous for Met50del IVS2 + 1(–G) mutation. Nine IDA patients were heterozygous for G71D G–A mutation and homozygous were not identified in both mutations.Controls were showing heterozygous frequency 1.8 and 2.1% of Met50del IVS2 + 1(–G) and G71D G–A mutations respectively. Mutation of HAMP (Met50del IVS2 + 1(–G) and G71D G–A) were clinically associated with IDA and act as modulator of disease.
Keywords: Genotype, HAMP, Ferritin, IDA
Introduction
Hepcidin is a protein found in humans and encoded by the HAMP (hepcidin antimicrobial peptide) gene. Hepcidin plays a key role in body iron metabolism by preventing the release of iron from macrophages and intestinal cells. Hepcidin is a key regulator of the entry of iron into the circulation in humans. Defective hepcidin synthesis causes iron loading, while overproduction results in defective reticuloendothelial iron release and iron absorption [1–3]. Inflammations resulted high level of hepcidin where serum iron falls due to iron trapping within macrophages and liver cells and decreased gut iron absorption and leads to anemia due to an inadequate amount of serum iron being available for developing red cells. Iron overload occurs due to excessive ferroportin mediated iron influx in hemochromatosis when the hepcidin level is abnormally low. Iron deficiency anemia (IDA) is caused by the failure of adequate iron absorption and studies have found that measuring hepcidin would be of benefit to establish optimal treatment in IDA, although as this is not widely available, C-reactive protein (CRP) is used as a surrogate marker [4–6]. Induced iron loading in the liver was found to be associated with increased HEPC gene expression in mice experiment [7]. A study reported that a complete lack of hepcidin in mice leads to progressive iron pooling similarly the iron overload of human hemochromatosis, with excess iron in hepatic and reticuloendothelial cells [8]. Overexpression of hepcidin in the transgenic animals’ liver have decreased body iron levels and presented at birth a severe microcytic hypochromic anemia [9]. The role of hepcidin as an iron regulator whose induction results in a decrease in both dietary iron absorption and transplacental iron transport [10]. Inactivation of HAMP gene in mice leads to severe iron overload, whereas its overexpression in transgenic mice leads to IDA which is similar as in humans [8, 9]. It has been recently, demonstrated that an inappropriately low expression of HAMP mRNA is constant in hemochromatosis related to the HFE gene, both in humans and in animal models [6, 11–15]. In normal condition, hepcidine inhibit excess iron absorption in gut mucosa and maintain normal level within the body, while in mutant condition it losses the function and lead iron overload.This is the preliminary study on genetic form of iron haemostasis and clinical association with IDA. Our aim was to correlate the genotype–phenotype behaviour of HAMP(Met50del IVS2 + 1(–G) and G71D G–A) mutant condition in IDA.
Materials and Methods
Study Subject and Base Line Investigation
Study participants were 500 IDA patients and 550 age and sex-matched healthy controls. Fifty-eight percent recruited subject and 52% controls were belonging in tribal populations. From recruited subjects and controls, five millilitres of venous blood were collected after obtaining the signed informed consent. Study was approved by the institutional ethics committee. All IDA patients were diagnosed by iron profiling and complete blood count analysis. Hepcidin, ferritin and CRP analysis was done by ELISA method while ESR analysis was done according to Wintrobe method. Hemogramanalysis was done by auto-analyzer (SYSMEX K-4500, Kobe, Japan).
Detection of Mutation
Total genomic DNA was isolated from peripheral blood leukocytes by the kit (Bioserve Frenchtown, NJ, USA) method. Two mutations in the HAMP gene, namely the IVS2 + 1(–G) splice site mutation at the end of exon 2 and a G to A (Gly71Asp) substitution at nucleotide 212 in exon 3 were analysed by PCR(BIORADC1000™USA)RFLP(restriction fragment length polymorphism) method. Sense primer was 5′AGCAAAGGGGAGGGGGCTCAGACCAC and antisense primer was 5′ TCCCATCCCTGCTGCCCTGCTAAGGAC for Met50del IVS2 + 1(–G) mutation and BstF5I (NEB, UK) restriction enzyme was used for restriction digestion. G71DG-A mutation sense primer was 5′ TTGCCGGGAGCCAGTCTCAGAGGTCCAC and antisense primer was 5′ TGCAAGGCAGGGTCAGGACAAGCTCTTAGC and AciI (NEB, UK) restriction enzyme used for restriction according to published literature with few modifications [16, 17]. Student’s t test was used to compare the means of groups using GraphPad software (version 3.06). P < 0.05 was considered statistically significant.
