Abstract
Thiamine or vitamin B1 is a well known coenzyme and nutrient necessary for the assembly and right functioning of several enzymes involved in the energy metabolism. The present study evaluates oxidative stress and prevalence of neurodegenerative conditions in the brain following TD. The study was carried out on mice (Musmusculus) in three groups, namely control and thiamine-deficient group for 8 (TD 8) and 10 (TD 10) days. Lipid peroxidation was determined in terms of reduced glutathione (GSH) and thiobarbituric acid reactive substance (TBARS). The level of antioxidant enzymes such as catalase (CAT), glutathione reductase, glutathione peroxidase (GPx), superoxide dismutase (SOD) and glutathione transferase (GST) were measured along with histopathological studies in all the groups. There was significant increase in the TBARS levels in group II (TD 8) and group III (TD 10) animals in comparison to controls (Group I). The GSH levels were found to be lower in both the treated groups. The level of antioxidant enzymes CAT (p < 0.001), glutathione reductase (p < 0.001), GPx (p < 0.001), SOD (p < 0.0001) were found to be significantly reduced in group III (TD 10) in comparison to controls. Histopathological studies showed moderated to extensive neuronal loss in group II and group III in comparison to control group. The increase in LPO and reduction in enzymes CAT, glutathione reductase, GPx, SOD, and GST following TD suggests mitochondrial dysfunction, neuronal loss acute oxidative stress that may impair the functioning of the brain along with the rise of neurodegenerative conditions in the affected animals.
Keywords: Thiamine, Brain, Mitochondria, Energy metabolism, Neurodegeneration
Introduction
Thiamine (vitamin B1) is water soluble vitamin which is required for assembly and proper functioning of several mitochondrial enzymes in cell function and energy production. Thiamine deficiency denoted as TD generates changes in brain function that closely model diseases with characteristics of neurodegeneration such as alcoholic brain disease, stroke, Alzheimer’s disease and multiple sclerosis. TD is one of the main causes of Wernicke’s encephalopathy. Mitochondria are the power house and key regulators of cell survival and death and are responsible for energy production of the cell. Thiamine is essential as cofactor of three mitochondrial enzyme complexes namely α-ketoglutarate dehydrogenase complex (KGDHC), pyruvate dehydrogenase complex (PDHC), and branched-chain α-keto acid dehydrogenase. These three enzyme complexes are central to mitochondrial energy production. Impaired energy metabolism in mitochondria due to reduced ATP production weakens calcium buffering and augments generation of reactive oxygen species (ROS) [1]. Impairment in mitochondria energy metabolism also severely affects the functioning of brain.
Though brain is only 2% of the human body weight yet it consumes 20% of the oxygen. It stores little energy but it is mostly reliant upon the constant supply of energy giving substrates. Any derailment in this pathway leads to alteration in neurological function, loss of consciousness and comma within minutes [2]. Brain is highly susceptible to oxidative imbalance due to its high oxygen utilization, energy demand and rich content of polyunsaturated fatty acids (PUFAs) that are highly vulnerable to lipid peroxidation, high levels of ROS and lower activity of antioxidant enzymes [3]. The main energy substrate of brain is glucose but under certain conditions brain has the ability to make use of other energy substrate such as ketone bodies during starvation and development [4] or lactate during periods of physical activity [5]. TD leads to multiple cascades of events such as impairment in brain glucose metabolism [6] and cerebral energy dysfunction, breakdown of the blood–brain barrier, distorted glutamate neurotransmission, buildup of amyloid precursor like protein, increased free radical production, and oxidative stress [7–9].
Mitochondria are main ATP generating organelle which is essential for cellular function [10]. During the production of energy, mitochondrial ROS is produced. Production and detoxification of mitochondrial ROS are firmly balanced. Oxidative stress occurs when cellular antioxidant defense mechanism are insufficient to keep ROS levels below a toxic threshold. When the production of ROS in mitochondrial exceeds and the ability of cell’s antioxidant system decreases, the levels of ROS rises which induces oxidative stress that can damage lipid, protein and DNA. These processes will eventually lead to cell injury and cell death [11]. The formation of ROS is prevented by antioxidant system: low molecular mass antioxidants (ascorbic acid and glutathione), enzymes regenerating the reduced forms of antioxidants, and ROS-interacting enzymes such as SOD, peroxidases and catalases [12]. These antioxidants enzymes prevent the formation of hydroxyl radical and protect the cell components from oxidative damage [13].
