Abstract
Systemic Lupus Erythematosus is an autoimmune disease with female preponderance. Anemia is found in 50% of Systemic Lupus Erythematosus patients. This is a cross sectional case control study with 30 female Systemic Lupus Erythematosus patients having inflammation associated anemia (Hemoglobin < 10.0 gm/dl) and 30 age matched controls with the aim to measure serum hepcidin and ferritin levels, correlate and study their role as homeostatic regulators of iron metabolism and utility as markers. Serum transferrin, ferritin, iron, total iron binding capacity, hsCRP, liver enzymes and renal parameters were analyzed by using automated analyser. Hepcidin levels were estimated by Sandwich-ELISA method. There was significant decrease in Iron (p < 0.0001), Iron Binding capacity (p < 0.0001), Transferrin (p < 0.0001) in patients, and a significant increase in inflammatory markers: hs-CRP (p < 0.0001), ESR (p < 0.0001) compared to controls. Significant increase in both Hepcidin (p < 0.0001) and Ferritin (p < 0.0001) was observed in patients with significant positive correlation (r = 0.711) with each other. Additionally, ferritin and hepcidin significantly positively correlated with hs-CRP and ESR (r = 0.526, 0.735); (r = 0.427, 0.742) respectively. Negative correlation with hemoglobin, iron, total iron binding capacity and transferrin with hepcidin (r = − 0.80, − 0.307, − 0.553, − 0.584) and ferritin (r = −0.722, − 0.22, − 0.654, − 0.728) was observed respectively. On ROC analysis both hepcidin and ferritin has sensitivity of 96.7%, specificity of 100% at cut-off values of 110 and 49 respectively. AUC of hepcidin was 0.993 and ferritin was 0.978. We have established a positive linear correlation between Hepcidin and Ferritin levels in disease activity and the changes correlated with the inflammatory state and anemia in patients, making them important mediators and potential markers of inflammation associated anemia.
Keywords: Ferritin, Hepcidin, Inflammation, Anemia of chronic disease, Systemic Lupus Erythematosus
Introduction
Systemic Lupus Erythematosus (SLE) is a prototypic autoimmune disease, affecting more than 300,000 in the United States and millions worldwide. It is characterized by a wide variety of clinical features and presence of numerous auto-antibodies, circulating immune complexes and widespread immunologically determined tissue damage [1]. Studies of racial tendencies showed that SLE more frequently affected non-Caucasian individuals such as African Americans, Hispanics and Asians [2]. The Copcord Bhigwan study, an ongoing, prospective population study from Pune, India, found a crude incidence rate of 1 per 25,000 persons i.e. 4 per 100,000 population per year.
SLE shows female preponderance in 80–90% of cases, with the median age of onset in Indian SLE being 24.5 years and the sex ratio (F:M) of 11:1 [3]. Of the two major scoring systems (SLEDAI, BILAG) to evaluate disease activity in SLE, the most commonly used is the SLE Disease Activity Index (SLEDAI) which comprises a list of 24 items, 16 clinical and 8 laboratory tests such as urinalysis, blood complement levels, anti-DNA antibody levels, low platelets and low white blood cell counts. A SLEDAI of 6 or more indicates an active disease requiring therapy.
By incorporating haemocytopenias into the revised American College of Rheumatology criteria for SLE, the experts of the field have acknowledged that the “haematologic system” is affected. Hematological manifestations of SLE are diverse [4] and the most frequent hematological manifestation is anemia, usually normochromic normocytic, reflecting chronic illness. Anemia is found in about 50% of SLE patients. Although it was initially suspected that anemia in SLE was a result of mainly antibody-induced damage of erythrocytes, evidence to date indicates that the causes of anemia in SLE vary and may be of immune or non-immune pathogenesis. In 60–80% cases of SLE, the cause of anemia is due to suppressed erythropoiesis from chronic inflammation [5]. Anemia of chronic disease (ACD), also known as “anemia of inflammation” response is due to increasing inflammatory cytokines and IL-6. A hallmark of ‘anemia of chronic disease’ is the development of disturbances of iron homeostasis, with increased uptake and retention of iron within cells of the reticulo-endothelial system. This leads to a diversion of iron from the circulation into storage sites of the reticulo-endothelial system, subsequent limitation of the availability of iron for erythroid progenitor cells and iron-restricted erythropoiesis which leads to hypoferremia.