Result
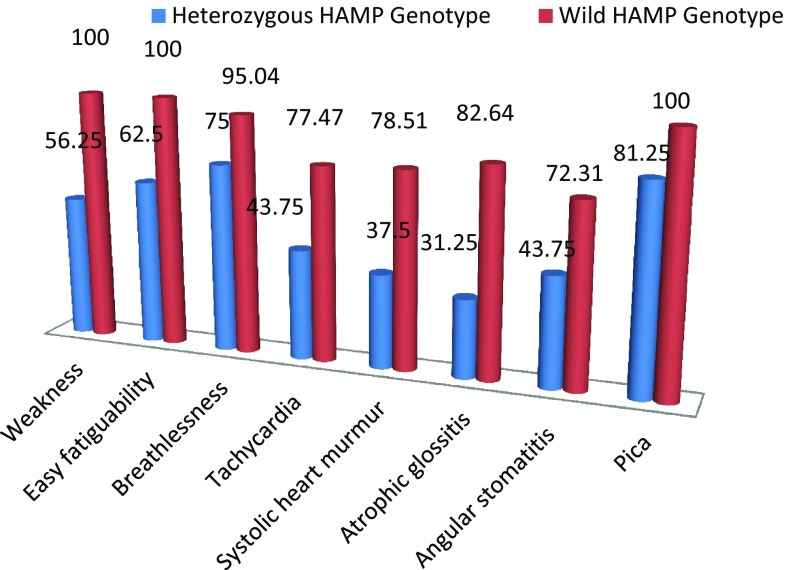
A total 500 IDA patients were evaluated (320 males and 180 females with a mean age of 23.7 ± 3.2 and 20.3 ± 2.6 years respectively). Out of 180 females, 88 were ingestation and 92 were in non gestation period. A complete blood count, iron profile, CRP and ESR were performed in all subjects as well as in age –sex matched controls (350 male and 200 female with mean age 23.1 ± 2.3 and 18.7 ± 3.6 years respectively). Out of 500 subjects studied in this study, 350 were tribal ethnic origin of Vindhyan region, Madhya Pradesh especially Kol, Gond, Panica and Khairwar tribes. Two HAMP gene mutations, namely the Met50del IVS2 + 1(–G) (c.148–150 + 1del splice site mutation) in intron 2 and a G to A substitution at nucleotide 212 position (p.Gly71Asp) (rs104894696) in exon 3 were analysed. Amongst the IDA patients, seven were heterozygous for Met50del IVS2 + 1(–G) mutation. Nine were heterozygous for G71D G–A mutation. Controls revealed heterozygous frequencies of 1.81 and 2.18% of Met50del IVS2 + 1(–G) andG71D G–A mutations respectively. Neither IDA patients nor controls were homozygous conditions for these mutations. There were no compound heterozygotes of two alleles. Comparative iron profile, value of hepcidin, CRP and ESR of heterozygous and wild genotype IDA patients is given in Table 1. Comparative frequency of clinical symptom is given in Fig. 1.
Table 1.
Laboratory findings according to the HAMP genotype in iron deficiency anemia
| Iron/biochemical profile | Mean ± SD | T test | |||
|---|---|---|---|---|---|
| Heterozygous variationa (N = 16) | Wild type (N = 484) | SE | 95% CI | P value | |
| Serum ferritin (μg/L) | 11.7 ± 2.5 | 10.8 ± 1.3 | 0.343 | 0.225 to 1.575 | 0.009 |
| TIBC (μg/dL) | 435 ± 6.8 | 490.6 ± 80.6 | 20.172 | − 95.232 to − 15.968 | 0.006 |
| % transferrin saturation | 14.7 ± 3.8 | 12.6 ± 2.4 | 0.957 | − 3.980 to − 0.220 | 0.028 |
| ESR (mm/h) | 19 ± 3 | 23 ± 4 | 1.010 | − 5.98 to − 2.02 | < 0.001 |
| CRP (mg/L) | 0.9 ± 0.5 | 1.2 ± 0.3 | 0.078 | − 0.454 to − 0.146 | < 0.001 |
| HGB (g/dL) | 7.4 ± 1.5 | 6.5 ± 1.3 | 0.332 | 0.24776 to 1.55224 | 0.006 |
| Hepcidine (ng/mL) | 25.6 ± 4.3 | 37 ± 6.5 | 1.638 | − 14.617 to − 8.183 | < 0.001 |
a Heterozygous for Met50del IVS2 + 1(–G) and G71D G–A, respectively
Fig. 1.