The sequential mechanistic pathway involving brain energy metabolism and oxidative due to TD cannot be revealed in autopsied human brain, however, experimental animals offer the opportunity to determine the plausible cause and extent of damage leading to neurodegeneration. The TD model will help to find out the combinatorial factors (biochemical and pathological) amounting to neuronal loss and oxidative damage in the brain. The sequential mechanism of events and their relationship in generating multiple cascading effects in TD and related neurodegenerative disorders is still unknown. Neurons are usually considered to be the most sensitive brain cells that are prone to disruption of oxidative metabolism by hypoxia, ischemia, or toxins. The aim of our study is to illustrate the oxidative stress related events in brain induced by TD which leads to the development of neurodegenerative conditions.
Materials and Methods
Chemicals
Pyrithiamine hydrobromide (P0256-1 mg) was purchased from Sigma-Aldrich. All others chemicals were purchased from Himedia (Mumbai, India) and all the chemicals were of analytical grade.
Animals
In the present experimental study, male Swiss mice (Mus musculus), 8–10 weeks old, and weighing 25 ± 5 g were used (DIPSAR, Delhi Institute of Pharmaceutical Sciences And Research, New Delhi). They were housed and fed with pelleted diet (Hindustan Unilever Limited) and water ad libitum. The animals were kept in a room and temperature was maintained between 28 and 32 °C. Animals were allowed to acclimatize in the environment for a week before the experiment. All the experiments protocols were followed according the guidelines laid down by the Animal Ethics Committee Rules and Regulations of the Institute.
Experimental Protocol and Induction of TD
In the present study mice were divided into three groups of animals. There were six animals in each group.
Group I Control
Group II Thiamine-deficient diet + pyrithiamine (TD 8 days)
Group III Thiamine-deficient diet + pyrithiamine (TD 10 days)
Experimental TD was induced in mice by providing them thiamine-deficient diet and animals were administered daily intraperitoneal injection of pyrithiamine (5 μg in 0.1 ml normal saline/10 g body weight) [14]. The control animals were given daily intraperitoneal injection of normal saline (0.1 ml saline/10 g body weight) and were fed with normal pelleted diet.
Tissue Preparation
Mice were sacrificed by cervical dislocation. Brains were removed from the sacrificed mice and cleaned with ice-cold saline. The mice brains were dissected in half for biochemical assays and the other half was used for histopathology. 10% tissue homogenate of the brain was prepared in the buffer containing 50 mM Tris–HCl, 1 mM EDTA, and 10% glycerol for estimation of biochemical parameters. All the experiments were replicated thrice.
Biochemical Analyses
Lipid peroxidation is the oxidative degradation of lipids. Lipid peroxide (LPO) levels were estimated by determining thiobarbituric acid reactive substances (TBARS) which are formed as a byproduct of lipid peroxidation by the method of Ohkawa et al. [15]. Reduced glutathione (GSH) levels were determined by Ellman method [16]. Enzymatic activity of catalase (CAT) was measured by the method of Claiborne [17]. The rate of decomposition of hydrogen peroxide (H2O2) was measured spectrophotometrically at 240 nm. Glutathione reductase activity was determined by the method of Carlberg and Mannervik [18]. The enzyme activity was measured by disappearance of NADPH at 340 nm. The glutathione peroxidase (GPx) activity was measured by determining the rate of oxidation of NADPH at 340 nm [19]. Superoxide dismutase (SOD) activity was measured by the method of Dhindsa et al. [20]. The change in absorbance was recorded at 560 nm due to formation of formazon, a reaction product of NBT. The activity of GST was measured by Habig et al. [21]. Protein estimation was done by Lowry’s method.
Histopathological Studies
For histopathological studies, brain was dissected and it was post fixed for 24 h in 10% formaldehyde and then rinsed and stored in PBS until sectioning. At the time of sectioning, brains were fixed in Bouin’s fixative and sections were prepared by using microtome at a thickness of 40 μm and stained with haematoxylin and eosin. Pathological changes were examined using a light microscope.
Biostatistics
The data were expressed as mean ± SEM. Student t test was used to compare the groups. The level of significance was chosen as p < 0.05.