Ferritin is an iron-binding molecule that stores iron in a biologically available form for vital cellular processes while protecting proteins, lipids, and DNA from the potential toxicity of this metal element. Ferritin is an acute-phase reactant and also plays an important role in iron storage and recycling. Two key factors that can regulate ferritin expression are iron and proinflammatory cytokines. Inflammation in SLE is likely to be a major driving force in up-regulating ferritin expression. These proinflammatory stimuli also induce the retention of iron in macrophages by down-regulating the expression of ferroportin, thus blocking the release of iron from these cells leading to anemia [6].
Hepcidin is a peptide hormone, also an acute phase protein, produced by the liver and appears to be the master regulator of iron homeostasis in humans and other mammals. The connection between hepcidin and iron metabolism was first made by Pigeon et al. [7] during studies of the hepatic responses to iron overload. Hepcidin functions is to regulate iron transport across the gut mucosa, thereby preventing excess iron absorption and maintaining normal iron levels within the body. Hepcidin also inhibits transport of iron out of macrophages. In states of high hepcidin levels, serum iron levels drops because iron is trapped inside macrophages. Hepcidin expression is induced by lipopolysaccharides (LPS) and IL-6 and is inhibited by TNFα [8]. Inflammation-induced hepcidin increase causes the hypoferremia that develops early during infections or inflammatory diseases.
The primary pathological findings in patients with SLE are those of inflammation, vasculitis, immune complex deposition and vasculopathy. In response to the inflammatory stimulus, C-reactive protein (CRP), an inducible protein is secreted which binds to pathogens and activates the complement to enhance opsonisation and clearance, even before the production of a specific IgM or IgG. CRP, a member of the pentraxin family of proteins, is the prototype of acute phase protein, secreted by the liver in response to inflammatory cytokines and can increase up to a 1000 fold in acute inflammations. Also CRP recognizes altered self and foreign molecules based on pattern recognition and is thought to act as a surveillance molecule for altered self and certain pathogens. This recognition provides early defense and leads to a proinflammatory signal and activation of the humoural, adaptive immune system. Hence measurement of CRP is used widely to monitor various inflammatory states [9]. In a recent study in hemophilic arthropathy patients with progressing joint destruction, hsCRP, a marker of acute inflammation is shown to be increased [10]. Erythrocyte sedimentation rate (ESR) though nonspecific, is a surrogate marker of inflammation and commonly used while CRP is a more sensitive acute phase reactant.
Iron deficient anemia (IDA), secondary to nutritional lack is common in Indian population, more so in women and is also common in patients with SLE. Serum ferritin provides an indirect estimate of body iron stores and is a significant positive predictor for IDA while serum Iron and RBC indices, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC) are not dependable to assess anemia. Serum Ferritin may be normal or elevated in inflammation making it difficult to differentiate between coexisting IDA from ACD. Hepcidin levels may help identify iron deficiency and institute therapy early.
The aim of present study is to measure the levels of Hepcidin and Ferritin in patients with SLE having inflammation associated anemia and to study their role as homeostatic regulators of iron metabolism and mediators of inflammation and correlate levels of hepcidin and ferritin with hemoglobin, inflammatory, and iron markers.
Materials and Methods
A cross sectional case control study of 30 female SLE patients presenting to the Department of Rheumatology, Nizam’s Institute of Medical Sciences, Hyderabad. The study was conducted in the Department of Biochemistry in collaboration with Department of Rheumatology, from April 2014 and July 2015. The study was approved by the hospital Institutional Ethical Committee (EC/NIMS/1396/2013). Detailed history and clinical examination was performed as per the devised study proforma.
Inclusion Criteria
All the female patients between the ages of 15 and 45 years, who were attending to our hospital, diagnosed as SLE according to Systemic Lupus International Collaborating Clinics (SLICC) Classification 2012 criteria with SLE Disease Activity Index (SLEDAI) ≥ 10.
Female SLE patients having ACD with Hemoglobin < 10 gm/dl. Subjects based on clinical and laboratory findings were diagnosed as having anemia in SLE patients by attending Rheumatologist.