Comparative clinical parameters of HAMP genotype (Met50del IVS2 + 1(–G) and G71D G–A)
Discussion
With objectives of this study, a clinical association between HAMP gene mutation and phenotypic expression in IDA have been studied. Identified genetic variants in the HAMP gene and associations between the genotypes were found in iron status parameters. Various forms of hemochromatosis appear due to hepcidin deficiency, ultimate cause of either due to mutations in the HAMP gene itself or due to mutations in the regulators of hepcidin synthesis. Hepcidin estimation as the pathogenic factor in most systemic iron disorders should provide important opportunities for improving their diagnosis and treatment [18]. HAMP gene expression modifications further suggest an important role for hepcidin in iron homeostasis under various pathophysiological conditions, which may support the pharmaceutical use of hepcidin agonists and antagonists in various iron homeostasis disorders [10].
We reported the significant elevation of serum ferritin and haemoglobin level in Met50del IVS2 + 1(–G) and G71D G-A heterozygote’s while decreasing level of ESR and CRP were observed. Level of hepcidin and TIBC were elevated in HAMP (Met50del IVS2 + 1(–G) and G71D G–A) wild type while % transferrin saturation level was elevated in heterozygous and p values were statistically significant.
A new mutation (C70R) of HAMP was discovered, which affects 1 of the 8 conserved cysteines that form the disulfide bonds and are critical for the stability of the polypeptide [19]. The c.–582A > G HAMP promoter variant was not associated with serum iron, transferrin or ferritin levels in the healthy population. The c.–153C > T variant showed a frequency high enough to be considered when a genetic analysis is done in iron overload patients [20]. HAMP expressions play a key role in modulation of systemic iron levels. Mutation of HAMP express the deficient production of hepcidin that differences in the level of its expression may partly account for the phenotypic variant in iron metabolism between individuals. Hepcidin has been explored as an indicator of iron status in more complex clinical scenarios [21].
Our controls had 1.8 and 2.1% heterozygous frequency of Met50del IVS2 + 1(–G) and G71D G–A mutations respectively while IDA patients were presenting 1.4 and 1.8% frequency of heterozygous for same mutations. This data suggested that frequencies of these mutations are approximately similar in controls as well as patients in Asian Indian populations. Another similar study did not report the HAMP genotype in their Indian populations [17]. G71D G–A mutation frequency was higher in patents as well as controls group. A study revealed the hepcidin is a diagnostic test for IDA and another study provides age- and sex-specific reference ranges of serum hepcidin concentration and indicates ferritin as the primary correlate of serum hepcidin concentration [22, 23]. Inactivating mutations of hepcidin result in a rare form of juvenile haemochromatosis whereas hepcidin overexpression in inflammation causes anemia of chronic diseases with features of iron restricted erythropoiesis in humans [24]. Concentrations of hepcidin can be used in the management iron-loading anemias. Hepcidin is key in the diagnosis of iron refractory IDA and can be used to the diagnosis of iron deficiency in patients with anemia of chronic diseases [25, 26]. Erythropoietin response and intravenous iron analysis can be guided by potential marker of hepcidin and might be used in treatment decision [27]. Diagnostic and therapeutic approaches of this new knowledge are beginning to emerge. The role of hepcidin in iron haemostasis could lead to new opportunities for therapies of hemochromatosis and anemia of inflammation [28].