Results
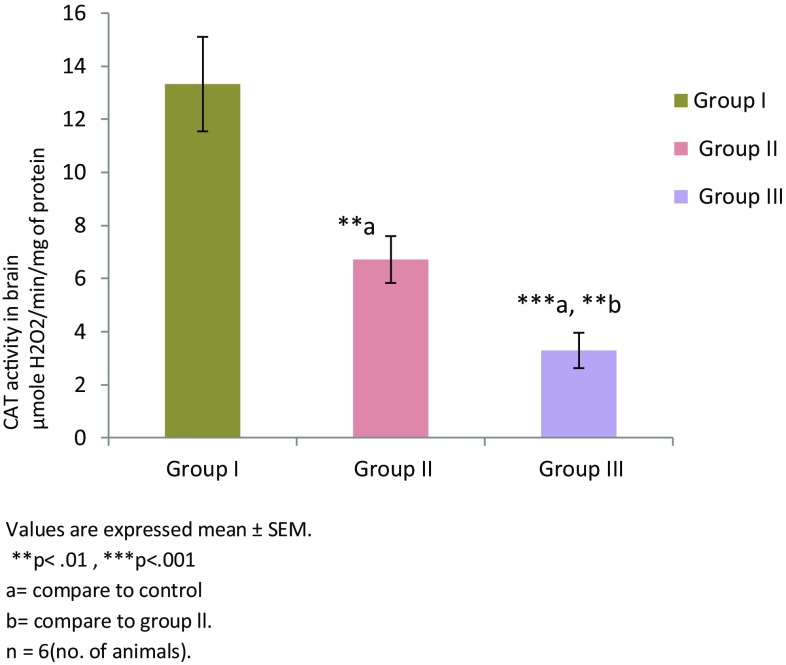
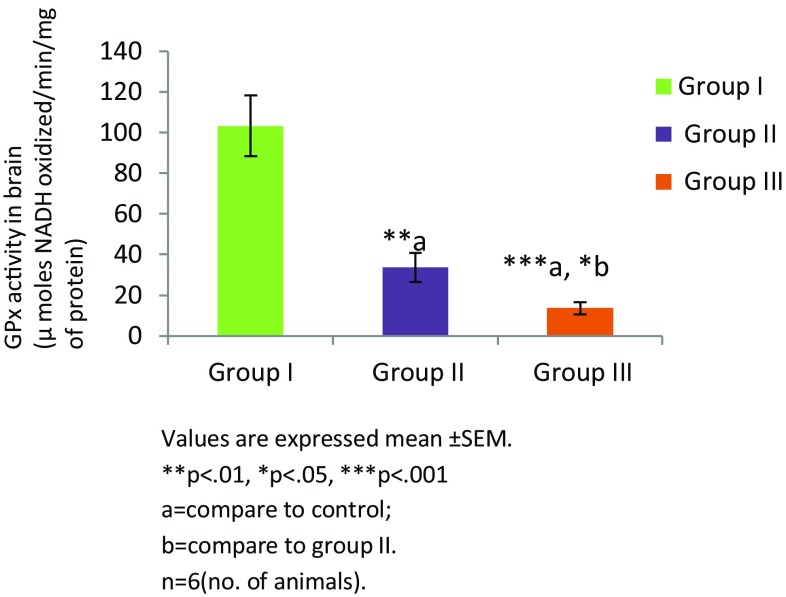
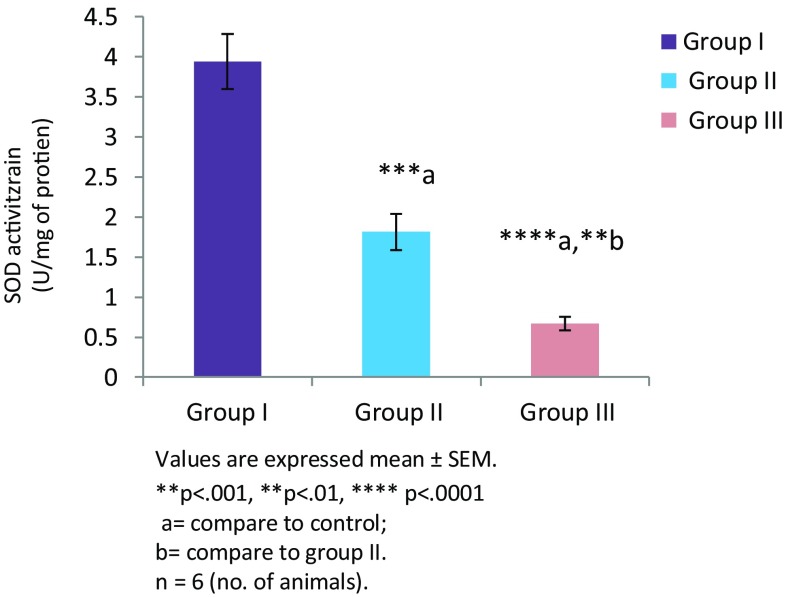
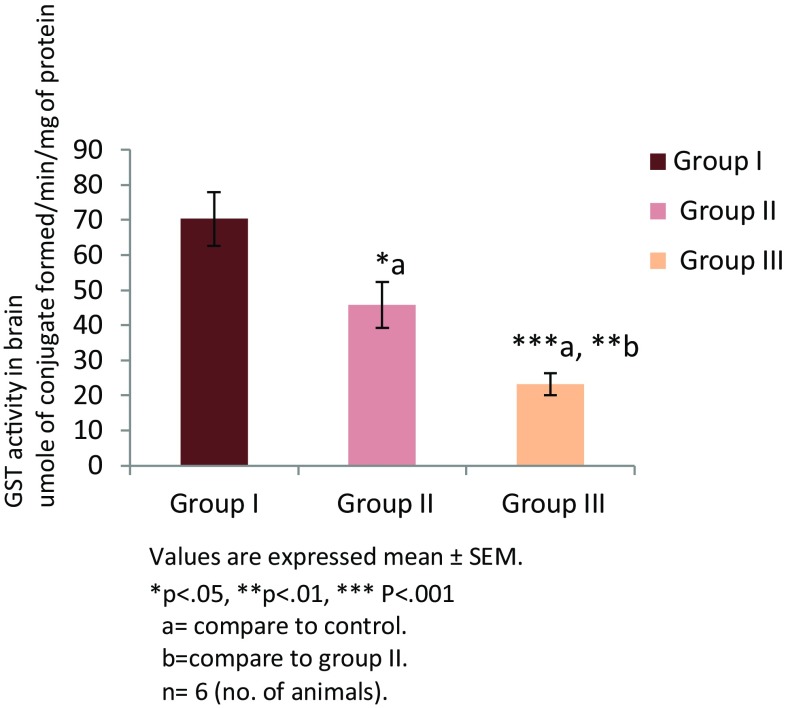
Thiamine deficiency altered enzymatic and non enzymatic defense mechanisms. Thiamine deficient treatment to mice for 8 and 10 days altered non enzymatic antioxidant LP level (Fig. 1). There was significant increase in the level of TBARS in group II (TD 8; **p < 0.01) and group III (TD 10; ****p < 0.0001) in comparison to group I (control). Among all the groups, significantly higher levels of malondialdehyde (MDA) were observed in group III (p < 0.001). The levels of reduced glutathione (GSH) were found to be decreased in group II and group III in comparison to the control group (Fig. 2). However, reduction in both the groups was not significant. Enzymatic activity of antioxidants was also found to be altered in thiamine deficient group of animals. A significant decline in the enzymatic activity of catalase (CAT) was seen in the TD brains of experimental groups (Fig. 3). The enzymatic activity was significantly lower in group II (**p < 0.01) and group III (***p < 0.001) animals in comparison to the control group. Similarly, a significantly reduction (Fig. 4) in activity of GR was also found in group II (p < 0.01) and group III animals (p < 0.001) which were made thiamine deficient. The enzymatic activity of glutathione peroxidase (GPx; Fig. 5) was also found to be significantly decreased in group II (**p < 0.01) and group III (***p < 0.001) animals in comparison to the control animals. A significant decline in the activity of superoxide dismutase (SOD) was also seen in group II (***p < 0.001) and group III animals (****p < 0.0001) in comparison to the control group (Fig. 6). Minimum activity of SOD was observed in group III among all experimental groups. The enzyme activity of glutathione transferase (GST) was also found to be significantly reduced (Fig. 7) in group II (p < 0.05) and in group III (p < 0.001) animals in comparison to the control group.
Fig. 1.
Levels of TBARS in brains of different experimental groups
Fig. 2.
Levels of reduced glutathione in brain of different experimental groups
Fig. 3.