Exclusion criteria Iron deficiency anemia (IDA) patients with ferritin < 20 ng/ml and TIBC > 400 μg/dl were excluded from the study. Female patients with active infections, multiple blood transfusions, hemolytic anemia, all inflammatory conditions other than SLE, chronic kidney disease, pregnancy, and chronic liver disease were excluded.
Controls Thirty controls, age matched females, were recruited from among normal apparently healthy population.
Inclusion criteria for controls Hemoglobin values > 12.0 g/dL, ESR- within normal limits and hsCRP < 3.0 mg/l.
Exclusion criteria for controls Adults who did not comply with the above, who had infections, blood transfusions, hemolytic anemia or any other inflammatory conditions, chronic kidney disease, pregnancy, chronic liver disease were excluded from the control group. Informed consent was obtained from all the study participants.
Sample collection Peripheral venous blood samples were collected in three tubes: tube without any anticoagulant was used for serum tests: Iron profile (transferrin, ferritin, iron, TIBC), hs-CRP, liver enzymes and renal parameters which were analyzed immediately using automated analyser (Roche cobas c501, USA), while serum samples for hepcidin estimation were stored at − 70 °C until analysis and later estimated by Sandwich-ELISA method (DRG Diagnostics, NJ, USA). Complete Blood Picture (CBP) was done immediately from the tube containing EDTA as anti-coagulant. ESR was estimated immediately by using ESR tube containing sodium citrate as anti-coagulant.
Statistical Analysis
The statistical analysis was done by Graph pad prism Version 6 and Medcalc-version 15.2.1 statistical software. In cases and controls, normally distributed variables were analyzed by independent t- test. Non-normally distributed variables were analyzed by Mann–Whitney U test. Correlation between variables was done by Spearman Rank correlation analysis. Comparison of Area under Curve (AUC) in Receivers Operating Characteristics (ROC) curves was performed. A p value of < 0.05 was taken as statistically significant.
Results
The mean ± SD of age in the female patients and female control subjects were 27 ± 9.5 and 29 ± 7.9 years respectively with no significant difference between the groups (Table 1). Out of 30 patients of SLE with ACD, 9 (30%) patients had thrombocytopenia, 7 (23.3%) patients have leucopenia, and 3 (10%) patients have pancytopenia.
Table 1.
Demography and comparison of iron profile, hemoglobin, ESR and hsCRP in controls and SLE patients
| Variables | Controls (n = 30) Mean ± SD or median (IQR) |
SLE patients (n = 30) Mean ± SD or median (IQR) |
p value |
|---|---|---|---|
| Age (mean ± SD, yrs) | 29 ± 7.9 | 27 ± 9.5 | 0.34 |
| Sex, N (%) | F = 30 (100%) | F = 30 (100%) | – |
| Iron (µg/dl) | 72.24 ± 26 | 51.3 ± 22.9 | 0.001a* |
| TIBC (µg/dl) | 339.9 ± 53.4 | 238.3 ± 71.7 | < 0.0001a* |
| Transferrin (mg/dl) | 287.5(243–321)# | 195 (162.5–214) | < 0.0001b* |
| %TIBC (saturation) | 21.2 ± 6.95 | 22 ± 10.3 | 0.72 |
| Hemoglobin (%) | 12.2 ± 0.2 | 8.3 ± 1.2 | < 0.0001a* |
| ESR (mm/h) | 14.2 ± 4.3 | 95 ± 42.5 | < 0.0001a* |
| hsCRP (mg/L) | 1.3 (0.4–3.1) | 18.5 (6.5–18.5) | < 0.0001a* |
Normally distributed data is expressed as Mean ± SD
p value was calculated between groups by : a unpaired student’s t test, b Mann–Whitney U test
*p < 0.05-statistical significance
#Non-normally distributed data expressed as Median (25th–75th percentile, interquartile range)
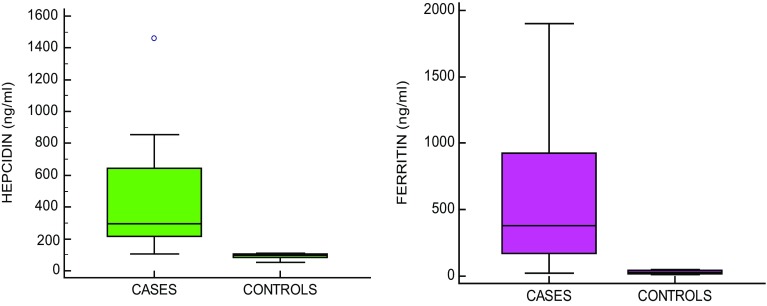
There is significant difference between the levels of hemoglobin and erythrocytes in SLE patients and controls; increase in the inflammatory markers hsCRP (p < 0.0001), ESR (p < 0.0001) and significant decrease in Iron (p < 0.0001), TIBC (p < 0.0001), Transferrin (p < 0.0001) in SLE patients as compared to controls (Table 1). A significant increase in both Hepcidin (p < 0.0001) and Ferritin (p < 0.0001) is observed in SLE patients as compared to controls (Table 2, Fig. 1).