Typical clinical symptoms of iron deficiency anaemia were less severe in HAMP heterozygous. Findings of this study showed the HAMP heterozygous mutation may be beneficial of iron deficiency anemia patients and need to be diagnosed by HAMP genotyping. HAMP genotyping could be used in treatment decision and overcoming from the iron overload and chelation therapy.
Conflict of interest
None.
Ethical Standards
Study financially supported by Indian Council of Medical Research (ICMR), New Delhi, India—No. 45/4/2014-Hum/BMS.
Ethical Approval
Study was approved by Awadhesh Pratap Singh University Ethics committee. Samples were collected after taken signed consent from patients.
References
- 1.Maria AM, Milena C, Rita C, Gabriella S, Susanna B, Antonio C, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93:1473–1479. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 2.Hunter HN, Fulton DB, Ganz T, Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277(40):37597–37603. doi: 10.1074/jbc.M205305200. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 4.Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93(1):90–97. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 5.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict no responsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88(2):97–101. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 6.Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120(12):4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new mouse liver specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is over expressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI0215686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, et al. Disrupted hepcidin regulation in HFE-associated hemochromatosis and the liver as a regulator of body iron homeostasis. Lancet. 2003;361:669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 12.Gehrke SG, Kulaksiz H, Herrmann T, Riedel HD, Bents K, Veltkamp C, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102:371–376. doi: 10.1182/blood-2002-11-3610. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, et al. Decreased liver hepcidin expression in the HFEknockout mouse. Blood Cells Mol Dis. 2002;29:361–366. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 14.Muckenthaler M, Roy CN, Custodio AO, Miñana B, deGraaf J, Montross LK, et al. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet. 2003;34:102–107. doi: 10.1038/ng1152. [DOI] [PubMed] [Google Scholar]
- 15.Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34:97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 16.Merryweather-Clarke AT, Cadet E, Bomford A, Capron D, Viprakasit V, Miller A, et al. Digenic inheritance of mutations in HAMP and HFE results in different types of Haemochromatosis. Hum Mol Genet. 2003;12(17):2241–2247. doi: 10.1093/hmg/ddg225. [DOI] [PubMed] [Google Scholar]
- 17.Shukla P, Julka S, Bhatia E, Shah S, Nagral A, Aggarwal R. HFE, hepcidin and ferroportin gene mutations are not present in Indian patients with primary haemochromatosis. Natl Med J India. 2006;19:20–23. [PubMed] [Google Scholar]
- 18.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 19.Antonella R, Filomena D, Paolo P, Roberta C, Cox Timothy M, Cazzola Mario, et al. Screening hepcidin for mutations in juvenile hemochromatosis: identification of a new mutation (C70R) Blood. 2004;103:2407–2409. doi: 10.1182/blood-2003-10-3390. [DOI] [PubMed] [Google Scholar]
- 20.Silvia P, Arturo G, Joaquín C, Celsa Q, Fernando D. Genetic study of the hepcidin gene (HAMP) promoter and functional analysis of the c.–582A > G variant. BMC Genet. 2010;11:110. doi: 10.1186/1471-2156-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 22.Pasricha S, McQuilten Z, Westerman M, Keller A, Nemeth E, Ganz T, et al. Serum hepcidin as a diagnostic test of iron deficiency in premenopausal female blood donors. Haematologica. 2011;96(8):1099–1105. doi: 10.3324/haematol.2010.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, Wetzels JF, Kiemeney LA, Sweep FC, den Heijer M, Swinkels DW. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117(25):e218–e225. doi: 10.1182/blood-2011-02-337907. [DOI] [PubMed] [Google Scholar]
- 24.Island ML, Jouanolle AM, Mosser A, Deugnier Y, David V, Brissot P. A new mutation in the hepcidin promoter impairs its BMP response and contributes to a severe phenotype in HFE related hemochromatosis. Haematologica. 2009;94:720–724. doi: 10.3324/haematol.2008.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113(21):5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 26.Swinkels DW, Wetzels JF. Hepcidin: a new tool in the management of anaemia in patients withchronic kidney disease? Nephrol Dial Transplant. 2008;23(8):2450–2453. doi: 10.1093/ndt/gfn267. [DOI] [PubMed] [Google Scholar]
- 27.Nemeth E, Ganz T. The Role of Hepcidin in Iron Metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]