CAT activities in brain of different experimental groups
Fig. 4.
Activity of glutathion reductase in brain of different experimental groups
Fig. 5.
Activity of glutathion peroxidase in brain of different experimental groups
Fig. 6.
Activity of SOD in brain of different experimental groups
Fig. 7.
Activity of GST in brain of different experimental groups
Histopathology of Brain
Brain sections of TD animals showed extensive damage as evidenced by necrosis, vacuolization and degeneration of neurons. The histological features of the brain in the control group showed a normal brain cortex architecture and cell structure. No evidence of inflammation or damage to any of the neuronal layers was seen (shown in Fig. 8a) in the cortex region. Brain sample from TD 8 group showed the shrinkage of several cortical neurons (indicated by arrows) as shown in the Fig. 8b. High power photomicrograph of brain sample from TD 10 group showed the shrinkage of several cortical neurons (indicated by arrows; HE × 100×) as shown in the Fig. 8c.
Fig. 8.
Histopathology of brain cortex. a High power photomicrograph of brain sample from control group showing the cortical neurons (Arrow). No evidence of inflammation or damage to any of the neuronal layers is seen. (HE × 100×). b High power photomicrograph of brain sample from TD 8 group showing the cortical neurons (Arrow). Shrinkage of several cortical neurons (Arrow) is seen. (HE × 100×). c High power photomicrograph of brain sample from TD 10 group showing the cortical neurons. Shrinkage of several cortical neurons (Arrow) is seen. (HE × 100×)
Discussion
The animal model of TD characterizes an established model of impaired cerebral oxidative metabolism that leads to selective neuronal loss and neurological symptoms [14]. Our study investigates the effects of TD on the brain oxidative machinery by determining alterations in enzymatic and nonenzymatic defense mechanisms. Thiamine is vital for functioning of cell as well as for energy production in the human body [22]. TD is induced in two groups (TD 8 and TD 10) by providing them with thiamine deficient diet in combination with treatment of thiamine antagonist’s pyrithiamine. Pyrithiamine inhibits transport of thiamine and demonstrate composite and diverse action on thiamine metabolism. It is a well recognized inhibitor of thiamine kinase. It provides a dependable method to make experimental TD in the mice that produce characteristics of oxidative stress, inflammation leading to neurodegeneration [23]. Mice start to lose weight after 8 or 9 days of pyrithiamine treatment. There is little or no neuronal loss on day 8 that is followed by ataxia, paralysis and extensive neuronal loss in select brain regions on day 9 or 10 [13].
TD diminishes the levels of reduced glutathione, which is the cofactor for glyoxalase [24]. Our results were also in agreement with these studies, levels of reduced glutathione GSH in the brain of thiamine deficient mice group [TD 8 days and TD 10 days] were found to be decreased in comparison to the control group. Glutathione (GSH) plays a key role in a many cellular processes including cell differentiation, proliferation, and apoptosis and therefore reduction in GSH will impact all these processes. As expected, in our studies the TBARS levels were found higher in the brain of mice of TD after 8 and 10 days. Reduced level of GSH promotes lipid peroxidation (LPO). Increased levels of LPO have also been reported by Madrigal and co-workers [25] in rat brain. The authors have reported that chronic stress induces increased level of LPO, glutathione exhaustion and causes mitochondrial dysfunction in rat brain.
The GSH reduction is also directly related to the rise of ROS/RNS, lipid peroxides and extremely reactive hydroxyl radicals that add to endorse free radical load and oxidative stress [26]. Oxidative stress is an outcome on account of differences between the production of free radicals or reactive oxygen species and capacity of body to detoxify their damaging effects through neutralization by anti-oxidants. Antioxidant enzymes such as CAT, SOD, GST, GPx and GR prevent generation of ROS and hydroxyl radicals and protect the cellular constituents from oxidative damage which leads to cell injury. In our studies a significant decrease was found in the activities of these enzymes in the brain of TD mice group for 8 and 10 days as compared to the control group. Antioxidant enzymes CAT and GPx convert H2O2 into water [27]. Another enzyme Glutathione reductase catalysis the reduction of oxidized GSH (GSSG) to glutathione (GSH). GSH present in the brain is a key antioxidant that protects cells from free radical generation [13]. It has long been recognized that high levels of free radicals or reactive oxygen species (ROS) can inflict direct damage to lipids. Lipid peroxidation produces a wide variety of oxidation products. Among the many different aldehydes which are formed as secondary products during lipid peroxidation are malondialdehyde (MDA), propanal, hexanal, and 4-hydroxynonenal (4-HNE). These changes will further impair cellular functions [28].