Table 2.
Comparison of Ferritin and Hepcidin in Controls and SLE patients
| Variables | Controls (n = 30) Median (IQR) |
SLE patients (n = 30) Median (IQR) |
p Value |
|---|---|---|---|
| Ferritin (ng/ml) | 28.5 (14.4–45)# | 380.5 (171.8–959)# | < 0.0001b* |
| Hepcidin (ng/ml) | 98.5 (83.7–104)# | 280 (213.8–622.5)# | < 0.0001b* |
p value was calculated between groups by: a unpaired student’s t test, b Mann–Whitney U test
* p < 0.05-statistical significance
#Non-normally distributed data expressed as Median (25–75th percentile, interquartile range)
Fig. 1.
Serum hepcidin and ferritin in SLE patients and controls. Significant increase in hepcidin (p < 0.0001) and ferritin (p < 0.0001) in SLE pateints compared to controls
Ferritin, hsCRP and ESR were positively correlated (r = 0.711, 0.526, 0.735) respectively; whereas Hb, iron, TIBC and transferrin were negatively correlated (r = − 0.80, − 0.307, − 0.553, − 0.584) respectively with hepcidin and these relations were statistically significant (Table 3). Hepcidin, hs-CRP and ESR were positively correlated (r = 0.711, 0.427, 0.742) respectively; whereas Hemoglobin, TIBC and transferrin were negatively correlated (r = − 0.722, − 0.654, − 0.728) respectively, with ferritin and these were statistically significant; iron is negatively correlated (r = − 0.22) with ferritin and not statistically significant (Table 4).
Table 3.
Correlation of hepcidin with variables
| Variables | Hepcidin (ng/ml) | |
|---|---|---|
| r-valuea | p value | |
| Hb (gm/dl) | − 0.80 | < 0.0001* |
| Ferritin (ng/ml) | 0.711 | < 0.0001* |
| hs –CRP (mg/l) | 0.526 | < 0.0001* |
| ESR (mm/h) | 0.735 | < 0.0001* |
| Iron (µg/dl) | − 0.307 | 0.01* |
| TIBC (µg/dl) | − 0.553 | < 0.0001* |
| %Saturation | 0.0576 | 0.66 |
| Transferrin (mg/dl) | − 0.584 | < 0.0001* |
a‘r’-value is calculated by spearman correlation test
* p-value < 0.05 is consider as significant
Table 4.