Increased level of oxidative stress may result in enhanced superoxide (O2 −) production and shortage in the antioxidant enzyme production. An excess production of ROS superoxide radical O2 − has been found to be involved in synaptic plasticity, impaired memory function and neuronal death. The superoxide dismutase enzymes defend neurons from high O2 − environment. Therefore reduced activity of SOD compromises defenses against oxidative stress and eventually enhances peroxynitrite production gradually as part of the secondary damage [29, 30]. H2O2 is the most toxic molecules in the brain and antioxidant enzymes such as CAT and GPx helps to get rid of H2O2. Ansari and Scheff, (2010) [31] have reported decrease activity of CAT in mitochondria that indicates loss of major antioxidant defenses against ROS. Glutathione peroxidase (GPx) activity was also low in TD animals. Our results are in conformity with the previous report that suggests that low amount of NADPH in TD decreases GPx activity in the heart tissue [32]. Another detoxification enzyme is Glutathione-S-transferase (GST), which lessens damage caused from the product of lipid peroxidation (4-HNE and acrolein) by catalyzing its conjugation with GSH [33]. Depletion of GST could result in increased protein modification or dysfunction and decline of GSH that will further aggravate the oxidative stress.
The cerebral cortex region in dogs has been found to be severely affected in thiamine pyrophosphate (TPP)-dependent enzymes accompanied by reduced mitochondrial mass and oxidative phosphorylation capacity and increase in the oxidative stress [34]. Similar to the above observations, studies on TD induced neurodegeneration has been confirmed by our histological studies in the cortex region of mice brains. Neurodegeneration in the tissues is characterized by morphological changes such as neuronal loss and vacuolization. TD induces various changes in microglia, mast cell, endothelial cells and astrocytes. All these elements combine and cause neuronal death. Clingason and Gibson 2000 [35] also suggested that TD induces changes in these cell types that lead to neuronal changes. The decline in the neuronal population was observed in TD. The loss of neurons progresses eventually and by 10 days there is acute neuronal loss and damage. These anatomical changes will profusely affect the brain physiology. However, further studies are warranted to find out the tissue specific changes in other regions of brain and their correlation with memory, behavioral and other cognitive deficits observed during the later phases of TD.
Conclusion
Thiamin is an antioxidant, necessary for the production of energy and right functioning of neurons. Thiamine deficiency (TD) models the impairment of oxidative metabolism and reduction in thiamin-dependent processes leads to neurodegenerative disorders. TD induced changes of oxidative metabolism advances oxidative stress that leads to neuronal damage in specific brain regions. Although TD induced changes has been underlined in neurological disorders with depleted metabolic processes in brain, a straight relation to the pathophysiology has been missing. Our studies have been able to link the TD induced biochemical changes in brain with the pathological findings.
Acknowledgements
We acknowledge Professor Aditya Shastri, Vice Chancellor, Banasthali University, Rajasthan for providing suitable facilities and funding to carry out the research in the department of BioScience and Biotechnology. We are also thankful to Department of Science and Technology (DST) for providing funding to Ms. Anisha Chauhan under women scientist scheme.