Correlation of ferritin with variables
| Variables | Ferritin (ng/ml) | |
|---|---|---|
| r-valuea | p value | |
| Hb (gm/dl) | − 0.722 | < 0.0001* |
| Hepcidin (ng/ml) | 0.711 | < 0.0001* |
| hs-CRP (mg/L) | 0.427 | 0.0007* |
| ESR (mm/h) | 0.742 | < 0.0001* |
| Iron (µg/dl) | − 0.220 | 0.09 |
| %Saturation | 0.195 | 0.13 |
| TIBC (µg/dl) | − 0.654 | < 0.0001* |
| Transferrin (mg/dl) | − 0.728 | < 0.0001* |
a‘r’-value is calculated by spearman correlation test
* p-value < 0.05 is taken as significant
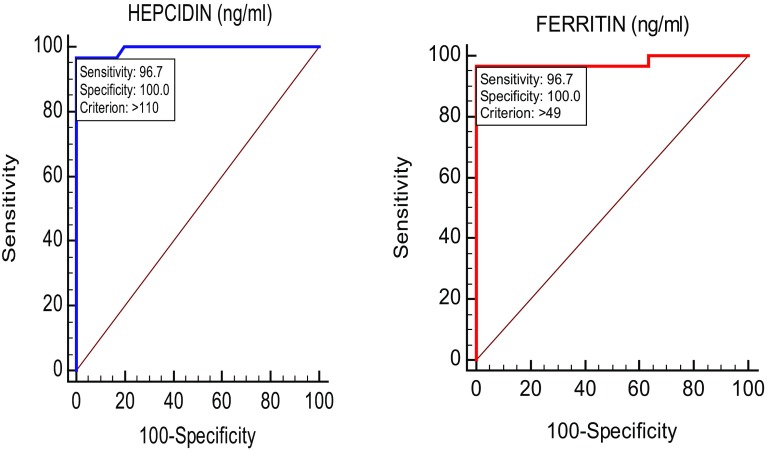
On ROC analysis both hepcidin and ferritin has sensitivity of 96.7%, specificity of 100% at cut-off values of 110 and 49 respectively. AUC of hepcidin was 0.993 and ferritin was 0.978 (Table 5, Fig. 2).
Table 5.
ROC analysis for ferritin and hepcidin
| Hepcidin (ng/ml) | Ferritin (ng/ml) | TIBC (µg/dl) | Transferrin (mg/dl) | |
|---|---|---|---|---|
| Sensitivity | 96.7% | 96.7% | 76.7% | 82.8% |
| Specificity | 100% | 100% | 93.3% | 100% |
| Area under curve (AUC) | 0.993 | 0.978 | 0.875 | 0.901 |
| Cut-off point | 110 | 49 | 282 | 225 |
Fig. 2.
ROC curves for hepcidin and ferritin. ROC analysis of hepcidin and ferritin: sensitivity is 96.7% and specificity is 100% at cut-off values of 110 and 49. AUC of hepcidin is 0.993 and ferritin is 0.978
Discussion
One of the molecules that is upregulated in SLE is ferritin: an acute phase protein that is produced in all tissues of reticulo-endothelial system. Previously, Shoenfeld and his coworkers provided evidence for hyperferritinemia in lupus [11], where it was suggested to be a biomarker in lupus. Our primary objective is to highlight the levels of two key proteins: ferritin and hepcidin in SLE patients and correlation of these proteins with each other, inflammatory status and hemoglobin levels in patients of ACD in SLE.
In our study, the median ferritin level in SLE patients is statistically significant (p < 0.0001) compared to controls, which is similar to a study done in population in western India (JAPI) [12]. Likewise, in a Japanese population, Nishiya et al. demonstrated elevated levels of serum ferritin in SLE patients as compared to RA patients and also reported that these increased levels are positively correlated with SLEDAI and those with SLEDAI scores ≥ 11 had significantly higher serum ferritin levels (p < 0.001) [13]. Similar studies in Turkish SLE patients [14] and a cluster of Korean SLE patients [15] yielded a positive correlation between SLEDAI and serum ferritin levels and suggested the use of serum ferritin as a marker of disease activity in SLE patients. There is increasing evidence that circulating ferritin levels may not only reflect an acute phase response but also may play a critical role in inflammation.
ESR and hsCRP which are non-specific markers of inflammation have shown a positive correlation with ferritin in our study {hs-CRP (r = 0.427, p = 0.0007) and ESR (r = 0.742, p = <0.0001)}. Mehmet and his colleagues have demonstrated that active SLE patients with hsCRP > 3.2 mg/l and ESR > 20 mm/h, have significantly higher ferritin levels [16]. Serum hsCRP and ferritin concentrations were increased in hemodialysis patients compared to controls and is attributed to the fact that serum ferritin being a potent acute phase reactant is usually associated with elevation of CRP levels and inflammation [17]. Taken together with the above mechanistic studies, our findings suggest that inflammation in SLE is likely to be a major driving force in up-regulating ferritin expression. A very recent study showed that serum hs-CRP and
IL-6 levels among active SLE were significantly higher as compared with inactive SLE [18].
Hepcidin, an acute phase protein also increases several folds during inflammation. Hepcidin is among the newer proteins identified to be involved in iron hemostasis. It acts as a systemic iron-regulatory hormone as it controls iron transport from iron-exporting tissues into plasma. Lee and his co-workers have noted that hepcidin transcription was stimulated not only by IL-6 but also by IL-1α and IL-1β in murine hepatocytes [19]. In human hepatocyte culture, hepcidin is induced by IL-6, but not IL-1 or TNF-α, indicating that hepcidin induction by inflammation is a type II acute phase response [20, 21]. The increase in TNF-α and IL-6 increases the hepatic secretion of hepcidin which represses the ferroportin expression and this situation stimulates iron retention in macrophages and prevents direct absorption of iron into circulation [22]. It was observed that, in decrease or absence of hepcidin, ferroportin was indicated to increase iron content by continuously absorbing iron [20].
Mehmet and his colleagues also observed that SLE patients have significant higher hepcidin levels, in active SLE group (hs-CRP > 3.2 mg/l, ESR > 20 mm/h) [16] similar to our study, corroborating the existence and influence of an inflammatory event. Hepcidin is thus a type II acute-phase protein that provides a molecular link between inflammation, resulting anemia, and the regulation of iron metabolism.
The present study showed, the levels of hepcidin in patients with anemia of chronic disease with SLE were found to be significantly high compared to healthy controls. We found the significant negative correlation between hemoglobin and hepcidin indicating active role of hepcidin in anemia. Urinary hepcidin peptide assayed in patients with anemia of inflammation due to chronic infections or severe inflammatory diseases, showed as much as a 100-fold increase in hepcidin excretion, with smaller increases in patients with less severe inflammatory disorders [23]. Also, Hepcidin levels were significantly higher in chronic disease anemia patients and reflected the underlying inflammatory event brought about by the increased IL-6 levels [8]. Though Roe and his colleagues demonstrated that hepcidin has no role in iron hemostasis in a study on healthy individuals [24], studies parallel to those showed that prohepcidin and hepcidin could play a central regulatory role in the hemostasis of iron in inflammation [24]. Hence in the pathobiology of inflammation and chronic disease anemia frequently encountered in SLE, hepcidin and cytokines are proved to play an active role together.
Thus, inflammation is well established to upregulate hepcidin-mediated degradation of the iron transport channel, ferroportin, thereby sequestering iron in the form of ferritin in the cells of reticuloendothelial system. This results in the unavailability of iron for hemoglobin production, which explains the anemia. Ferritin synthesis is regulated by intracellular iron at both the transcriptional and translational levels. When iron levels are low, ferritin synthesis is reduced and vice versa. Inflammation causes increase in intracellular iron, by iron sequestration leading to increase ferritin synthesis and making less availability of iron for hemoglobin production. In the present study, we found a significant increase in ferritin levels in SLE patients having ACD compared to healthy controls and a significant negative correlation between ferritin and hemoglobin levels. Significant correlation between hepcidin and ferritin in our study, confirmed that these 2 molecules are inter dependent: Hepcidin being the cause and Ferritin being the effect, similar to a study conducted by Mehmet et al. in 40 RA patients with anemia, who had higher hepcidin and ferritin levels than those with IDA and controls [22]. Ferritin level is the most frequently used parameter used to distinguish between chronic disease anemia and iron deficiency anemia as differential diagnosis. Studies have also suggested the use of the ratio of the the concentration of soluble transferring receptor to the log of the ferritin levels to differentiate between IDA coexisting with anemia of chronic disease [25]. In our study we excluded IDA in SLE anemia patients accepting ferritin < 20 ng/ml and TIBC > 400 µg/dl as cut off value. Iron-deficiency anemia and ‘anemia of chronic disease’ both exhibit reduced serum iron but are very different otherwise. Whereas anemia of chronic disease is marked by elevated ferritin but reduced transferrin levels, iron deficiency anemia exhibits the reverse profile. Indeed, the SLE patients described in this study exhibit ‘anemia of chronic disease’ rather than iron deficiency anemia.
In our study, we found significant lower levels of transferrin and TIBC in SLE patients having ACD compared to controls and a significant negative correlation was found between ferritin and transferrin. A similar study conducted in SLE patients, revealed elevated urine ferritin and transferrin, which correlated with each other, as well as with disease activity, inflammatory status and anemia while serum transferrin levels were lower in active SLE patients [26].