References
- 1.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 2.Zielke HR, Zielke CL, Baab PJ. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. J Neurochem. 2009;09(Suppl 1):24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alz Dis. 2006;10:59–73. doi: 10.3233/JAD-2006-10110. [DOI] [PubMed] [Google Scholar]
- 4.Magistretti PJ. Brain energy metabolism. In: Squire LR, Berg D, Bloom FE, Du Lac S, Ghosh A, Spitzer NC, editors. Fundamental neuroscience. San Diego: Academic Press; 2008. pp. 271–293. [Google Scholar]
- 5.Van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 6.Hakim AM, Pappius HM. The effect of thiamine deficiency on local cerebral glucose utilization. Ann Neurol. 1981;9(4):334–339. doi: 10.1002/ana.410090404. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa H, Watanabe IS, Fursue T, Iwasaki Y, Satoyoshi E, Sumi T, et al. Low energy levels in thiamine deficient encephalopathy. J Neuropathol Exp Neurol. 1984;43:276–287. doi: 10.1097/00005072-198405000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Harata N, Iwasaki Y. Evidence for early blood-brain barrier breakdown in experimental thiamine deficiency in the mouse. Metab Brain Dis. 1995;10:159–174. doi: 10.1007/BF01991863. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins P, Butterworth RF. Pathogenesis of selective neuronal loss in Wernicke–Korsakoff syndrome: role of oxidative stress. In: Jordan F, Patel MS, editors. Thiamine: catalytic mechanisms and role in normal and disease states. New York: Marcel Dekker; 2003. pp. 339–347. [Google Scholar]
- 10.Koopman WJ, Distelmaier F, Smeitink JA, Willems PH. OXPHOS mutations and neurodegeneration. EMBO J. 2013;32(1):9–29. doi: 10.1038/emboj.2012.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Reyes I, Cuezva JM. The H(+)-ATP synthase: a gate to ROS-mediated cell death or cell survival. Biochim Biophys Acta. 2014;1837(7):1099–1112. doi: 10.1016/j.bbabio.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Bist R, Bubber P. Thiamine deficiency induces oxidative stress in brain mitochondria of Mus musculus. J Physiol Biochem. 2013;69:539–546. doi: 10.1007/s13105-013-0242-y. [DOI] [PubMed] [Google Scholar]
- 14.Bubber P, Ke ZJ, Gibson GE. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem Int. 2004;45:1021–1028. doi: 10.1016/j.neuint.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohshi N, Yagi K. Assay or lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC handbook of methods in oxygen radical research. Boca Raton, FL: CRC Press; 1985. pp. 283–284. [Google Scholar]
- 18.Carlberg I, Mannervik B. Glutathione reductase levels in rat brain. J Biol Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- 19.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller D. Differential distribution of glutathione and glutathione related enzymes in rabbit kidneys: possible implication in analgesic neuropathy. Can Res. 1984;44:5086–5091. [Google Scholar]
- 20.Dhindsa RH, Plumb-Dhindsa P, Thorpe TA. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jokoby WB. Glutathione-S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 22.Jhala SS, Hazell AS. Modeling neurodegenerative disease path physiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem Int. 2011;58:248–260. doi: 10.1016/j.neuint.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Gibson GE, Ksiezak-Reding H, Sheu KF, Mykytyn V, Blass JP. Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem Res. 1984;9(6):803–814. doi: 10.1007/BF00965667. [DOI] [PubMed] [Google Scholar]
- 24.Shangari N, Depeint F, Furrer R, Bruce WR, O’Brien PJ. The effects of partial thiamin deficiency and oxidative stress (i.e., glyoxal and methylglyoxal) on the levels of alpha-oxoaldehyde plasma protein adducts in Fischer 344 rats. FEBS Lett. 2005;579:5596–5602. doi: 10.1016/j.febslet.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Madrigal JL, Olivenz R, Moro MA, Lizasoin I, Lorenza P, Rodrigo J, et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. J Neuropsychopharmacol. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 26.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425(2):313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 28.Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, Oliveira CR. Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer’s disease: from pathogenesis to biomarkers. Int J Cell Biol. 2012;2012:735206. doi: 10.1155/2012/735206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callio J, Oury TD, Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J Biol Chem. 2005;280:18536–18542. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, et al. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- 31.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69(2):155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioda CR, Tde Oliveira Barreto, Primola-Gomes TN, de Lima DC, Campos PP, Capettini LSA, et al. Cardiac oxidative stress is involved in heart failure induced by thiamine deprivation in rats. Am J Physiol Heart Circ Physiol. 2010;298:H2039–H2045. doi: 10.1152/ajpheart.00820.2009. [DOI] [PubMed] [Google Scholar]
- 33.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernau K, Napoli E, Wong S, Ross-Inta C, Cameron J, Bannasch D, et al. Thiamine deficiency-mediated brain mitochondrial pathology in Alaskan Huskies with mutation in SLC19A3.1. Brain Pathol. 2015;25(4):441–453. doi: 10.1111/bpa.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calingasan NY, Gibson GE. Vascular endothelium is a site of free radical production and inflammation in areas of neuronal loss in thiamine-deficient brain. Ann N Y Acad Sci. 2000;903:353–356. doi: 10.1111/j.1749-6632.2000.tb06386.x. [DOI] [PubMed] [Google Scholar]