Transferrin was reported to be more useful than serum iron and TIBC in determining ACD. In our study, on ROC analysis, we found hepcidin and ferritin has AUC of 0.993 and 0.978 respectively, whereas transferrin and TIBC has AUC of 0.901 and 0.875 respectively. The ROC curve analysis indicated that the hepcidin and ferritin concentrations provided the highest diagnostic accuracy for discriminating ACD individuals (Fig. 2). Serum transferrin and TIBC were significantly poorer discriminators when compared to hepcidin and/or ferritin,. At the cutoff value for hepcidin (110 ng/ml) and ferritin (49 ng/ml) at which diagnostic accuracy was highest, the sensitivity and specificity were 96.7% and 100% respectively. The serum hepcidin may be of value for determining whether a low or borderline low hemoglobin concentration is due to the suppression of erythropoiesis by mediators of an inflammatory response as occurs in ACD. Further studies are required to establish the use of serum hepcidin levels as an early indicator of underlying anemia associated with inflammation. Given that most SLE patients with anemia have ‘anemia of chronic disease’ rather than iron-deficiency anemia, it is important to manage this accordingly in the clinics. Targeting the underlying inflammation rather than treating it with iron supplements may be a more fruitful approach in these patients.
Conclusion
We have established a positive linear correlation between hepcidin and ferritin levels in SLE disease activity, thus bolstering previous reports in other ethnic groups. Most importantly, the changes in hepcidin and ferritin levels correlated with the inflammatory state and anemia in SLE, making them potential markers of disease activity. Taken together, inflammation, elevated hepcidin, elevated ferritin, and “anemia of chronic inflammation” may constitute an ominous tetrad in SLE.
Conflict of interest
Neeraja Kunireddy, Rachel Jacob, Siraj Ahmed Khan, B. Yadagiri, K.S.S. Sai Baba, I. Rajendra Vara Prasad and Iyyapu Krishna Mohan declare that we have no conflict of interest.
Ethical Approval
The study was approved by the hospital Institutional Ethical Committee (EC/NIMS/1396/2013).
References
- 1.Laurence J, Wong JE, Nachman R. The cellular hematology of systemic lupus erythematosus. In: Lahita RG, editor. Systemic lupus erythematosus. 2. New York: Churchill Livingstone; 1992. p. 480. [Google Scholar]
- 2.Ramsey-Goldman R, Manzi S. Systemic lupus erythematosis. Women and health. Cambridge: Academic Press; 2000. p. 704. [Google Scholar]
- 3.Malaviya AN, Chandrasekaran AN, Kumar A, Sharma PN. Occasional series-lupus round the world: systemic lupus erythematosus in India. Lupus. 1997;6:690–700. doi: 10.1177/096120339700600903. [DOI] [PubMed] [Google Scholar]
- 4.Sasidharan PK. SLE as a hematological disease. In: Agarwal MB, editor. Hematolgy today. Mumbai: Vikas Publications; 2010. pp. 953–966. [Google Scholar]
- 5.Liu H, Ozaki K, MatsuzakiY Abe M, Kosaka M, Saito S. Suppression of haematopoiesis by IgG autoantibodies from patients with systemic lupus erythematosus (SLE) Clin ExpImmunol. 1995;100(3):480–485. doi: 10.1111/j.1365-2249.1995.tb03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 7.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig. 2004;113:1271–1276. doi: 10.1172/JCI200420945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuy AM, Terrier N, Sénécal L, Morena M, Leray H, Canaud B, Cristol JP. Is C-reactive protein a marker of inflammation? Nephrologie. 2003;24(7):337–341. [PubMed] [Google Scholar]
- 10.Hua B, Olsen EHN, Sun S, Gudme CN, Wang L, Vandahl B, Roepstorff K, Kjelgaard-Hansen M, Sørensen BB, Zhao Y, Karsdal MA, Manon-Jensen T. Serological biomarkers detect active joint destruction and inflammation in patients with haemophilicarthropathy. Haemophilia. 2017 doi: 10.1111/hae.13196. [DOI] [PubMed] [Google Scholar]
- 11.Zandman-Goddard G, Orbach H, Agmon-Levin N, Boaz M, Amital H, Szekanecz Z, Szucs G, Rovensky J, Kiss E, Corocher N, Doria A, Stojanovich L, Ingegnoli F, Meroni PL, Rozman B, Gomez-Arbesu J, Blank M, Shoenfeld Y. Hyperferritinemia is associated with serologic antiphospholipid syndrome in SLE patients. Clin Rev Allergy Immunol. 2013;44(1):23–30. doi: 10.1007/s12016-011-8264-0. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan V, Pandit P, Rajadhyaksha A, Patwardhan M, Surve P, Kamble P, Lecerf M, Bayry J, Kaveri S, Ghosh K, Nadkar MY. Association of serum ferritin levels with hematological manifestations in systemic lupus erythematosus patients from Western India. J Assoc Physicians India. 2016;64(5):14–18. [PubMed] [Google Scholar]
- 13.Nishiya K, Hashimoto K. Elevation of serum ferritin levels as a marker for active systemic lupus erythematosus. Clin Exp Rheumatol. 1997;15:39–44. [PubMed] [Google Scholar]
- 14.Beyan E, Beyan C, Demirezer A, Ertugrul E, Uzuner A. The relationship between serum ferritin levels and disease activity in systemic lupus erythematosus. Scand J Rheumatol. 2003;32:225–228. doi: 10.1080/03009740310003712. [DOI] [PubMed] [Google Scholar]
- 15.Lim MK, Lee CK, Ju YS, Cho YS, Lee RS, Yoo B, Moon HB. Serum ferritin as a serologic marker of activity in systemic lupus erythematosus. Rheumatol Int. 2001;20:89–93. doi: 10.1007/s002960000083. [DOI] [PubMed] [Google Scholar]
- 16.Dagli M, Yilmaz S, Sivrikaya A, Öztürk B. Role of prohepcidin andhepcidin in anemia associated with systemic lupus erythematosus. World Appl Sci J. 2011;13(9):2032–2036. [Google Scholar]
- 17.HyeSung W, Hyun G, Yu S. IL-6 is an independent risk factor for resistance to erythropoiesis-stimulating agents in hemodialysis patients without iron deficiency. Hemodial Int. 2012;16:31–37. doi: 10.1111/j.1542-4758.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 18.Umare V, Nadkarni A, Nadkar M, Rajadhyksha A, Khadilkar P, Ghosh K, Pradhan VD. Do high sensitivity C-reactive protein and serum interleukin-6 levels correlate with disease activity in systemic lupus erythematosuspatients? J Postgrad Med. 2017;63(2):92–95. doi: 10.4103/0022-3859.188550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12(2):107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Mok CC, Birmingham D, Ho LY, Hebert L, Rovin B. THU0302. Hepcidin, Interleukin-6 and Anemia of Chronic Inflammation in Systemic Lupus Erythematosus (SLE) Ann Rheum Dis. 2013;72:A268. doi: 10.1136/annrheumdis-2012-201393. [DOI] [Google Scholar]
- 22.Demirag MD, Haznedaroglu S, Sancak B, Konca C, Gulbahar O, Ozturk MA, Goker B. Circulating hepcidin in the crossroads of anemia and inflammation associated with rheumatoid arthritis. Inter Med. 2009;48:421–426. doi: 10.2169/internalmedicine.48.1578. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acutephase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 24.Roe MA, Spinks C, Heath AL, Harvey LJ, Foxall R, Wimperis J, Wolf C, Fairweather-Tait SJ. Serum prohepcidin concentration: no association with iron absorption in healthy men; and no relationship with iron status in men carrying HFE mutations, hereditary haemochromatosis, patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr. 2007;97(3):544–549. doi: 10.1017/S0007114507336829. [DOI] [PubMed] [Google Scholar]
- 25.Chogle AR. Utility of serum ferritin levels in the differenciation of anemia in Systemic Lupus Erythematosus. JAPI. 2016;64(5):11–12. [PubMed] [Google Scholar]
- 26.Vanarsa K, Ye Y, Han J, Xie C, Mohan C, Tianfu W. Inflammation associated anemia and ferritin as disease markers in SLE. Arthritis Res Ther. 2012;14:R182. doi: 10.1186/ar4012. [DOI] [PMC free article] [PubMed] [Google Scholar]